Abstract
Transcription factors of the Forkhead Box, class O (FOXO) family promote cell-cycle arrest and/or apoptosis in a variety of cell types. Mitogenic stimuli inactivate FOXO function by way of an evolutionarily conserved pathway involving the activation of phosphoinositide 3-kinase (PI3K) and its downstream effector, Akt. Although PI3K activation is required for B-lymphocyte proliferation, it is not known whether PI3K-dependent inactivation of FOXO proteins is important for cell-cycle progression and survival of these cells. Here, we show that B-cell receptor (BCR) engagement triggers PI3K-dependent phosphorylation and nuclear export of FOXO1. Furthermore, forced expression of PI3K-independent variants of FOXO1 or FOXO3a in activated B cells induces partial arrest in G1 phase of the cell cycle and increases apoptosis. These findings establish that FOXO inactivation is a functionally important consequence of PI3K signaling in primary B cells.
Introduction
Signaling through the B-cell receptor (BCR) initiates events leading to proliferation and survival. One key signal is the activation of the phosphoinositide 3-kinase (PI3K) pathway.1-4 Lipid second messengers produced by PI3K activate a number of kinases, including Akt.5,6 Many proteins that influence cell-cycle progression and survival have been defined as Akt substrates in other cellular systems. One such group is the Forkhead Box, class O (FOXO) family of transcription factors, which includes FOXO1, FOXO3a, and FOXO4.7,8 Transcriptional targets of FOXO proteins include genes encoding proapoptotic and/or antiproliferative proteins such as Bim, Fas ligand, p27kip,7 and Rb2/p130.9-13 Phosphorylation by Akt and other kinases, including Sgk, results in FOXO inactivation. This results in part from shuttling out of the nucleus and into the cytoplasm, where they are sequestered by binding to 14-3-3 proteins and/or degraded.7,8,14 FOXO1 is phosphorylated following BCR stimulation of a B-cell line.15 Here, we address the question of whether PI3K/Akt pathway-mediated inhibition of FOXO function contributes to proliferation and survival of nontransformed mature B cells.
Study design
Mice
Animals were housed and studied in accordance with protocols approved by the institutional animal care and use committee. Balb/c mice and Eμ-Bcl-2 transgenic mice (mixed background containing C57Bl/6, 129SvEv, and Balb/c) were bred in our colony.
RNA expression analysis (microarray and Q-PCR)
RNA was prepared from resting and activated splenic B cells (purified as previously described16 ) and analyzed by microarray (Affymetrix, Santa Clara, CA) as described.17 Expression patterns for cyclin G2 and pRb2/p130 were validated by quantitative real-time polymerase chain reaction (Q-PCR) as described.17
Antibodies and immunoblots
Anti-FOXO1 and antiphospho-FOXO1 (Ser256) were from Cell Signaling Technologies (Beverly, MA). Anti-FOXO3a was from Upstate (Charlottesville, VA). Anti–β-actin was from Sigma-Aldrich (St Louis, MO). Purified Balb/c B cells at 2 × 106/mL were incubated at 37°C for 1 hour in B-cell medium,16 treated for 15 minutes with LY294002 (LY; 10 μM) or vehicle control (0.1% EtOH), then stimulated with anti-IgM (immunoglobulin M) F(ab′)2 (Jackson Immunoresearch, West Grove, PA). Nuclear and cytoplasmic extracts were prepared by using Pierce NE-PER kit (Rockford, IL), diluted in 2 × sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer and boiled for 5 minutes. Separations were verified by blotting for cytoplasmic and nuclear markers (tubulin and lung Krüppel-like factor [LKLF]). Whole-cell lysates were made by resuspending cells in 1 × sample buffer and boiling for 5 minutes. Immunoblotting was performed as previously described.18 Blots were quantitated by using NIH Image 1.61 (Bethesda, MD).
Retroviral transduction and vectors
FLAG–tagged wild-type FOXO1 and FOXO1(A3) (gift from Kun-Liang Guan, University of Michigan) and hemagglutin (HA)–tagged FOXO3a and FOXO3a(A3) (gift from Dr Boudewijn Burgering, University of Utrecht, the Netherlands) were subcloned into a retroviral vector, MSCV-IRES-Thy1.1 (pMIT, gift from P. Marrack, National Jewish Medical and Research Center, Denver, CO), as previously described.18 The A3 mutants have all 3 Akt/Sgk phosphorylation sites (FOXO1: T24, S256, S319; FOXO3a: T32, S253, S3157 ) mutated to alanine. Retroviral stocks were produced and titered as described.18 Primary B cells from Balb/c and Bcl-2 transgenic mice were purified and stimulated for 24 hours with lipopolysaccharide (LPS; 10 μg/mL). Retroviral transduction was performed by centrifuging cells with viral supernatant in a 24-well plate for 45 minutes at 450g at 30° C in the presence of 2 μg/mL polybrene. Cells were stained and analyzed by fluorescence activated cell sorting (FACS) for apoptosis (Annexin V) in Thy1.1+ populations. Cell-cycle analysis was also performed on Thy1.1+ cells by measuring DNA content as previously described.16 Data were analyzed by Flowjo (TreeStar, Ashland, OR).
Results and discussion
Previously, we used DNA microarrays to gain a global understanding of how PI3K regulates gene expression changes in activated B cells.17 Several genes were found to be down-regulated following BCR engagement in a PI3K-dependent manner. Protein products of 2 of these genes, Rb2/p130 and cyclin G2, have known growthsuppressive activity.19-22 We used Q-PCR to verify PI3K-dependent down-regulation of p130 and cyclin G2 mRNA in murine B cells activated with anti-IgM (Figure 1A). The genes encoding human Rb2/p130 and cyclin G2 have been shown to be direct targets of FOXO proteins.12,23 This led us to explore the hypothesis that FOXO protein inactivation by PI3K/Akt signaling contributes to proliferation and survival following B-cell activation.
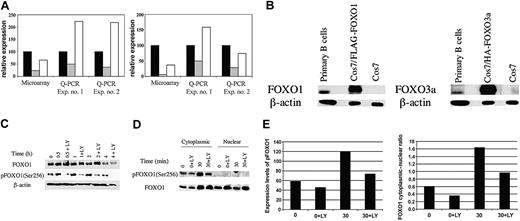
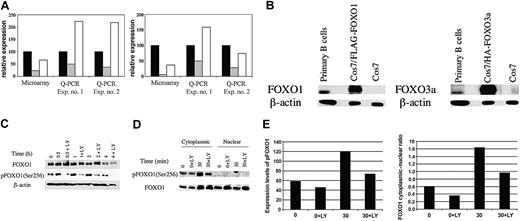
BCR crosslinking leads to PI3K-dependent down-regulation of gene expression accompanied by phosphorylation and nuclear export of FOXO1. (A) Independent RNA samples derived from separate preparations of murine B cells were analyzed by microarray (Affymetrix) or Q-PCR for expression of cyclin G2 (left) or pRb2/p130 (right). Relative expression in microarray data refers to the mean hybridization signal from replicate experiments after normalization of overall chip signals. For Q-PCR data, relative expression was calculated from standard curves and normalized to β-actin mRNA levels. Q-PCR experiments used RNA from Balb/c B cells stimulated with anti-IgM in the presence of a maximal inhibitory concentration (10 μM) of the PI3K inhibitor LY294002 (LY), whereas a suboptimal LY concentration (3 μM) was used in the microarray experiment.17 Data are plotted as percentage of control, defined as the expression level in fresh unstimulated B cells. Relative expression at T = 0 minute (▪), T = 2 hours (▦) , and T = 2 hours + LY (□) is shown. (B) Whole-cell lysates were prepared from resting primary B cells and either Cos7 cells transfected with FLAG-tagged FOXO1 and HA-FOXO3a or untransfected. These samples were then resolved by SDS-PAGE and immunoblotted for total FOXO1 and FOXO3a. The slower mobility of the transfected samples is the result of the epitope tags. Anti–β-actin was used to determine equal loading. (C) B cells were either lysed immediately (T = 0 minute) or stimulated with anti-IgM for the indicated times in the presence or absence of LY (15-minute pretreatment). Whole-cell lysates were resolved by SDS-PAGE and immunoblotted for phospho-FOXO1 (Ser256) and total FOXO1. The blot was also probed for β-actin to verify equivalent loading. (D) B cells were either harvested immediately (T = 0 and T = 0 + LY) or stimulated for 30 minutes (T = 30 and T = 30 + LY) with anti-IgM in the presence or absence of 10 μM LY294002. Nuclear and cytoplasmic lysates were loaded on the basis of cell equivalents and resolved by SDS-PAGE, followed by immunoblotting for phospho-FOXO1 (Ser256) and total FOXO1. (E) (Left) Expression levels of FOXO1 and phospho-FOXO1 (pFOXO1) in panel D were quantitated by using NIH Image 1.61. Expression is shown in arbitrary units. (Right) Ratio of cytoplasmic to nuclear FOXO1 expression. Left panel shows expression of phospho-FOXO1 in the cytoplasm. For immunoblots, similar results were obtained in 2 to 4 replicate experiments.
BCR crosslinking leads to PI3K-dependent down-regulation of gene expression accompanied by phosphorylation and nuclear export of FOXO1. (A) Independent RNA samples derived from separate preparations of murine B cells were analyzed by microarray (Affymetrix) or Q-PCR for expression of cyclin G2 (left) or pRb2/p130 (right). Relative expression in microarray data refers to the mean hybridization signal from replicate experiments after normalization of overall chip signals. For Q-PCR data, relative expression was calculated from standard curves and normalized to β-actin mRNA levels. Q-PCR experiments used RNA from Balb/c B cells stimulated with anti-IgM in the presence of a maximal inhibitory concentration (10 μM) of the PI3K inhibitor LY294002 (LY), whereas a suboptimal LY concentration (3 μM) was used in the microarray experiment.17 Data are plotted as percentage of control, defined as the expression level in fresh unstimulated B cells. Relative expression at T = 0 minute (▪), T = 2 hours (▦) , and T = 2 hours + LY (□) is shown. (B) Whole-cell lysates were prepared from resting primary B cells and either Cos7 cells transfected with FLAG-tagged FOXO1 and HA-FOXO3a or untransfected. These samples were then resolved by SDS-PAGE and immunoblotted for total FOXO1 and FOXO3a. The slower mobility of the transfected samples is the result of the epitope tags. Anti–β-actin was used to determine equal loading. (C) B cells were either lysed immediately (T = 0 minute) or stimulated with anti-IgM for the indicated times in the presence or absence of LY (15-minute pretreatment). Whole-cell lysates were resolved by SDS-PAGE and immunoblotted for phospho-FOXO1 (Ser256) and total FOXO1. The blot was also probed for β-actin to verify equivalent loading. (D) B cells were either harvested immediately (T = 0 and T = 0 + LY) or stimulated for 30 minutes (T = 30 and T = 30 + LY) with anti-IgM in the presence or absence of 10 μM LY294002. Nuclear and cytoplasmic lysates were loaded on the basis of cell equivalents and resolved by SDS-PAGE, followed by immunoblotting for phospho-FOXO1 (Ser256) and total FOXO1. (E) (Left) Expression levels of FOXO1 and phospho-FOXO1 (pFOXO1) in panel D were quantitated by using NIH Image 1.61. Expression is shown in arbitrary units. (Right) Ratio of cytoplasmic to nuclear FOXO1 expression. Left panel shows expression of phospho-FOXO1 in the cytoplasm. For immunoblots, similar results were obtained in 2 to 4 replicate experiments.
First, we studied the expression and regulation of FOXO proteins in primary B cells. Both FOXO1 and FOXO3a were detectable by using isoform-specific antibodies, but their relative expression levels could not be inferred by this analysis (Figure 1B). Phosphospecific antisera that recognize Akt phosphorylation sites on FOXO1, FOXO3a, and FOXO4 detected a predominant band comigrating with FOXO1 (Figure 1C; data not shown), suggesting that this is the most abundant isoform in resting B cells. Phospho-FOXO1 was consistently detected in unstimulated B cells but increased on BCR stimulation in a LY-sensitive manner (Figure 1C). Consistent with the model that FOXO phosphorylation promotes nuclear exit, phospho-FOXO1 was found exclusively in the cytoplasm (Figure 1D-E). Furthermore, the ratio of cytoplasmic to nuclear FOXO1 increased following BCR engagement, and this increase was significantly diminished in LY-treated cells (Figure 1D-E). Although it appears that the amount of FOXO1 protein in the cytoplasm of activated cells was similar in the absence or presence of LY, this could be explained by degradation of the highly phosphorylated FOXO1 in cells with active PI3K.14 Consistent with this, the amount of FOXO1 protein in total lysates decreased over time, and this was partially delayed by pretreatment with LY (Figure 1C). Other kinases can phosphorylate FOXO proteins, some in a PI3K-independent manner,7,8 and may contribute to FOXO degradation.
To test whether FOXO proteins influence B-cell proliferation and/or survival, FOXO factors were overexpressed in proliferating Balb/c B cells. We used the pMIT retroviral vector that allows bicistronic expression of the gene of interest and the surface marker, Thy 1.1. Overexpression of wild-type FOXO1 caused a modest increase in cell death, whereas FOXO3a had no consistent effect (Figure 2A). Conversely, FOXO3a but not FOXO1 caused a reproducible arrest or delay in cell-cycle progression, as judged by a decrease in the fraction in S phase and an increase in the fraction in G1 phase (Figure 2A). Cell size analysis did not suggest induction of G0 by either FOXO isoform (data not shown).
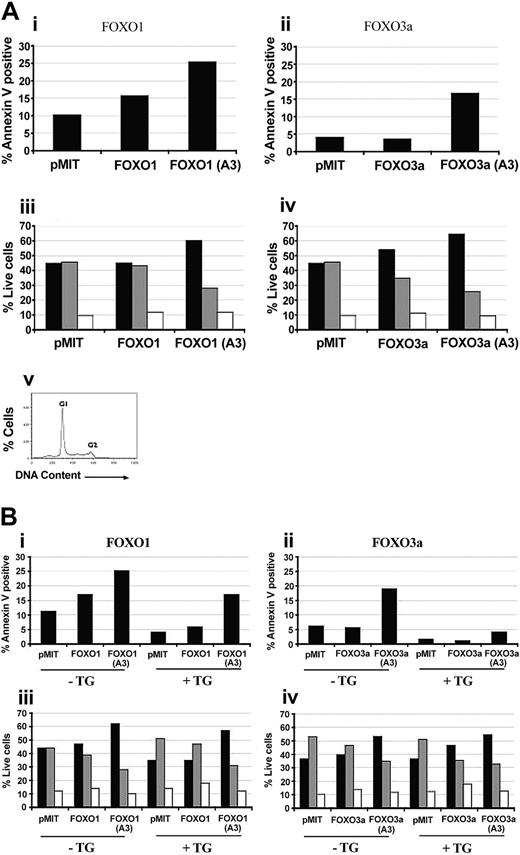
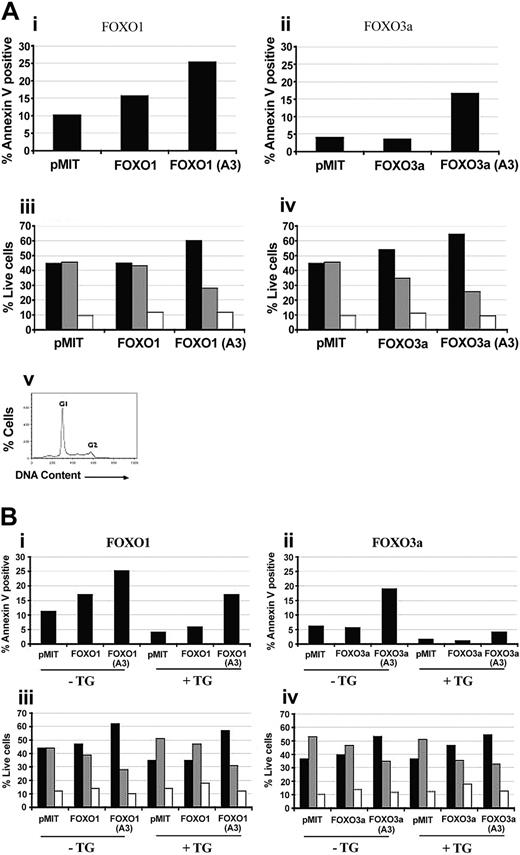
FOXO overexpression in primary B cells causes cell-cycle arrest and increases apoptosis in a manner opposed by PI3K/Akt signaling. (A) Purified B cells from Balb/c mice were stimulated with 10 μg/mL LPS for 24 hours. The cells were then retrovirally transduced with either vector alone (pMIT), or wild-type or A3 mutant versions of FOXO1 (i,iii) or FOXO3a (ii,iv). (i-ii) Sixty-four hours after infection the cells were stained with anti-Thy1.1–biotin followed by Streptavidin-CyChrome to identify positively transduced cells and Annexin V–phycoerythrin (PE) to identify cells undergoing apoptosis. Bar graphs depict the percentage of apoptotic (Annexin V–positive) cells in the population expressing high levels of Thy1.1. (iii-iv) Thirty-six hours after infection, cells were stained with anti-Thy1.1–biotin and Streptavidin-FITC, then analyzed for DNA content by using propidium iodide. The percentage of live Thy1.1 cells in G1 (▪), S (▦) , and G2 (□) phases of the cell cycle was determined by using Flowjo. (v) A representative cell-cycle graph. Three to 4 independent experiments with similar results were obtained for both cell death and cell cycle. (B) Purified B cells from nontransgenic littermates (–Tg) and Bcl2 Tg (+Tg) mice were stimulated and retrovirally transduced as for panel A. Cell death (i-ii) and cell-cycle status (iii-iv) were monitored at 64 hours and 36 hours after infection, respectively, as for panel A.
FOXO overexpression in primary B cells causes cell-cycle arrest and increases apoptosis in a manner opposed by PI3K/Akt signaling. (A) Purified B cells from Balb/c mice were stimulated with 10 μg/mL LPS for 24 hours. The cells were then retrovirally transduced with either vector alone (pMIT), or wild-type or A3 mutant versions of FOXO1 (i,iii) or FOXO3a (ii,iv). (i-ii) Sixty-four hours after infection the cells were stained with anti-Thy1.1–biotin followed by Streptavidin-CyChrome to identify positively transduced cells and Annexin V–phycoerythrin (PE) to identify cells undergoing apoptosis. Bar graphs depict the percentage of apoptotic (Annexin V–positive) cells in the population expressing high levels of Thy1.1. (iii-iv) Thirty-six hours after infection, cells were stained with anti-Thy1.1–biotin and Streptavidin-FITC, then analyzed for DNA content by using propidium iodide. The percentage of live Thy1.1 cells in G1 (▪), S (▦) , and G2 (□) phases of the cell cycle was determined by using Flowjo. (v) A representative cell-cycle graph. Three to 4 independent experiments with similar results were obtained for both cell death and cell cycle. (B) Purified B cells from nontransgenic littermates (–Tg) and Bcl2 Tg (+Tg) mice were stimulated and retrovirally transduced as for panel A. Cell death (i-ii) and cell-cycle status (iii-iv) were monitored at 64 hours and 36 hours after infection, respectively, as for panel A.
Mutation of 3 serine and threonine residues on FOXO1 or FOXO3a to alanine (“A3 mutants”) converts these proteins to Akt independence and enhances the ability of FOXO factors to oppose cell cycle and survival in many cell types.7,9-13 In activated B cells, FOXO1(A3) and FOXO3a(A3) markedly augmented apoptosis and cell-cycle arrest/delay (Figure 2A). The enhanced effectiveness of the A3 mutants relative to their wild-type counterparts indicates that PI3K in the activated B cells can partially inactivate exogenous wild-type FOXO.
To determine if A3 mutants of FOXO1 and FOXO3a use distinct mechanisms to promote cell-cycle arrest and death, we studied B cells from transgenic mice overexpressing Bcl-2. Consistent with the antiapoptotic activity of Bcl-2, transgenic B cells showed lower spontaneous death compared with littermate controls (Figure 2B). Overexpression of Bcl-2 also diminished death induced by expression of FOXO proteins, more strikingly when FOXO3a(A3) was used (Figure 2B) but did not block FOXO-mediated cell-cycle arrest (Figure 2A-B). These findings could suggest that in activated B cells FOXO proteins exert their effects through up-regulation of both proapoptotic genes such as Bim, as described in primary T cells,13 along with antiproliferative genes such as Rb2.
PI3K activation plays a pivotal role in B-cell proliferation and survival,1-3 yet the downstream targets of this signaling pathway in B cells are not fully defined. We have established that PI3K-dependent inactivation of FOXO protein function is important for cell-cycle progression and survival in activated B cells. Following BCR cross-linking, PI3K/Akt-mediated phosphorylation of FOXO1 increases, the protein exits the nucleus, and its total expression decreases. These findings indicate that FOXO regulation in B cells adheres to the paradigm established in other cell types.7,8 A critical question that remains to be answered is whether FOXO proteins function in resting B cells to promote quiescence rather than death, as suggested by the elevated expression of Rb2/p130.12 Resting B cells are refractory to current gene delivery methods; therefore, dissecting FOXO function in naive B cells will require alternative approaches such as transgenesis.
Prepublished online as Blood First Edition Paper, April 6, 2004; DOI 10.1182/blood-2003-09-3071.
Supported by a grant from the National Institutes of Health (NIH) (AI50831 [D.A.F.]) and by an NIH Training Grant (T32 CA09054 [M.G.K.]).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Pratibha Sareen, Cattleya Buranasombati, and Travis Moore for mouse colony maintenance and genotyping; Boudewijn Burgering, Kun-Liang Guan, and Philippa Marrack for DNA constructs; and Craig Walsh for helpful discussions.