Abstract
Since interferon-α and imatinib (IM; STI571, Glivec, Gleevec) are effective for the treatment of chronic myeloid leukemia (CML), and their mechanisms of action are different, we designed an exploratory study investigating the effects of a standard IM dose (400 mg/d) and a variable pegylated interferon-α (PegIFN) dose (50 μg/wk, 100 μg/wk, and 150 μg/wk). The criteria for dose adjustment were designed so as to ensure the delivery of the IM dose and to protect life quality. There were 76 patients with previously untreated Philadelphia (Ph)–positive CML enrolled in the study. There were 3 patients who discontinued IM and 45 patients who discontinued PegIFN. The severity of adverse events increased with increasing PegIFN dose. The IM dose could be administered to the patients who were assigned to receive 50 μg/wk or 100 μg/wk PegIFN but not to those who were assigned to receive 150 μg/wk. The median administered dose of PegIFN ranged between 32 μg/wk and 36 μg/wk. The cytogenetic response was 70% complete (Ph-neg 100%) and 83% major (Ph-neg > 65%). The BCR/ABL transcript was reduced by at least 3 logs in 68% of complete cytogenetic responders. These data of toxicity, compliance, and efficacy may assist in the design and preparation of prospective studies.
Introduction
The introduction of imatinib (IM; STI571, Glivec, Gleevec), a protein tyrosine kinase inhibitor that suppresses Philadelphia-positive (Ph-pos) cells by a specific interference with the leukemogenic protein encoded by the bcr/abl fusion gene, has brought about a revolution in the treatment and the management of Ph-pos chronic myeloid leukemia (CML).1-10 In the short term, the superiority of IM over interferon-α (IFN-α), the first-choice agent for several years, is overwhelming.11-14 However, IFN-α is effective and may significantly prolong survival. This is particularly true in low-risk patients, where the cytogenetic response (CgR) to IFN-α may be higher than 50% and the survival of complete responders may be longer than 10 years, even if the disease remains detectable at a molecular level.15-17 On the other hand, the mechanism and the spectrum of IM activity leave doubts that IM alone may be sufficient to control leukemia in the long term. The doubts are based on the observation that the response to IM is related to the phase and the risk of the disease,12,13,18 and that resistance may develop through several mechanisms, including point mutations in the abl part of the gene, bcr/abl gene amplification/overexpression, and the occurrence of additional genetic abnormalities.19-25 While the mechanism of action of IM is rather well established,1-4 the mechanism of action of IFN-α is less known, and is likely to be multifactorial and different from that of IM. IFN-α can restore the adhesion of leukemic stem cells to marrow stroma, down-regulate the expression of the bcr/abl gene, and activate several transcriptional factors that regulate cell proliferation, maturation, and apoptosis.26-31 Moreover, and maybe more importantly, IFN-α can enhance the recognition and the elimination of the leukemic cells by the immune system.32-35 Although preclinical in vitro studies on the effects of the exposure of Ph-pos cells to IM and IFN-α have provided contrasting data,36,37 there is a wide clinical consensus that the combination of IM and IFN-α is worth testing, to prevent the emergence of resistance and to control and eliminate minimal residual disease. In preparation of a prospective randomized study, we designed an exploratory study to provide information on the side effects of the combination, on the deliverable doses, and on patient compliance. The results of this study were the subject of a preliminary report at the last meeting of the American Society of Hematology (ASH),38 and are now reported in full in this paper.
Patients and methods
This was an exploratory, phase 2 study of the Italian Cooperative Study Group (ICSG) on CML (currently GIMEMA Working Party on CML) to investigate the treatment of CML with IM and IFN-α. The purpose of the study was to explore which dose of IFN-α could be combined with a standard dose of IM, and to investigate the toxicity profile of the combination as well as the compliance to the treatment. The response to treatment was also assessed.
The protocol was designed, promoted, and sponsored by the ICSG with the support of Novartis Pharma and Schering Plough, which ensured the supply of the study drugs, free of charge, for the duration of the study. The study and the protocol were approved by the independent ethics committee of S. Orsola-Malpighi Hospital in Bologna, hence by the ethic committees of all participating centers, and was designed and performed according to the Declaration of Helsinki and to good clinical practice.
Patients
Patients were eligible if they had a confirmed diagnosis of Ph-pos CML in early chronic phase (CP), were previously untreated with either study drug, were younger than 70 years old, if their performance status (according to the World Health Organization [WHO]) was 0-1, and if they were able and willing to provide written informed consent, including a specific mention to pregnancy and to procreation. Exclusion criteria were hepatitis B virus (HBV), hepatitis C virus (HCV), or HIV positivity, alcohol or drug addiction, and severe unrelated disease.
Study drugs and treatment protocol
Study drugs were IM (also known as STI571, currently registered as Glivec or Gleevec; Novartis Pharma, East Hanover, NJ), and a pegylated preparation of human recombinant interferon-α2b (PegIFN; PegIntron; Schering Plough, Kenilworth, NJ). PegIFN had already been tested in the treatment of CML39-41 and was preferred to conventional IFN-α because of the weekly injection schedule.
The treatment protocol was designed so as to ensure that all the patients could receive the standard or recommended dose of IM (400 mg/d). The scheduled dose of PegIFN was 50 μg/wk in a first cohort of 27 patients, 100 μg/wk in a second cohort of 18 patients, and 150 μg/wk in a third cohort of 31 patients. The number of cases in each cohort was not identical because the enrollment was competitive, with a grace period of 15 days after the 20th case of each cohort, when additional patients could be enrolled. The 2 drugs were begun simultaneously (day 1). Treatment trial time was 1 year. Subsequent treatment was free, with the recommendation to continue with both drugs in case of response and if tolerated.
Dose adaptation and discontinuation
The basic principle of dose adaptation was to save the dose of IM over that of PegIFN. Stringent criteria were adopted for safety requirements, with attention to the severity of the adverse events (AEs) and also to the recurrence of the events in the same patient (Table 1).
For operational purposes, AEs were divided into hematologic (Hema) and nonhematologic (non-Hema). All the Hema AEs were attributed a priori to both drugs. In case of a Hema AE grade 1 or 2, no action was taken. Notice that in case of a Hema AE grade 3 third time, or grade 4 first time, PegIFN was allowed to be reassumed and continued only at the lowest dose level (50 μg/wk), and that in case of a Hema AE grade 3 fourth time, or grade 4 second time, PegIFN was discontinued forever. In case of a non-Hema AE, the investigators were required to identify which drug was responsible for that event. In case of a grade 3 non-Hema AE, PegIFN was allowed to be reassumed and continued only at the lowest dose level (50 μg/wk), while in case of repeated grade 3 or grade 4 non-Hema AEs, PegIFN was discontinued forever. IM was discontinued forever only after 2 grade 3 or one grade 4 non-Hema AEs attributable to IM itself (Table 1).
Required studies
A visit, with blood counts and differential, was scheduled before treatment. Visits were weekly during the first month, every 2 weeks during the second and third months, and monthly thereafter. The karyotype of bone marrow cells was examined by standard banding techniques before treatment, every 3 months during the first year of treatment, and every 6 months thereafter. Fluorescent in situ hybridization (FISH) analysis for 9q deletion was performed prior to treatment in 66 of 76 cases.
Molecular studies were performed on marrow cells prior to treatment, every 3 months during the first year of treatment, and every 6 months thereafter.
Molecular methods
Patient samples were shipped by overnight courier to the Group Central Laboratory in Bologna where the cell pellets were isolated and stored. The samples were divided into 3 groups and each group was assigned for molecular assay to one of the 3 reference laboratories of the ICSG, in Bologna, Turin, and Naples. All the samples of the same patient were analyzed in the same laboratory. Quantitative assays were performed using a real-time quantitative reverse transcriptase–polymerase chain reaction (QRT-PCR) assay, which has been described and reported in detail elsewhere.42-44 Briefly, leukocyte pellets were isolated from bone marrow aspirates by lysis of red blood cells upon their arrival at the Bologna laboratory. Cell pellets were washed twice in saline solution and resuspended in aliquots of 1 × 106 in 600 μL of 4 M guanidium isothiocyanate solution. The aliquots were stored at -20°C until shipment to the other reference laboratories (Naples and Turin). The QRT-PCR method independently measured in each sample the copy number of the mRNAs for the P210BCR/ABL protein and for β2-microglobulin (β2m), which served as a control gene to verify and adjust for sample-to-sample RNA quality variations. Each sample was analyzed in triplicate, and threshold cycle (Ct; number of PCR cycles necessary to achieve a target-specific fluorescence detection threshold) values were averaged. Reaction conditions and primer and probe sequences for QRT-PCR of BCR/ABL and β2m transcripts were designed, tested, and standardized within the framework of a European Union (EU) concerted action.42,43 For each amplification run, a BCR/ABL and β2m standard curve were independently generated by assaying, in parallel with the samples, 10-fold serial dilutions (from 106 to 102 copies, each in triplicate) of plasmid DNA calibrators containing the target sequences diluted in a solution of E coli RNA (20 ng/μL). Plasmid dilutions were purchased from IPSOGEN (Marseille, France). Given the mean Ct values for each sample, the copy number of BCR/ABL and β2m transcripts was derived by interpolation to the appropriate standard curve, and the result was expressed as the ratio of BCR/ABL mRNA copies to β2m mRNA. Since the level of β2m mRNA is approximately 2 logs higher than the level of the other 2 control genes selected within the EU concerted action, ABL and beta-glucuronidase, the ratio of BCR/ABL to β2m was multiplied by 100. The lowest limit of sensitivity of the method was set at 0.000 01 (corresponding to a Ct of 38.5 for the BCR/ABL transcript). Patient RNA samples that repeatedly gave β2m Cts higher than 25.7 (corresponding approximately to 10 000 molecules) were operationally considered degraded and were excluded from further evaluation. This ensured that in all samples assayed, the dynamic range of detectability of the technique was at least 4 logs.
Definitions
Chronic phase (CP) was distinguished from accelerated phase and blastic phase (AP, BP) on the basis of the percentage of blast cells in peripheral blood (less than 10% vs more than 10%) and of the involvement of nonhematopoietic tissues or organs (not involved vs involved), as in prior studies.45 The relative risk of the patients was calculated and defined as low, intermediate, or high, according to both the Sokal and Hasford (European) formulations.46,47 The hematologic response was defined as complete (CHR) if the white blood cell (WBC) count was less than 10 × 109/L, if the platelet count was less than 450 × 109/L, and if no granulocyte precursors were counted in the differential. Moreover, it was required that the spleen was not palpable. The cytogenetic response was based on the evaluation of a minimum of 20 marrow cell metaphases and was graded according to the proportion of Ph-negative (Ph-neg) metaphases, as complete (100% Ph-neg), partial (66%-99% Ph-neg), minor (34%-65% Ph-neg), and minimal or none (< 34% Ph-neg). The term major CgR (MCgR) grouped together both partial and complete CgRs (65%-100% Ph-neg). Molecular response was graded according to the log reduction of BCR-ABL transcript amount. Survival and progression-free survival were determined according to the method of Kaplan and Meier.48 Namely, survival was calculated from the date of enrollment to the date of death. Progression-free survival was calculated from the date of enrollment to the date of progression to AP or BP, or to death. AEs were identified and graded according to the WHO scale and were divided into Hema AEs and non-Hema AEs. The frequency of AEs was calculated and expressed either as the percent of the patients who suffered at least once from a given AE or as the number of AEs per patient, because several patients complained of the same or of different AEs at the same time or more than once. The compliance to the doses was calculated as the ratio between the administered dose and the scheduled dose, taking into account all dose reductions and all the cases of discontinuation, both temporary and permanent.
Results
Patients
During a 5-month period, 18 centers enrolled 78 patients. There were 2 patients who were enrolled in the second cohort who were not eligible: one patient was BCR/ABL-positive but Ph-neg, and one patient was already in AP. There were 27 patients enrolled in the first cohort (PegIFN 50 μg/wk), 18 patients enrolled in the second cohort (PegIFN 100 μg/wk), and 31 patients enrolled in the third cohort (PegIFN 150 μg/wk). Details of the patients are reported in Table 2. All were in early CP, less than 3 months from diagnosis. There were 59 patients (78%) who had been pretreated with hydroxyurea. About 50% were low risk (45% by Sokal score, 51% by Hasford score), while high-risk patients were 24% by Sokal's score and 12% by Hasford's score. Additional cytogenetic abnormalities were found in 19 cases (25%), and included 9q deletions (12 cases), variant translocations (5 cases), and others (4 cases) (data not shown in Table 2).
Adverse events (AEs)
Neutropenia, grades 3 and 4, was recorded in 63% of cases, with a frequency of 1.69 events per patient (Table 3). It increased in frequency from the first cohort (1.37) to the second and the third cohort (1.66 and 1.99, respectively). Thrombocytopenia (grade 3 only) occurred in 28% of cases, with a frequency of 0.22 events per patient in the first cohort, 0.44 events per patient in the second cohort and 0.52 events per patient in the third cohort. Grade 3 anemia occurred in one patient only.
Non-Hema AEs, grades 2 to 4, were recorded in 79% of cases, with a frequency of 2.97 events per patient (Table 3). The frequency of these AEs was not different in the 3 cohorts, but the severity increased along with the increasing dose of PegIFN (grade 3 non-Hema AEs were reported in 22%, 33%, and 55% of patients of the first, second, and third cohorts, with a frequency of 0.59, 1.00, and 1.32, respectively; Table 3). Among non-Hema AEs, constitutional events (pain, fatigue, asthenia, fever, flu-like syndrome) were the most frequent and occurred in 43% (grade 2) and 36% (grade 3) of cases, with a clear increase of grade 3 events from the first cohort (11% of cases) to the third cohort (60% of cases; Table 4). Skin AEs, including rash and pruritus, occurred in 39% of cases (22% grade 2 and 17% grade 3) without any detectable relationship with PegIFN dose. Neurologic or psychiatric problems, mainly depression, were experienced by 22% of patients. Nausea, oral mucositis, abdominal pain, or diarrhea developed in 21% of patients. Edema, generalized or local, was recorded in 16% of cases, with only one case of grade 3 (Table 4).
Treatment discontinuation and dose reduction
IM was permanently discontined in 3 of 76 patients (4%) because of generalized edema and skin rash (one patient) and of skin rash (2 patients). PegIFN was permanently discontinued in 45 of 76 cases (59%), of which 15 of 27 cases (55%) were in the first cohort, 11 of 18 (61%) were in the second cohort, and 19 of 31 (61%) were in the third cohort. Hema AEs (neutropenia and thrombocytopenia) caused PegIFN discontinuation in 30% of cases, but only after the first quarter. Non-Hema AEs caused PegIFN discontinuation in 29% of cases, and that occurred more frequently during the first quarter (14%), than during the second quarter (8%) and the second half-year (7%).
PegIFN dose was reduced to 50 μg/wk in 5 of 18 patients (28%) of the second cohort (and all these 5 patients did not tolerate and discontinued the reduced dose), and in 17 of 31 patients (55%) of the third cohort (and 9 of these 17 patients did not tolerate, and therefore discontinued the reduced dose).
The ratio between the administered and the scheduled dose of each drug is reported in Table 5, showing that the dose of IM was maintained at 87% to 90% of the scheduled dose in the first 2 cohorts, but was decreased (77%) in the third cohort. In contrast, the median administered dose of PegIFN was always substantially lower than scheduled, and was similar in all 3 cohorts, ranging between 32 μg/wk and 36 μg/wk.
Response and course
A CHR was achieved in all but 2 patients, who progressed to AP after 3 months. A CCgR was obtained in 53 of 76 patients (70%) and a PCgR was obtained in the other 10 patients, for a MCgR rate of 63 of 76 (83%). The CgR rate was not different in the 3 PegIFN cohorts and was not different in the patients who discontinued PegIFN versus those patients who did not discontinue PegIFN. All 13 patients with a 9q deletion and/or a variant translocation achieved an MCgR, with only one exception. On the contrary, only 55% of high-risk patients (as calculated according to either the Sokal or Hasford score)46,47 achieved an MCgR.
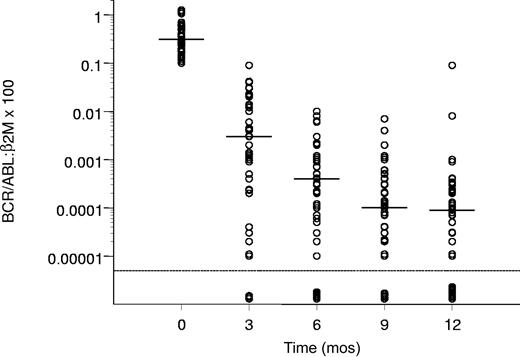
The molecular response of the patients who achieved a CCgR is reported in Figure 1, showing a progressive decrease of the amount of the BCR/ABL transcript from a median value of 0.31 at baseline to 0.000 09 after 12 months.
Molecular response in the patients who achieved a CCgR. Horizontal bars represent median values. The molecular response is assessed as the ratio between BCR/ABL and β2m RNA × 100, as described in “Patients and methods.” The lowest limit of sensitivity of the method was set at 0.000 01.
Molecular response in the patients who achieved a CCgR. Horizontal bars represent median values. The molecular response is assessed as the ratio between BCR/ABL and β2m RNA × 100, as described in “Patients and methods.” The lowest limit of sensitivity of the method was set at 0.000 01.
In 47% of all 76 patients and 68% of CCgRs, there was a reduction of the BCR/ABL transcript level of 3 logs or more, and at 12 months the amount of the transcript was below the level of sensitivity of the method in 11 of 76 cases (14%).
Six months after the end of the study (18 months after the first dose of IM and PegIFN), overall survival was 98.7%, progression-free survival was 97.4%, and 70% of patients were in continuous CCgR.
Discussion
This study was planned and designed with the aim of evaluating the feasibility of the treatment of Ph-pos CML with a combination of IM standard dose and PegIFN, and to provide information on 3 points. The first point concerned the hemopoietic toxicity. Although neither drug has a great hematologic toxicity, the combination could result in cases of marrow aplasia or could lead to an undesired reduction of IM dose due to recurrent or chronic neutropenia or thrombocytopenia. The second point concerned the nonhematologic side effects of the combination. IFN is a lymphokine that has a number of side effects. Some of these side effects, although less severe, are seen also with IM. The combination could result in an increase of the frequency and/or the severity of the side effects, lead to an undesired reduction of drug doses (particularly of IM dose), or even require treatment discontinuation. A third important point was the identification of the PegIFN dose, and in particular, of a dose being both safe and free from undesirable and recurrent AEs.
The main result of this study is that 45 of 76 patients (59%) required a permanent discontinuation of PegIFN during the first year of treatment. This figure is about 3 times higher than the figures of IFN-α discontinuation in many prior studies of IFN-α alone or in combination with low dose arabinosyl cytosine (LDAC).45,49-53 The causes of discontinuation of PegIFN were multifactorial in many cases, but were accounted for almost equally by Hema (30%) and non-Hema AEs (29%).
Neutropenia, grade 3 or 4, was very frequent in all 3 cohorts. However, there were neither cases of severe or prolonged hemopoietic failure nor cases of severe infection. Grade 4 non-Hema AEs were rare (3% of cases, 0.03 events per patient) and no treatment-related death occurred. Therefore, we can conclude that the combination treatment was safe, at least with a careful application of the rules for dose adaptation and treatment discontinuation, as described in Table 1. However, the frequency of grade 3 Hema AEs and of grades 2 and 3 non-Hema AEs was remarkable, required frequent visits, and contributed substantially to limit patient compliance.
One would have expected the frequency and the severity of all AEs to rise with increasing the scheduled dose of PegIFN. As a matter of fact, the severity of AEs increased, being maximal in the third cohort (PegIFN 150 μg/wk), whereas the frequency of all AEs did not increase. Also, the rate of PegIFN discontinuation was not different in the 3 cohorts (55%, 61%, and 61%, respectively). This may be explained by the treatment plan, which accounted for PegIFN dose reduction before discontinuation (Table 1). Thus, the dose of PegIFN was decreased to 50 μg/wk in 28% of the second cohort patients and in 55% of the third cohort patients, reducing substantially the dose difference that was originally scheduled, so that the median PegIFN dose that was actually delivered was similar in the 3 cohorts (Table 5). However, IM dose was not the same in the 3 cohorts, with a significant reduction of the dose in the third cohort (median 310 mg/d, corresponding to 77% of the scheduled dose), suggesting that the attempt to deliver a higher PegIFN dose (150 μg/wk) resulted in a reduction of the deliverable IM dose, which may compromise efficacy.
The major types of non-Hema AEs (Table 4) were those that can be expected from IFN-α alone and from IM alone. We may try to offer a comparison of the frequency of the side effects of this combination with the frequency of the side effects of IM alone, using the data of a study of 191 late CP patients with CML who were treated with IM alone at the same institutions44 (Table 6). In the present study (IM+PegIFN), the frequency of all non-Hema AEs was 3 to 5 times greater than in the prior study of IM alone, with the exception of liver toxicity. Interestingly, the events that may be attributed mainly to IM were more frequent with the combination. Thus, skin rash and related events occurred with a frequency of 0.74 events per patient versus 0.13 with IM alone. Edema, generalized and superficial, occurred with a frequency of 0.21 events per patient versus 0.08 with IM alone. Therefore, it is wise to consider the possibility that the simultaneous administration of the 2 agents may enhance the toxicity of either agent. Another possibility that is worth discussing is that the combination was poorly tolerated because it was not perceived by patients and doctors as the best available treatment modality. This probably occurred in the recent International Randomized Study of Interferon and STI571 (IRIS) study, where the compliance to control treatment, IFN plus LDAC, was lowered by the perception that this treatment was less effective, and was outdated.11 We do not think that these considerations apply to the present study, because all the patients received the best perceived drug (IM) at a time when it was not yet available for CML treatment, and the treatment proved to be effective very rapidly, since within 3 months all patients but 2 were in CHR and 61% of them had already achieved an MCgR.
Even though we have also reported the data on response, there are no means to compare the results of this study to the results that have been reported with IM alone as a first-line treatment of CP CML. However, it may be of interest to point out that in the IM arm of the international IRIS study, after a median treatment time of 19 months, the CgR rate was 76% complete and 87% major, and a molecular response of 3 logs or more was achieved in 57% of all patients.11,12 In a pilot study of 30 patients who received first-line treatment with IM (400 mg/d) and LDAC, the CCgR at one year was 70% and the MCgR was 83%.54
In conclusion, we have found that a combination of IM standard dose (400 mg/d) with PegIFN at a dose ranging from 50 μg/wk to 150 μg/wk was devoid of severe AEs and did not result in an unpredictable toxicity, but was associated with considerable neutropenia, thrombocytopenia, and non-Hema toxicity. This required a reduction of the dose of PegIFN to 50 μg/wk in many of the patients who were assigned to receive 100 μg/wk or 150 μg/wk and a permanent discontinuation of PegIFN in 59% of all patients. We also observed that administering PegIFN at 150 μg/wk might cause a substantial reduction of IM dose. It is possible that modifying the dose and the schedule of either agent, as well as using conventional IFN-α or other pegylated IFN-α preparations, the toxicity profile, the compliance, and the results may change, in one sense or in another. Also, it should not be overlooked that we report here only on the short-term, one-year response to the treatment. Although the response was similar to that reported with IM alone,11,12 it is not possible to anticipate if there would be any effects on progression-free survival or survival. In any case, these data may assist in the design and preparation of prospective studies.
Appendix
The following members of the Italian Cooperative Study Group (ICSG) on CML (currently known as the GIMEMA Working Party on CML) have actively participated in this study: Antonio Peta and Franco Iuliano (Catanzaro), Renato Fanin and Mario Tiribelli (Udine), Tiziano Barbui and Renato Bassan (Bergamo), Eugenio Gallo and Patrizia Pregno (Torino), Francesco Lauria and Monica Bocchia (Siena), Pietro Leoni and Serena Rupoli (Ancona), Antonio Bosi and Valeria Santini (Firenze), Marco Gobbi and Maurizio Miglino (Genova), Giorgina Specchia (Bari), Federico Papineschi (Pisa), Giuliana Alimena (Roma), Dario Ferrero (Torino), Anna Marina Liberati (Perugia), Ester Pungolino (Milano), Elisabetta Abruzzese (Roma), and Adele Capucci (Brescia).
Prepublished online as Blood First Edition Paper, August 19, 2004; DOI 10.1182/blood-2004-03-0826.
Supported by COFIN 2003, Fondazione del Monte di Bologna e Ravenna, University of Bologna, Italian Association for Cancer Research (AIRC), National Research Council, Regione Campania, and European LeukemiaNet. A complete list of the members of the GIMEMA Working Party on Chronic Myeloid Leukemia appears in the “Appendix.”
D.A. is an employee of Novartis Pharma, Italy, and G.F. was an employee of Schering-Plough, Italy. These companies supplied the drugs used in this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are indebted to Barbara Giannini, Emanuela Ottaviani, Carolina Terragna, and Chiara Nicci (Bologna) for technical help. We acknowledge Ursula Giussani (Cytogenetic Laboratory, Bergamo), Gian Carlo Discepoli (Cytogenetic Laboratory, Pediatric Hospital “Salesi”), and Ancona and Mario Sessarego (DIMI, Genova), for their contributions to molecular cytogenetic studies. The technical assistance of Mrs Katia Vecchi and Mrs Maira Marsili (Bologna) is kindly acknowledged.