Abstract
This study investigated the role of several chemokines and their receptors on malignant B lymphocytes recovered from 13 patients with chronic lymphocytic leukemia (CLL), 9 with hairy cell leukemia (HCL), 5 with mantle cell lymphoma (MCL), 5 with marginal zone B-cell lymphoma (MZL), 6 with small lymphocytic lymphoma (SLL), and 5 with follicular cell lymphoma (FCL). Flow cytometry analysis demonstrated that CXCR4 and CXCR5 were expressed on all malignant and normal B cells. Considering CC receptors, CCR1 was expressed in 70% of patients with CLL and 40% of those with HCL but was lacking in patients with MCL, MZL, SLL, and normal B cells. CCR2 showed a heterogeneous pattern of expression. CCR3 was found in almost all patients with CLL and in the majority of those with HCL, whereas it was usually lacking in patients with MZL and SLL and in healthy subjects. CCR5 was expressed in patients with HCL and MCL. Migration assays showed that different chemokines, mainly CXCL12 and CXCL13, are able to trigger migration of malignant B lymphocytes. Some of these chemokines induce calcium mobilization. These data indicate that different patterns of chemokine receptor expression identify different malignant B-cell subsets and that these receptors are functional and might play a role in malignant B-cell circulation. (Blood. 2004;104:502-508)
Introduction
Chemokines represent a group of molecules that regulate cell migration and can be distinguished in inflammatory and homeostatic chemokines. These latter are involved in the homeostasis of the immune system.1-8 Cells are exposed to a complex pattern of chemoattractant signals that, through a gradient of chemokine concentrations, drive the migration of lymphocytes to target tissues or lymphoid organs. Different chemokine receptors are able to bind the same chemokine and, in turn, different chemokines are able to bind the same receptor, indicating that redundancy is a definite feature of the chemokine network. Chemokine-receptor interactions are of crucial importance for homeostatic functions within the immune system, particularly for establishing the complex architecture of the secondary lymphoid organs.3,4,9
The role of chemokines for B-cell lymphopoiesis has been primarily demonstrated for CXCL12 (stromal-derived factor 1α [SDF-1α]) and its receptor CXCR4.10 CXCL12 can induce chemotaxis of B-cell progenitors, indicating that the CXCL12/CXCR4 system may be important in directing the migration of B-cell progenitors to the appropriate bone marrow microenvironment.11-13 In addition, it is highly expressed on B lymphocytes and it is likely to play a key role in the architecture of spleen and lymph nodes. To date, most information in terms of chemokines and their receptors has been provided on normal human B cells and in the mouse; little data are available on the role of these molecules on malignant B lymphocytes.14-17 In particular, it has been demonstrated that malignant B cells express CXCR3 in different neoplastic conditions and mediate migration following the binding of the chemokines CXCL10 (inflammatory protein 10 [IP-10]) and CXCL9 (Mig).14,16 Furthermore, CXCR4, a receptor constitutively expressed on normal B lymphocytes, has been detected on malignant B lymphocytes from patients with chronic lymphocytic leukemia (CLL).15
In this study, we investigated the expression and functional role of a large spectrum of chemokine receptors, including CCR1 to CCR6 and CXCR1 to CXCR5, in several B-cell non-Hodgkin lymphomas (NHLs). We demonstrated that these receptors recognize different B-cell malignancies and mediate chemotaxis following binding to their own ligands.
Patients, materials, and methods
Patient samples
Forty-three patients with different B-cell malignancies were studied at the time of diagnosis.18 This study was approved by the Università of Padova institutional review board. Informed consent was provided according to the Declaration of Helsinki. Thirteen patients (10 men and 3 women, ages 46-78 years) with the diagnosis of B-CLL were graded according to the Rai staging system19 as follows: stage 0 (1 case) stage I (5 cases), stage II (4 cases), stage III (2 cases), stage IV (1 case); the total lymphocyte count ranged from 21 to 98 × 109/L (21 000-98 000/mm3).
Nine patients with hairy cell leukemia (HCL; 6 men and 3 women, ages 46-75 years) were investigated. The diagnosis was established on the basis of clinical, morphologic, cytochemical, histologic, and immunologic features.18
Twenty-one patients with different histologic NHL entities (5 with mantle cell lymphoma [MCL], 5 with marginal zone lymphoma [MZL], 6 with small lymphocytic lymphoma [SLL], and 5 with follicular cell lymphoma [FCL]) in the leukemic phase were also included in the study.18
Preparation of cell suspensions
Peripheral blood mononuclear cells (PBMCs) from patients with NHL were obtained from freshly heparinized blood samples by centrifugation on Ficoll/Hypaque (F/H) gradient.20 Normal B lymphocytes were obtained from 5 tonsils after mechanic disruption.21 Mononuclear cells, recovered following centrifugation on F/H gradient, were washed 3 times with phosphate-buffered saline (PBS) and resuspended in endotoxin-free RPMI 1640 medium (Sigma Chemical, St Louis, MO) supplemented with 20 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) and l-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% fetal calf serum (FCS; ICN Flow, Costa Mesa, CA).
Cell samples with a percentage of monocytes, T cells, and natural killer (NK) cells more than 5% were further enriched in B lymphocytes by rosetting with neuroaminidase-treated (Sigma) sheep red blood cells (SRBCs) and by removing residual CD3+, CD16+, CD56+, and CD14+ cells using magnetic separation columns (Miltenyi Biotec, Bergisch Gladbach, Germany), as previously described.22 Following this multistep negative selection procedure, more than 98% of the resulting cell population was CD19+.
mAbs and cytokines
CC and CXC receptor analysis was performed using anti-CCR and anti-CXCR (mouse or rat) monoclonal antibodies (mAbs) purchased from R&D Systems (Minneapolis, MN). The chemokines CXCL12 and CXCL13 were purchased from R&D Systems and the chemokines CCL3 (macrophage inflammatory protein 1α [MIP-1α]), CCL23 (MIP-3), CCL20 (MIP-3α), CCL24 (eotaxin-2), and CCL5 (regulated on activation and normal T cell expressed [RANTES]) were purchased from PeproTech (London, United Kingdom).
Flow cytometry analysis of CCR and CXCR
The expression of the antigens on B cells was assessed by flow cytometry analysis using direct or indirect immunofluorescence assay, as previously described.23 Following incubation of cells with anti-CCR and anti-CXCR mAbs or the control-matched mAb, they were washed, and a fluorescein isothiocyanate-phycoerythrin (FITC/PE)-conjugated antimouse or antirat mAb (Technogenetics, Turin, Italy) was added. After this last incubation, cells were washed and analyzed.
For fluorescence-activated cell sorting (FACS) analysis, 1 × 104 cells were acquired and the analysis was determined by overlaying the histograms of the samples stained with the different reagents. Cells were scored using a FACScan analyzer (Becton Dickinson, Heidelberg, Germany) and data were processed using CELLQuest software program (Becton Dickinson). For each marker, the threshold of positivity was found beyond the nonspecific binding observed in the presence of irrelevant control antibody. Mean log fluorescence intensity (MFI) values were obtained by subtracting the MFI of the isotype control from the MFI of the positively stained sample. To evaluate whether the differences between the peaks of cells were statistically significant with respect to control, the Kolmogorov-Smirnov (K-S) test for analysis of histograms was used, according to the CELLQuest software guide (Becton Dickinson). K-S tests report significant differences between histograms; therefore, positive cases have been defined according to D/s parameter obtained from this statistical test, which has been considered significant for values greater than 10.
Migration assay
Cell migration was measured in a 48-well modified Boyden chamber.14 Chemokines were diluted in RPMI 1640 medium at different concentrations and were used to evaluate the chemotactic properties of B lymphocytes from healthy donors and patients. Polyvinylpyrrolidone-free polycarbonate membranes with 5-μm pores were coated with fibronectin before use. Then 30 μL chemokine or control medium was added to the bottom wells, and 50 μL 5.0 × 106 cells/mL B cells, resuspended in RPMI 1640, were added to the top wells. The chemokines were used at the following concentrations: CXCL12 (both SDF-1α and SDF-1β) at 100 ng/mL, CXCL13 at 1 ng/mL, CCL3 at 5 ng/mL, CCL23 at 5 ng/mL, CCL20 at 5 ng/mL, CCL24 at 100 ng/mL, and CCL5 at 1 ng/mL. The concentrations indicated represent the doses that elicited the highest migration index in a dose-response curve with different chemokine concentrations (0.1, 1, 5, 10, 100 ng/mL). At these concentrations the migration index was as follows: 0, 0, 0, 2 to 3, 5 to 15 for CXCL12; 2 to 3, 7 to 10, 10 to 12, 10 to11, 9 to 12 for CXCL13; 0, 4 to 6, 10 to 30, 18 to 22, 20 to 24 for CCL3; 2 to 3, 8 to 10, 10 to 11, 10 to 12, 12 to 14 for CCL5; 0, 2 to 5, 8 to 15, 10 to 12, 8 to 12 for CCL20; 0, 2 to 4, 10 to 15, 10 to 12, 8 to 10 for CCL23; and 0, 0, 2 to 3, 10 to 40 for CCL24.
The chamber was incubated at 37°C with 5% CO2 for 2 hours. The membranes were then removed, washed with PBS on the upper side, fixed and stained with DiffQuik (Dade, Düdingen, Switzerland). Cells were counted at × 400 magnification in 3 fields/well. All assays were performed in triplicate.
Cytosolic calcium measurement
Changes in the intracellular calcium concentration [Ca2+]i were measured in B lymphocytes from 10 patients with B-cell malignancies (6 CLL, 2 MZL, 1 HCL, 1 MCL) and in 2 healthy subjects, using the fluorescent indicator fura-2/am, as previously described.24 Briefly, 20 × 106 cells were incubated with 2 μM fura-2/am at 37°C for 40 minutes. After the loading procedure, aliquots of the cells (2 × 106) were rapidly washed and resuspended in a magnetically stirred thermostatted cuvette. The incubation medium contained 1 mM CaCl2. Excitation and emission wavelengths were 340 and 500 nm, respectively; the excitation slit width was 5 nm, and the emission slit was 10 nm. The inhibitor of organic anion transport sulfinpyrazone was added in experiments during [Ca2+]i measurements at a final concentration of 250 μM to prevent fura-2 release into the medium.25 Control experiments without sulfinpirazone gave essentially the same results except for a slowly increasing baseline due to fura-2 leakage.25 The chemokines were used at 100 ng/mL; anti-Ig was used at 500 ng/mL.
Statistical analysis
Data are expressed as mean ± SE of the mean and comparisons between values were made using the analysis of variance (ANOVA) test. A P value of less than .05 was considered as significant.
Results
Expression of CC and CXC chemokine receptors on normal and malignant B cells
The expression of chemokine receptors was analyzed by flow cytometry analysis on normal B lymphocytes obtained from healthy donors and on tumor B cells recovered from patients with different NHLs. Flow cytometry profiles for representative patients are shown in Figures 1 and 2 and the overall results are detailed in Tables 1 and 2. These latter report the number of positive cases for each receptor in different series of patients.
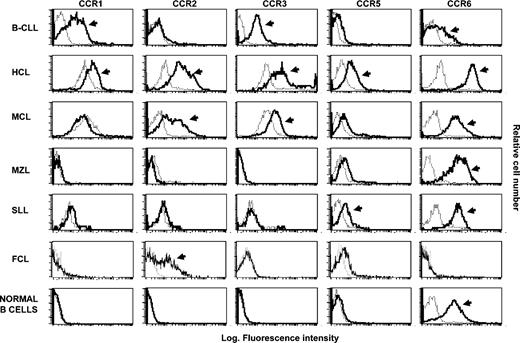
Flow cytometry analysis of CCR shows a distinct pattern of expression of these receptors among different B-NHLs. The histograms were obtained from a representative healthy subject and from malignant B lymphocytes recovered from representative patients with different types of NHL. The analysis was performed by flow cytometry on purified B lymphocytes following incubation with anti-CCR receptor mAb (bold histograms) and control isotype mAb (dotted histograms). Arrows denote positive cases on the basis of the Kolmogorov-Smirnov test.
Flow cytometry analysis of CCR shows a distinct pattern of expression of these receptors among different B-NHLs. The histograms were obtained from a representative healthy subject and from malignant B lymphocytes recovered from representative patients with different types of NHL. The analysis was performed by flow cytometry on purified B lymphocytes following incubation with anti-CCR receptor mAb (bold histograms) and control isotype mAb (dotted histograms). Arrows denote positive cases on the basis of the Kolmogorov-Smirnov test.
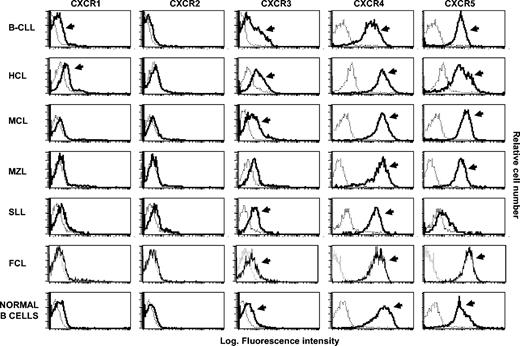
Flow cytometry analysis of CXCR shows distinct pattern of expression of these receptors among different B-NHLs. The histograms were obtained from a representative healthy subject and from malignant B lymphocytes recovered from representative patients with different types of NHL. The analysis was performed by flow cytometry on purified B lymphocytes following incubation with anti-CXCR receptor mAbs (bold histograms) and control isotype mAbs (dotted histograms). Arrows denote positive cases on the basis of Kolmogorov-Smirnov test.
Flow cytometry analysis of CXCR shows distinct pattern of expression of these receptors among different B-NHLs. The histograms were obtained from a representative healthy subject and from malignant B lymphocytes recovered from representative patients with different types of NHL. The analysis was performed by flow cytometry on purified B lymphocytes following incubation with anti-CXCR receptor mAbs (bold histograms) and control isotype mAbs (dotted histograms). Arrows denote positive cases on the basis of Kolmogorov-Smirnov test.
Considering the CC chemokine receptors, the data reported in Table 1 and Figure 1 demonstrate that CCR1 is usually expressed in B-CLL (9 of 13 patients tested) and in some HCL patients (4 of 9), whereas its expression is lacking on normal B lymphocytes and on malignant cells from patients with MCL, MZL, SLL, and FCL. CCR2 was heterogeneously found on normal B cells and in different groups of patients, being, however, consistently undetectable on tumor cells from patients with MZL. CCR3 was present in all patients with CLL and in the majority of those with HCL, whereas normal B cells and malignant B lymphocytes from patients having other NHLs (MCL, MZL, SLL, and FCL) were almost negative for CCR3. CCR5 is expressed in all subjects with MCL and in the majority of patients with HCL (78%), but barely detectable on B lymphocytes from control subjects and on malignant B cells from patients with CLL (7.7%). CCR6 is constitutively expressed on different B-cell subsets, both normal and malignant, with the exception of FCL.
The analysis of the CXC chemokine receptors (Table 1; Figure 2) showed that CXCR4 and CXCR5 receptors are constitutively present both on normal B cells from healthy subjects and on tumor B cells from patients with most NHLs, whereas a heterogeneous pattern of expression has been observed for CXCR1 and CXCR2 receptors. These 2 receptors are never found on malignant cells from MCL. CXCR3 expression was consistent with our data previously reported.14
We also evaluated the intensity of chemokine receptors by analyzing the MFI; data are reported in Table 2. Among different B-cell subsets that bear a particular receptor, the MFI changed from one B-cell population to another. In particular, CXCR4, a receptor expressed on all B lymphocytes under study, showed a lower MFI in patients with MZL and SLL compared with normal B lymphocytes and other NHLs (P < .05). Again, malignant cells from patients with CLL and HCL showed a higher expression of CXCR5, compared with patients with SLL (P < .05). Considering patients with B-CLL, there were not any statistically significant differences in the expression of CC and CXC receptors among subjects in different stages of the disease.
Migratory assay of CC chemokines
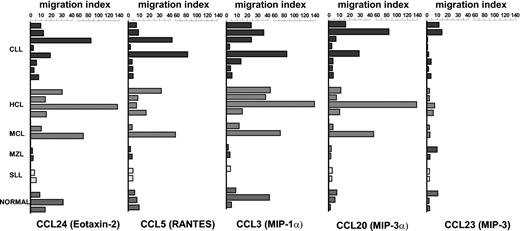
Due to the expression of CC chemokine receptors on malignant B cells, the migratory assay was performed in the presence of several CCL chemokines, including CCL24, CCL5, CCL3, CCL23, and CCL20. The assay was performed in 18 patients with NHL and in 3 healthy subjects, and the findings are reported in Figure 3. CCL24, a chemokine that binds CCR3, induced a high migration of malignant B cells recovered from all patients with HCL and in some with CLL. The effect on normal B lymphocytes was lower than that observed in patients with HCL. In addition, the migration induced in patients with MZL or SLL was very low or absent. This negative result is consistent with the lack of this receptor in these types of NHL (Tables 1 and 2).
CC receptors were functionally investigated in chemotactic assay and were observed to display chemotaxis in some patients with NHL following binding to relative chemokines. Chemotactic activity of CC chemokines (CCL3 at 5 ng/mL, CCL23 at 5 ng/mL, CCL20 at 5 ng/mL, CCL24 at 100 ng/mL, and CCL5 at 1 ng/mL) was tested on normal B cells obtained from 3 healthy subjects and on malignant B lymphocytes obtained from 18 patients with NHL. The concentrations were chosen in relation to a dose-response curve (see “Patients, materials, and methods”). The assay was performed in triplicate.
CC receptors were functionally investigated in chemotactic assay and were observed to display chemotaxis in some patients with NHL following binding to relative chemokines. Chemotactic activity of CC chemokines (CCL3 at 5 ng/mL, CCL23 at 5 ng/mL, CCL20 at 5 ng/mL, CCL24 at 100 ng/mL, and CCL5 at 1 ng/mL) was tested on normal B cells obtained from 3 healthy subjects and on malignant B lymphocytes obtained from 18 patients with NHL. The concentrations were chosen in relation to a dose-response curve (see “Patients, materials, and methods”). The assay was performed in triplicate.
CCL5 binds several receptors, including CCR1, CCR3, and CCR5. The migration induced by this chemokine was again more effective in patients with HCL or CLL as compared to normal B lymphocytes and patients with MZL or SLL.
The migration induced by CCL3, which binds CCR1 and CCR5 receptors, was principally induced on tumor cells obtained from patients with HCL and in some patients with CLL or MCL lymphoproliferative disorders (Figure 3).
CCL20, which selectively binds CCR6, induced migration of malignant B cells obtained from a small number of patients suffering from NHL. CCL23, which preferentially binds CCR1, seemed to display very low migration as compared to the other molecules tested.
Migratory assay of CXC chemokines
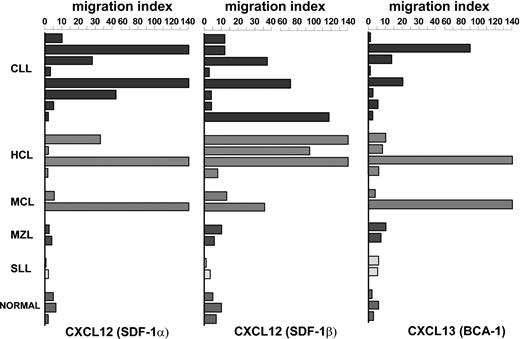
To further characterize the biologic properties of CXC receptors, normal and malignant B cells were assessed for their migratory properties in the presence of several chemokines. The data reported in Figure 4 were obtained at the concentration giving the highest migration in a previously determined dose-response curve (not shown). Figure 4 illustrates the migratory effect of CXCL chemokines, that is, CXCL12 (SDF-1α and SDF-1β) and CXCL13, on normal B cells recovered from 3 healthy subjects, and on malignant B lymphocytes obtained from 18 patients with NHL. CXCL12 (SDF-1α and SDF-1β), 2 chemokines that specifically bind to CXCR4 receptor, displayed a low migratory capability on normal B lymphocytes, whereas the effect on B-cell migration was stronger in tumor cells from patients with CLL and HCL. The ability to induce migration in other NHLs was much lower in patients with MZL and SLL, whereas it was heterogeneous in patients with MCL. Even though cell chemotaxis was triggered by both molecules, the migration index was higher with SDF-1β rather than that obtained with SDF-1α.
CXC receptors were functionally investigated in chemotactic assay and were observed to display chemotaxis in some patients with NHL following binding to relative chemokines. Chemotactic activity of CXC chemokines (CXCL12: SDF-1α at 100 ng/mL, SDF-1β at 100 ng/mL, CXCL13 at 1 ng/mL) was tested on normal B cells obtained from 3 healthy subjects and on malignant B lymphocytes obtained from 18 patients with NHL. The concentrations were chosen in relation to a dose-response curve (see “Patients, materials, and methods”). The assay was performed in triplicate.
CXC receptors were functionally investigated in chemotactic assay and were observed to display chemotaxis in some patients with NHL following binding to relative chemokines. Chemotactic activity of CXC chemokines (CXCL12: SDF-1α at 100 ng/mL, SDF-1β at 100 ng/mL, CXCL13 at 1 ng/mL) was tested on normal B cells obtained from 3 healthy subjects and on malignant B lymphocytes obtained from 18 patients with NHL. The concentrations were chosen in relation to a dose-response curve (see “Patients, materials, and methods”). The assay was performed in triplicate.
Another chemokine receptor of particular interest for the recirculation of B lymphocytes is CXCR5. This receptor was widely expressed in both healthy subjects and in patients with lymphoproliferative disorders and mediates chemotaxis following binding of the relevant chemokine CXCL13 (Figure 4). Our data demonstrate that this chemokine displayed a discrete migratory capability in a few patients with B-CLL, HCL, and MCL malignancies, whereas it was unable to induce migration of normal B lymphocytes obtained from healthy subjects or of malignant cells recovered from patients with MZL and SLL.
Cytosolic calcium measurement
We examined the ability of CXC and CC chemokines to stimulate functional responses in malignant B cells from patients with NHL through the evaluation of cytosolic Ca2+ levels. The analysis was performed in B lymphocytes from 10 patients with NHL and 2 healthy subjects. The data are reported in Table 3 and 3 representative cases are shown in Figure 5. Our data demonstrate that a consistent increase in [Ca2+]i was observed on CXCL12 (100 ng/mL) addition. This effect was observed both on tumor B cells obtained from patients as well as on normal B lymphocytes obtained from healthy subjects. CXCL13 also displayed an increase in [Ca2+]i in patients with NHL and in healthy subjects; the effect, however, was very low when compared with the increase in [Ca2+]i observed with CXCL12 addition. Concerning the other chemokines we investigated, no [Ca2+]i increase was observed, with the only exception being CCL5 that displayed [Ca2+]i mobilization in a few patients with NHL (3 of 10 subjects tested, 2 CLL and 1 MZL). In all cases addition of anti-Ig resulted in an increase in [Ca2+]i, indicating that the cells we were dealing with were normal.
The capability of CC and CXC chemokines to stimulate functional properties through the evaluation of cytosolic Ca2+ level shows a consistent mobilization of Ca2+ in the presence of some chemokines. The effect of several chemokines on cytosolic Ca2+ concentration in malignant B cells obtained from 2 representative NHL patients and in normal B lymphocytes obtained from a healthy subject. The assay was performed in incubation medium containing 1 mM CaCl2. A discrete Ca2+ increase was observed following crosslinking surface membrane immunoglobulins (at a concentration of 500 ng/mL), as a positive control. Where indicated by arrows, the chemokines were added at a concentration of 100 ng/mL. At the end of each experiment 2 mM EGTA (ethylene glycol tetraacetic acid) was added and successively the Ca2+ ionophore ionomycin (1 μM) was added to induce a massive [Ca2+]i increase. The values are expressed as ratio between the intensity of the fluorescence signal obtained by exciting cells alternatively at 340 and 380 nm. An increase in ratio reflects an increase in [Ca2+]i.
The capability of CC and CXC chemokines to stimulate functional properties through the evaluation of cytosolic Ca2+ level shows a consistent mobilization of Ca2+ in the presence of some chemokines. The effect of several chemokines on cytosolic Ca2+ concentration in malignant B cells obtained from 2 representative NHL patients and in normal B lymphocytes obtained from a healthy subject. The assay was performed in incubation medium containing 1 mM CaCl2. A discrete Ca2+ increase was observed following crosslinking surface membrane immunoglobulins (at a concentration of 500 ng/mL), as a positive control. Where indicated by arrows, the chemokines were added at a concentration of 100 ng/mL. At the end of each experiment 2 mM EGTA (ethylene glycol tetraacetic acid) was added and successively the Ca2+ ionophore ionomycin (1 μM) was added to induce a massive [Ca2+]i increase. The values are expressed as ratio between the intensity of the fluorescence signal obtained by exciting cells alternatively at 340 and 380 nm. An increase in ratio reflects an increase in [Ca2+]i.
Discussion
This study was designed to investigate the expression and functional role of CC and CXC chemokine receptors and their ligands in patients with NHL. Our data indicate that some receptors, that is, CCR1, CCR2, and CCR5, are expressed in patients affected by a limited number of histologic types of NHL and are usually absent on normal B lymphocytes. CXCR4, CXCR5, and CCR6 are constitutively expressed on normal and malignant B lymphocytes recovered from patients with different B-cell malignancies, whereas other receptors, that is, CXCR1 and CXCR2, are expressed in some malignancies but not in normal B lymphocytes. On functional grounds, migration in vitro assay demonstrated that these structures behave as fully functional receptors because they transduce chemotactic activity after binding relevant chemokines. Furthermore, some of these receptors are able to induce [Ca2+]i mobilization following binding of their specific ligands.
The trafficking and homing of normal B lymphocytes is a multistep process that requires the involvement of adhesion molecules and chemokine receptors. CXCL12 represents a CXC chemokine constitutively expressed by bone marrow cells and plays a key role in B-cell development and traffiking.10-12,26-29 Our data demonstrated that the CXCL12 receptor (CXCR4) is expressed in all B-cell NHLs, thus extending the observations reported on normal and on malignant B-CLL cells15,30 to a large group of B-cell disorders. The analysis of the MFI and migratory assays suggests that this receptor may be functionally relevant when expressed at high density on the cell surface of malignant B lymphocytes, irrespective of its presence or absence on a defined cell population. In this context, patients with MZL or SLL showed a lower expression of CXCR4 on the membrane of tumor B cells and displayed a very low migration index to CXCL12. In addition, although CXCR4 is expressed with superimposable intensity on normal B lymphocytes and in some B-cell malignancies (CLL, HCL, and MCL), it transduces a low migratory activity on normal B lymphocytes with respect to malignant cells obtained from NHL. This observation on normal B cells is consistent with data in the literature30,31 showing a low migratory capability of germinal center B cells in response to CXCL12, whereas a migratory response was acquired when they were differentiated in vitro into memory B cells. In addition, a lower percentage of mature B lymphocytes migrates to CXCL12 than pro-B cells, indicating that responsiveness to CXCL12 decreases during cell differentiation, and this effect does not seem to be related to the binding affinity of CXCR4 for CXCL12.31 In conclusion, the observations herein reported on malignant B lymphocytes emphasize that CXCR4 is a fully functional receptor in some B-cell malignancies, thus permitting an efficacious chemoattraction. [Ca2+]i mobilization findings indicate that the binding of CXCR4 to CXCL12 induces the same [Ca2+]i increase in malignant as well as in normal B cells, thus supporting a dissociation between the 2 functional assays considered in this study, that is, [Ca2+]i mobilization and migration assay. This finding might be related to the functional CXCR4 expression on different cell types and to the number of receptors on the cell surface.
B-cell attracting chemokine 1 (CXCL13) selectively drives the migration of B lymphocytes via CXCR5.32 This receptor is commonly expressed on resting B cells33,34 and is important for enabling the formation of B- and T-cell compartments of secondary lymphoid organs. In fact, mice lacking CXCR5 do not develop inguinal lymph nodes, have few Peyer patches, and have an altered spleen structure, as a consequence of the impairment of B-cell migration.35 In addition, mice with a deletion of tumor necrosis factor (TNF), TNFR1, or lymphotoxin gene have decreased CXCL13 expression in follicular stromal cells of the spleen, a finding that is in agreement with the role of CXCL13 and CXCR5 in the homing of B lymphocytes.35 This observation suggests that the regulation of CXCL13 is under the control of some cytokines, such as TNF.35 This latter is constitutively expressed and produced by malignant lymphocytes from some chronic lymphoproliferative disorders (CLDs), that is, CLL and HCL.36-41 It might be suggested that the abnormal production of TNF by the transformed clone plays a role in the regulation of CXCL13 production and in the control of cell accumulation in lymphoid organs. The data herein provided showed that malignant B lymphocytes of different origin constitutively express CXCR5. The evidence that this receptor is not expressed in immature B lymphocytes33 and the demonstration that CXCR5 is down-regulated in differentiated IgG-producing B cells suggests that cells from our patients are in an advanced maturation stage. On clinical grounds, the expression of CXCR5 does not seem to correlate with a particular organ involvement. All patients with HCL, MCL, and MZL showed an enlargement of the spleen, whereas others (CLL) preferentially showed lymphadenopathy. This observation might suggest that other chemokines, possibly in association with CXCL13, might regulate lymphocyte compartmentalization in the spleen or in the lymph nodes. In this regard, CCL21 has been observed to be involved in lymphocyte recruitment in the spleen.35 Our data on patients with NHLs are also in agreement with recent findings reporting that mucosa-associated lymphoid tissue (MALT) lymphoma, a disease of the marginal zone, is characterized by the expression of CXCR5 on transformed cells.17 The evidence that these cells also express high levels of CXCL13 prompted the authors to hypothesize that MALT lymphomas have a relatively low propensity to infiltrate neighboring tissues, as a consequence of the in situ production of CXCL13. It will be interesting to verify whether or not this chemokine is produced at different sites of disease involvement (bone marrow, spleen, lymph nodes) to understand why some diseases spread to several tissues (ie, CLL) and others (ie, HCL) are restricted to a limited number of organs.
Concerning the CC chemokine receptors, CCR6 represents the receptor for CCL20 that is constitutively expressed on all normal42,43 and the majority of malignant B lymphocytes recovered from patients with NHLs with the exception of FCL. This finding is in agreement with the observation recently reported by Rodig and coworkers.44 Only in a few cases was migration elicited by CCL20. Whether this receptor affects the malignant B-cell accumulation remains to be defined. Concerning other CCL chemokines, little information is presently available on their role in normal B lymphocytes. Flow cytometry data herein provided indicate that these receptors (CCR1, CCR2, CCR3, CCR5) show a different pattern of expression in malignant B cells. This observation deserves 2 main considerations. One is the possible clinical use of the analysis of CC receptors to discriminate several B-cell malignancies. Second, the analysis of these receptors might help us to understand the different functional responsiveness of malignant B cells to chemokines. Molecules belonging to this family are effectively more promiscuous than CXC receptors because the same receptor can bind several chemokines. Among these, CCL3 was observed to display the highest migration activity on malignant B lymphocytes,45,46 supporting the concept that these cells express CCR1 and CCR5 receptors. The migration induced by this chemokine (Figure 4) was induced mainly on tumor cells obtained from patients with HCL as well as in some patients with CLL and MCL. Due to the different expression of CCR1 and CCR5 on tumor B lymphocytes, the effect of this chemokine may be transduced by different receptors in these malignancies. In particular, it can be hypothesized that in CLL the migration induced by CCL3 is preferentially mediated by CCR1 rather than by CCR5; this latter is in fact usually absent on CLL cells. By contrast, in patients with MCL it can be induced following binding to CCR5, because these malignant B lymphocytes are usually negative for CCR1. In HCL, it can be induced following binding to both CCR1 and CCR5.
In conclusion, we demonstrated that transformed tumor B lymphocytes obtained from patients with NHLs are equipped with different chemokine receptor profiles that might drive the recirculation of transformed cells from one site of the body to another. Future directions of research in this field rest on the study of the chemokines produced at several sites of involvement of NHL, that is, lymph nodes, spleen, and bone marrow. This might help us to understand the clinical presentation of different NHLs and might help us to design new therapeutic strategies using antagonistic molecules with the ultimate goal of limiting the spread of the disease.
Prepublished online as Blood First Edition Paper, March 4, 2004; DOI 10.1182/blood-2003-09-3103.
Supported by the Italian Association for Cancer Research (AIRC, Milan), by the Ministero dell'Università e della Ricerca Scientifica e Tecnologica (MURST), and by the Ministero della Salute (Rome).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors wish to thank Prof Tullio Pozzan for critical reading of the manuscript and Mr Martin Donach for his help in the preparation of the manuscript.

![Figure 5. The capability of CC and CXC chemokines to stimulate functional properties through the evaluation of cytosolic Ca2+ level shows a consistent mobilization of Ca2+ in the presence of some chemokines. The effect of several chemokines on cytosolic Ca2+ concentration in malignant B cells obtained from 2 representative NHL patients and in normal B lymphocytes obtained from a healthy subject. The assay was performed in incubation medium containing 1 mM CaCl2. A discrete Ca2+ increase was observed following crosslinking surface membrane immunoglobulins (at a concentration of 500 ng/mL), as a positive control. Where indicated by arrows, the chemokines were added at a concentration of 100 ng/mL. At the end of each experiment 2 mM EGTA (ethylene glycol tetraacetic acid) was added and successively the Ca2+ ionophore ionomycin (1 μM) was added to induce a massive [Ca2+]i increase. The values are expressed as ratio between the intensity of the fluorescence signal obtained by exciting cells alternatively at 340 and 380 nm. An increase in ratio reflects an increase in [Ca2+]i.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/2/10.1182_blood-2003-09-3103/6/m_zh80140464210005.jpeg?Expires=1769276378&Signature=Zpr0AIjEC8-cZD4alAlRC1SQsFYIvWQOlLdncdjkPmLvtC0EZMZHpqe9osIsoWFe16KOTAt3sUJzeLUi~YARJCiV-ZytaZ1ZHqjhsdrxxNyvoFGpCLC3XFNYYMFHEZKGDPzQ9oXD12aRqfz0H4vnHjJvVjDQ08hTBY5ZjaRdgXBOOcJCS6GeBPUh4lh8yPa-BZ0myA49EIYn~jGXEc59OBp7R24tZMA-QuJCg87VZxAnWMqH4In-bML-V7wO7865TYErNB4dLp0zVajFPb6riXYw9BnDSakG2w05GcZAn3GKZKzQKeSh-WE93zenwXf9LP~AtTrBWpBGYUln08OpFA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
