Abstract
We previously found that reduced glutathione (GSH) or a mixture of GSH/glutathione disulfide (GSSG) potentiated platelet aggregation. We here report that GSSG, when added to platelets alone, also potentiates platelet aggregation. Most of the GSSG was converted to GSH by a flavoprotein-dependent platelet surface mechanism. This provided an appropriate redox potential for platelet activation. The addition of GSSG to platelets generated sulfhydryls in the β subunit of the αIIbβ3 fibrinogen receptor, suggesting a mechanism for facilitation of agonist-induced platelet activation.
Introduction
Glutathione, an important modulator of the cellular redox environment, is found in blood where it could modulate the redox environment of platelets. Cells contain millimolar concentrations of glutathione, mostly in the reduced form. Plasma contains only 8 to 25 μM glutathione,1-6 also predominantly in the reduced form. This contrasts with other low-molecular-weight thiols that are mostly in disulfide forms.2,6 Plasma glutathione levels and GSH/glutathione disulfide (GSSG) ratios in plasma are altered in disease states.4,5,7 However, little is known about the role of plasma glutathione in the regulation of redox reactions. We recently found that physiologic concentrations of GSH generate thiols from disulfide bonds in αIIbβ3 and potentiate platelet aggregation.7
Recent reports have also raised the possibility that platelets have a transplasma membrane oxidoreductase that, like GSH, can reduce extracellular disulfide bonds in redox-sensitive membrane proteins.7-9 Although there is little information about such systems in cells, a form of a cell membrane nicotinamide adenine dinucleotide (NADH) oxidase10,11 in plant cells has been reported to have disulfide reductase activity.12 Cells and platelets contain flavoprotein-electron transport systems such as nicotinamide adenine dinucleotide phosphate (NADPH) oxidase on neutrophils; however, the electron acceptor is molecular oxygen (not disulfide bonds) and the product is superoxide anion (O–2).13-15 There are no reports of transplasma membrane electron transport systems that reduce disulfide bonds in mammalian cells. We here report that nonstimulated platelets have the ability to reduce the disulfide bond in extracellular GSSG. This results in the production of GSH, the generation of thiols in the αIIbβ3 integrin, and the facilitation of platelet aggregation.
Study design
Platelet aggregation, measurement of extracellular sulfhydryls in platelet samples, and sulfhydryl labeling of extracellular of platelets were performed as described elsewhere.7
Results and discussion
We previously found that GSH or a mixture of GSH/GSSG potentiated platelet aggregation stimulated by agonists such as collagen.7 When GSSG (5 μM) was used alone as a control for the collagen-induced aggregation studies, surprisingly, it also enhanced platelet aggregation (Figure 1). As expected, GSSG alone did not contain any GSH by the 5,5′-dithiobis-(2-nitrobenzoid) acid (DTNB) assay (not shown). When the concentration of agonist gave partial or submaximal aggregation, a low concentration of GSSG (2 μM) further stimulated aggregation (Figure 1B). Thus, under appropriate conditions low concentrations of glutathione, similar to those found in blood, substantially potentiate aggregation.
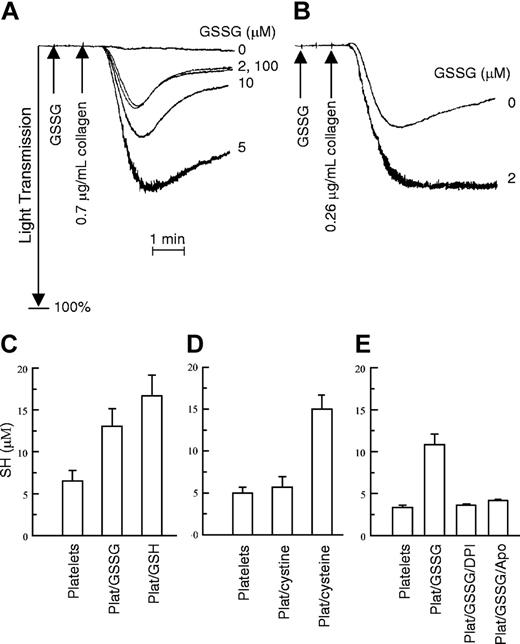
Effect of GSSG on platelet aggregation and effect of platelets on GSSG. (A) Gel-filtered platelets were incubated for 1 minute at 37° C with the indicated concentrations of GSSG. Collagen was then added at a concentration insufficient to induce aggregation by itself. (B) Collagen alone caused partial aggregation that a low concentration of GSSG (2 μM) further potentiated. These experiments were performed 3 times with similar results. (C-E) Gel-filtered platelets were incubated with GSSG, or under other conditions, and total sulfhydryl (SH) content in the sample was determined using DTNB as described.7 (C) Results of platelets incubated with GSSG. Platelets alone are in column 1. Column 2 shows the results of platelets preincubated with GSSG (5 μM) for 15 minutes at 24° C before the DTNB was added. Column 3 shows the results for GSH (10 μM) with platelets (Plat/GSH). For these experiments, n = 5. (D) The lack of effect of platelets on cystine (disulfide of cysteine) is seen. Column 1 again shows the amount of SH in platelets. Column 2 shows the SH content of platelets incubated with 5 μM cystine (Plat/cystine). Also shown are platelets with cysteine (10 μM; Plat/cysteine). For these experiments, n = 7. (E) The effect of NADPH oxidase inhibitors on conversion of GSSG to GSH is seen. The first column shows the SH content of platelets. Column 2 is the SH content of the platelet/GSSG mixture; columns 3 and 4 are the same as column 2 except that the flavoprotein NADPH oxidase inhibitors DPI (10 μM) or apocynin (100 μM; Apo) were added to the platelets for 30 minutes prior to the addition of GSSG. For these experiments, n = 2 to 4 for each sample. The results are ± 1 SE.
Effect of GSSG on platelet aggregation and effect of platelets on GSSG. (A) Gel-filtered platelets were incubated for 1 minute at 37° C with the indicated concentrations of GSSG. Collagen was then added at a concentration insufficient to induce aggregation by itself. (B) Collagen alone caused partial aggregation that a low concentration of GSSG (2 μM) further potentiated. These experiments were performed 3 times with similar results. (C-E) Gel-filtered platelets were incubated with GSSG, or under other conditions, and total sulfhydryl (SH) content in the sample was determined using DTNB as described.7 (C) Results of platelets incubated with GSSG. Platelets alone are in column 1. Column 2 shows the results of platelets preincubated with GSSG (5 μM) for 15 minutes at 24° C before the DTNB was added. Column 3 shows the results for GSH (10 μM) with platelets (Plat/GSH). For these experiments, n = 5. (D) The lack of effect of platelets on cystine (disulfide of cysteine) is seen. Column 1 again shows the amount of SH in platelets. Column 2 shows the SH content of platelets incubated with 5 μM cystine (Plat/cystine). Also shown are platelets with cysteine (10 μM; Plat/cysteine). For these experiments, n = 7. (E) The effect of NADPH oxidase inhibitors on conversion of GSSG to GSH is seen. The first column shows the SH content of platelets. Column 2 is the SH content of the platelet/GSSG mixture; columns 3 and 4 are the same as column 2 except that the flavoprotein NADPH oxidase inhibitors DPI (10 μM) or apocynin (100 μM; Apo) were added to the platelets for 30 minutes prior to the addition of GSSG. For these experiments, n = 2 to 4 for each sample. The results are ± 1 SE.
We hypothesized that the GSSG was being converted to GSH by the platelets. To test this we incubated GSSG with gel-filtered platelets and measured total thiols in the sample. The combination of GSSG with platelets gave substantially more free thiols as detected by DTNB than could be accounted for by either platelets or GSSG alone (Figure 1C). The amount of sulfhydryls on platelets alone corresponded to about 9.0 × 10–18 mol thiol/platelet (similar to what has been reported).16,17 Platelets with GSH (10 μM) gave the additive results of platelets alone plus GSH alone (column 3). Platelets with GSSG (5 μM) gave about double what was expected from platelets alone (about 6.5 μM additional SH; P < .05; n = 5). This suggests that 3 to 4 μM of the GSSG added is being converted to GSH by platelets. Therefore, the addition of GSSG to platelets would result in a ratio of GSH/GSSG of approximately 3:1 to 4:1. This ratio is similar to that found in blood and to the GSH/GSSG ratio that provides maximal potentiation of platelet activation.7 In contrast to the effect of platelets on GSSG, when cystine (the disulfide form of cysteine) was incubated with platelets no increase in sulfhydryls was found (Figure 1D). This indicates some specificity of the reaction.
The conversion of GSSG to GSH requires the addition of 2 electrons to reduce the disulfide bond. In cells NADPH serves as the electron donor for GSSG in the following reaction: GSSG + NADPH + H+ → 2 GSH + NADP+. Therefore, the generation of GSH by platelets in the absence of external reducing agents implicates a transmembrane electron transport system that uses a cytoplasmic electron donor, presumably NAD(P)H. NADPH oxidase13,14 and NADH oxidase10-12,18,19 are plasma membrane oxidoreductase systems found in cells. To begin to explore these possibilities, we tested the effect of diphenyleneiodonium (DPI), an inhibitor of flavin-containing enzymes, as well as a structurally unrelated inhibitor of NADPH oxidase, apocynin.20 Both DPI (10 μM) and apocynin inhibited the GSSG reductase activity (Figure 1E). Because the reaction is inhibited by relatively low concentrations of DPI, the mechanism likely involves a flavoprotein.
Time-course studies on the conversion of GSSG to GSH were performed at 37° C, the conditions under which GSSG enhanced platelet aggregation. The platelet reaction with GSSG was complete by 1 minute (Figure 2A). This suggests that the effect of GSSG on platelet aggregation occurs after some GSSG is converted to GSH, and thus after an appropriate redox potential for platelet activation is reached. To test for this possibility we compared the effect of GSSG added 1 minute before collagen to GSSG added simultaneously with collagen. Figure 2B shows that GSSG (5 μM) added simultaneously with collagen did not potentiate aggregation (the 0-second tracing). In contrast to the lack of effect of GSSG added with collagen, GSSG incubated for 1 minute with platelets as expected facilitated aggregation (the 60-second tracing). As expected from previous studies,7 controls of GSH (10 μM) or a mixture of GSH (10 μM) and GSSG (2 μM) potentiated aggregation when added simultaneously with collagen (not shown). These results imply that platelets convert GSSG to GSH and this can be part of the stimulus for platelet activation.
Time-course studies on GSSG and platelets and the effect of GSSG on sulfhydryl labeling of αIIbβ3. (A) Time course of GSSG conversion to GSH at 37° C. In these studies GSSG was added to platelets for the indicated times, and then DTNB was added. Total SH in the sample was measured as described for Figure 1C. Each point in the graph represents the mean of duplicate samples. (B) GSSG (5 μM) was added either simultaneously (0 seconds) with a subthreshold concentration of collagen (0.1 μg/mL) or 1 minute (60 seconds) before the subthreshold dose of collagen. (Controls not shown demonstrated the stimulatory effect of GSH (10 μM) or GSH (10 μM) with GSSG [2 μM], added simultaneously with the same dose of collagen.) These experiments were performed a minimum of 2 times with similar results. (C) Platelets were incubated without GSH (lane 1), with GSH (10 μM; lane 2), or with GSSG (5 μM; lane 3) for 5 minutes at 24° C under nonstirring conditions. The sulfhydryl reagent 3-N-maleimidylpropionyl biotin (MPB; 50 μM) was added in excess of the GSH, and the labeling performed under conditions that cause disruption of the αIIbβ3 receptor (5 mM EDTA [ethylenediaminetetraacetic acid], 60 minutes, 37° C). Equal amounts of protein were added to lanes 1, 2, and 3. (D) Densitometry of the individual bands was used to obtain quantitative results. The first 2 columns show the ratio of labeling of the β3 subunit with GSH or GSSG to the β3 subunit without GSH (± 1 SE, n = 8). As a control for possible translocation of αIIbβ3 the third and fourth columns give the ratio of labeling using a label for primary amines, sulfosuccinimido-biotin (SSB) in the β3 subunit with GSH or GSSG to the β3 subunit without GSH (± 1 SE).
Time-course studies on GSSG and platelets and the effect of GSSG on sulfhydryl labeling of αIIbβ3. (A) Time course of GSSG conversion to GSH at 37° C. In these studies GSSG was added to platelets for the indicated times, and then DTNB was added. Total SH in the sample was measured as described for Figure 1C. Each point in the graph represents the mean of duplicate samples. (B) GSSG (5 μM) was added either simultaneously (0 seconds) with a subthreshold concentration of collagen (0.1 μg/mL) or 1 minute (60 seconds) before the subthreshold dose of collagen. (Controls not shown demonstrated the stimulatory effect of GSH (10 μM) or GSH (10 μM) with GSSG [2 μM], added simultaneously with the same dose of collagen.) These experiments were performed a minimum of 2 times with similar results. (C) Platelets were incubated without GSH (lane 1), with GSH (10 μM; lane 2), or with GSSG (5 μM; lane 3) for 5 minutes at 24° C under nonstirring conditions. The sulfhydryl reagent 3-N-maleimidylpropionyl biotin (MPB; 50 μM) was added in excess of the GSH, and the labeling performed under conditions that cause disruption of the αIIbβ3 receptor (5 mM EDTA [ethylenediaminetetraacetic acid], 60 minutes, 37° C). Equal amounts of protein were added to lanes 1, 2, and 3. (D) Densitometry of the individual bands was used to obtain quantitative results. The first 2 columns show the ratio of labeling of the β3 subunit with GSH or GSSG to the β3 subunit without GSH (± 1 SE, n = 8). As a control for possible translocation of αIIbβ3 the third and fourth columns give the ratio of labeling using a label for primary amines, sulfosuccinimido-biotin (SSB) in the β3 subunit with GSH or GSSG to the β3 subunit without GSH (± 1 SE).
The effect of GSH (10 μM) and GSSG (5 μM) on sulfhydryl generation in αIIbβ3 is shown in Figure 2C. Using immunoprecipitation the bands shown were previously identified as αIIb and β3.7 GSH, previously found to generate sulfhydryls in the β3 subunit, increased sulfhydryl labeling in β3 by 2-2.5 fold. A concentration of GSSG (5 μM) that potentiated platelet aggregation increased sulfhydryl labeling in the β3 subunit by almost 3-fold (Figure 2D). This is similar to the effect of GSH (10 μM) and confirms a mechanism in αIIbβ3. A 1.6- to 1.7-fold increase in labeling of αIIb was also found with both GSH and GSSG (not shown). Because GSH by itself does not cause platelet aggregation,7 the sulfhydryl generation in β3 apparently does not by itself activate the receptor. An additional stimulus is necessary.
In summary, the conversion of GSSG to GSH by platelets implicates a novel surface flavoprotein GSH reductase activity that may be a marker for a more general disulfide reductase activity. This activity appears to provide the appropriate redox potential for platelet activation. This reaction may modulate the local redox environment at sites of vascular injury where platelets are found in high concentrations. Redox-sensitive protein on platelets whose function may be modulated by the redox environment include αIIbβ3 and protein disulfide isomerase.21
Prepublished online as Blood First Edition Paper, May 13, 2004; DOI 10.1182/blood-2004-03-1097.
Supported by the American Heart Association (Heritage and Texas affiliates).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 2. Time-course studies on GSSG and platelets and the effect of GSSG on sulfhydryl labeling of αIIbβ3. (A) Time course of GSSG conversion to GSH at 37° C. In these studies GSSG was added to platelets for the indicated times, and then DTNB was added. Total SH in the sample was measured as described for Figure 1C. Each point in the graph represents the mean of duplicate samples. (B) GSSG (5 μM) was added either simultaneously (0 seconds) with a subthreshold concentration of collagen (0.1 μg/mL) or 1 minute (60 seconds) before the subthreshold dose of collagen. (Controls not shown demonstrated the stimulatory effect of GSH (10 μM) or GSH (10 μM) with GSSG [2 μM], added simultaneously with the same dose of collagen.) These experiments were performed a minimum of 2 times with similar results. (C) Platelets were incubated without GSH (lane 1), with GSH (10 μM; lane 2), or with GSSG (5 μM; lane 3) for 5 minutes at 24° C under nonstirring conditions. The sulfhydryl reagent 3-N-maleimidylpropionyl biotin (MPB; 50 μM) was added in excess of the GSH, and the labeling performed under conditions that cause disruption of the αIIbβ3 receptor (5 mM EDTA [ethylenediaminetetraacetic acid], 60 minutes, 37° C). Equal amounts of protein were added to lanes 1, 2, and 3. (D) Densitometry of the individual bands was used to obtain quantitative results. The first 2 columns show the ratio of labeling of the β3 subunit with GSH or GSSG to the β3 subunit without GSH (± 1 SE, n = 8). As a control for possible translocation of αIIbβ3 the third and fourth columns give the ratio of labeling using a label for primary amines, sulfosuccinimido-biotin (SSB) in the β3 subunit with GSH or GSSG to the β3 subunit without GSH (± 1 SE).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/5/10.1182_blood-2004-03-1097/5/m_zh80170465770002.jpeg?Expires=1766229733&Signature=zvRKLGJmvGLXToRsMDT3hmKf3jPQHPd~MKhdWUelGwPHEib2F8To5lGYBviyLQ8ZyuQqpq4aP4LrsrPbZwYsnHAwVajPRnPSMMpyeoRpHikopSxKDEWMo0nIijTaiQEigDsUz3mi~qNJxcFmudeYWgbmrploPvpu3akNgy-7sF8rmU-eWDdg7AUmZmdU4BiQOtOMGEoHS42eiI-BrD-h7YMBU4xhiDxTZe9~2nL9PBpt224vkePG~N9C~LaUCGDBNk9iR0qwKNDVYJfaYGyyaGff6xja2vWGeHmXAYjQURHj1640UQGKQupuC44hjtZgOMBiGcikPKb35AYEVj~YsQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
