Abstract
We have isolated a novel cell surface molecule, the human homolog of osteoclast-associated receptor (OSCAR). Unlike mouse OSCAR, hOSCAR is widely transcribed in cells of the myeloid lineage. Notably, hOSCAR is expressed on circulating blood monocytes and CD11c+ dendritic cells but not on T and B cells. hOSCAR is continually expressed during differentiation of CD14+ monocytes into dendritic cells and maintained after maturation. hOSCAR associates with the FcRγ as shown by translocation of FcRγ to the cell surface in presence of hOSCAR and coimmunoprecipitation from transfected cell lines and ex vivo cells. Engagement of hOSCAR with specific mAb leads to Ca2+ mobilization and cytokine release, indicators of cellular activation. Endocytosis of the receptor in dendritic cells was observed, followed by passage of the internalized material into Lamp-1+ and HLA-DR+ compartments, suggesting a role in antigen uptake and presentation. Dendritic cells were able to stimulate a T-cell clone specific for an epitope of mouse IgG1 after uptake and processing of the hOSCAR-specific antibody, demonstrating the capacity of this receptor to mediate antigen presentation. hOSCAR thus represents a novel class of molecule expressed by dendritic cells involved in the initiation of the immune response.
Introduction
Early immune responses are mediated by a number of cell surface receptors on immune cells. Toll-like receptors (TLRs)1,2 are among the most potent receptors known for the detection and response to pathogen-derived products. Engagement of TLRs leads to the activation of nuclear factor (NF)κB and the induction of genes for chemokines and cytokines, as well as genes involved in cellular activation. A growing number of receptors from the lectin or immunoglobulin superfamily (IgSF) recently were shown to also play a role in the activation of myeloid cells. These activating receptors associate with transmembrane adapters (CD3ζ, FcRγ, DAP10, DAP12) containing immunoreceptor tyrosine-based activation motif (ITAM) sequences (consensus: D/Ex7D/Ex2Yx2L/Ix7Yx2L/I).3-5 Upon receptor ligation and ITAM phosphorylation, these transmembrane adapters recruit protein tyrosine kinases, leading to the triggering of phosphorylation cascades and cellular activation.6,7 It has been recently shown that triggering receptor expressed by myeloid cells (TREM)-2, associated with DAP12,8,9 can up-regulate the expression of CCR7 and promote maturation and survival of dendritic cells (DCs).10 TREM-2 recognizes pathogen-derived products such as lipopolysaccharide (LPS), lipoteichoic acid (LTA), and peptidoglycan (PGN).11 Additionally, binding of whole bacteria to cells expressing TREM-2 has been observed.11
Frequently studied ITAM-linked receptors are receptors for the Fc portion of immunoglobulins (FcR). Activating FcR (CD16, CD32a, CD64, FcαR/CD89, and FcϵRI) are expressed on cells of the myeloid lineage but are often down-regulated on differentiation toward DCs, particularly in human. These receptors induce cellular activation and functions of activated cells such as cytokine secretion, antigen-dependent cytotoxicity12 and the capture of antibody-opsonized pathogens. On DCs these receptors also have the ability to mediate antigen processing and both major histocompatibility complex (MHC) class I– and II–restricted presentation.13,14 The ITAM motif of FcRγ has been shown to permit endocytosis via activating FcR15 and to direct the receptor-ligand complex to antigen processing and presentation compartments in antigen-presenting cells (APCs).16-18 The expression pattern of FcR among the different DC subpopulations is highly heterogeneous in humans.14 In contrast to mouse, human DCs, particularly monocyte-derived DCs (mono-DCs), have low to negligible expression of FcR and thus have probably evolved alternative uptake pathways.
We have recently isolated a novel cell surface molecule, the human homolog of mouse osteoclast-associated receptor (hOSCAR).19 We show in this report that unlike mouse OSCAR, hOSCAR is widely expressed in cells of the myeloid lineage; in particular, at all stages of DC differentiation and maturation. As suggested by the presence of a charged residue in the putative transmembrane region, known to indicate the association with an ITAM-adapter,20 hOSCAR associates with FcRγ and not with other signaling molecules. The gene for hOSCAR has been mapped to the leukocyte receptor complex (LRC)21 on chromosome 19q13. Other molecules in the LRC include the immunoglobulin-like transcript/leukocyte Ig-like receptor (ILT/LIR) family of molecules, some of which are also associated with FcRγ.
We have explored the role of hOSCAR-mediated engagement at the cell surface of human monocytes and DCs. Association of hOSCAR with FcRγ allows this receptor to promote cellular activation. It also allows processing and presentation of exogenous antigens. We thus extend the family of myeloid and DC-encoded molecules involved in both innate and adaptive immune responses.
Materials and methods
Cell culture
The following factors were used at optimal conditions of 25, 200, 10, 25, 25, and 2.5 ng/mL, respectively: rhG-CSF (R&D Systems, Abington, United Kingdom), rhGM-CSF (Schering-Plough Research Institute, Kenilworth, NJ), rhIL-4 (Schering-Plough Research Institute), rhM-CSF (R&D Systems), rhSCF (R&D Systems), and rhTNF-α (Genzyme, Boston, MA). HEK293T and U937 cell lines were obtained from American Type Culture Collection (Rockville, MD).
Cells used for the reverse transcriptase–polymerase chain reaction (RT-PCR) panel were produced as described in Bates et al.22 All cells but HEK293T were cultured in RPMI 1640 (GIBCO BRL, Gaithersburg, MD) supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum (FBS) (Flow Laboratories, Irvine, United Kingdom), 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 2 mM l-glutamine, 100 μg/mL gentamicin (Schering-Plough, Levallois-Perret, France) (hereafter referred to as complete medium). HEK293T was cultured in DMEM-F12 with glutamax (GIBCO BRL) supplemented with 10% (vol/vol) heat-inactivated FBS.
Human peripheral blood samples were obtained according to institutional guidelines. Peripheral blood mononuclear cells (PBMCs) were purified by Ficoll-Hypaque centrifugation. Monocytes were purified by immunomagnetic depletion of low-density PBMCs isolated on a 52% Percoll gradient as described elsewhere.22 Mono-DCs was produced by culturing purified monocytes for 5 days in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4).23
Semi-quantitative RT-PCR
Total RNA extraction, cDNA synthesis, and PCR were described previously.22,24 cDNAs were quantified with respect to β-actin. The primers GACCCTCTGGCCTCTGTGTCA (forward primer) and CAACCTGAGTGGCGGAGAAGC (reverse primer) were used to amplify a fragment specific to OSCAR. Cycle conditions were 94°C, 40 seconds; 55°C, 1 minute; 72°C, 2 minutes, for 30 cycles.
Northern blot
Human multiple tissue (MTN blots I to IV Clontech, Palo Alto, CA) and human immune system multiple tissue (MTN blot II Clontech) blots were hybridized with the specific PCR fragment. Probe synthesis, purification, and membrane hybridization were performed as previously described.25 Autoradiography was performed with Biomax MR (Kodak, Rochester, NY) for 7 days.
Chromosomal localization
Chromosomal localization was performed with the Stanford G3 RH medium resolution panel (Research Genetics, Huntsville, AL) as described.25 PCR was performed as above. The results were scored manually and analysis was performed with the RHmapper program (http://shgc-www.stanford.edu).
Expression cloning and transfection in U937 and HEK293T
Full-length OSCAR cDNA was cloned into the HindIII and BamHI sites of pcDNA3.1(+) vector (Invitrogen, San Diego, CA) after PCR amplification from PBMC cDNA using the primers ACGTAAGCTTCAGCTCTAGCGGGTATCTG (forward) and GCTAGGATCCGTGAGTAGACGGCAGTGCTG (reverse). The GeneEditor mutagenesis system (Promega Bioscience, San Luis Obispo, CA) was used to generate mutants. Oligonucleotide primers, which encoded H231 (CAC) or V231 (GTC) instead of R231 (CGC), were designed to generate hOSCAR (R/H) and hOSCAR (R/V) respectively. FcRγ was amplified from PBMC cDNA using the primers CTAGAGATCTGAGCCTCAGCTCTGCTATAT (forward) TAATGCGGCCGCATCCGTAAACAGCATCTGAGC (reverse) and was cloned into the BglII-NotI sites of pDisplay (Invitrogen) (HA-γ-pDisplay). The FLAG-DAP12-pMX construction was a generous gift from L.L. Lanier (University of California, San Francisco).8
HEK293T cells were transfected with 10 μg hOSCAR-pcDNA3.1 or mutants and HA-FcRγ-pDisplay or FLAG-DAP12-pMX using calcium phosphate transfection kit (Invitrogen). After 24 hours the cells were harvested for cytometer and biochemical analysis. hOSCAR-pcDNA3.1 was transfected into U937 by electroporation. Stable transfectants were selected in hygromycin B–containing medium and by isolation using anti-hOSCAR mAb and goat anti–mouse IgG microbeads (Miltenyi Biotec, Bergish Gladbach, Germany).
Production of recombinant hOSCAR and generation of anti-hOSCAR mAbs and F(ab′)2
Soluble hOSCAR was produced as a fusion protein consisting of the extracellular domain of the receptor (amino acids 1-227) fused to the human IgG constant regions (hOSCAR-HuIgG) or to the phosphatase alkaline enzyme (hOSCAR-AP) cloned into pCDM-8. Fusion proteins were generated as described.25,26
Briefly, mice were immunized subcutaneously 3 times at 2-week intervals with 10 μg purified hOSCAR-HuIgG. Three days before fusion mice were boosted intravenously with 5 μg purified hOSCAR-HuIgG. Spleen cells were fused to myeloma X63.Ag8 cells and hybridoma supernatants were screened by enzyme-linked immunosorbent assay (ELISA) using hOSCAR-AP as detecting protein. ELISA-positive hybridoma supernatants were then tested after 2 cycles of cloning by limiting dilutions by flow cytometry on peripheral blood monocytes and mono-DCs.
Three clones were selected for their ability to label these cells: 11.1CN5, B71.01C1, and 51.1CN1, all of isotype IgG1κ. In all experiments the 3 clones behaved similarly. F(ab′)2 fragments of 11.1CN5 mAb were prepared using the F(ab)/F(ab′)2 kit (Pierce, Rockford, IL) based on ficin digestion. The F(ab′)2 were separated from Fc portions and undigested mAbs on an anion exchange column UnoQ1 (Bio-Rad Laboratories, Hercules, CA).
Flow cytometry
For single staining, cells were incubated with 10 μg/mL anti-hOSCAR mAb 11.1CN5 then labeled with phycoerythrin (PE)–conjugated goat anti–mouse (GAM) (Dako, Glostrup, Denmark). For double staining, cells were incubated with biotinylated anti-hOSCAR mAb and fluorescein isothiocyanate (FITC)–conjugated anti-CD3, anti-CD11c (both from Dako), anti-CD14, anti-CD19, or anti-CD56 mAbs (all from Becton Dickinson, Mountain View, CA), followed by streptavidin-PE (Dako). Four-color labeling was performed on PBMCs. Cells were first incubated with FITC-conjugated anti-CD3 (Dako), anti-CD14, anti-CD16, anti-CD19 (Becton Dickinson), anti-CD35 (BD Biosciences Pharmingen, San Diego, CA), anti-CD56, anti–glycophorin A (Immunotech, Marseille, France), PE-Cy5–conjugated anti-CD4 (Immunotech), APC-conjugated anti-CD11c (Becton Dickinson), and biotinylated anti-hOSCAR, followed by streptavidin-PE (Dako). CD4-positive, FITC-negative cells were gated.
Cell radiolabeling, immunoprecipitation, and deglycosylation
Cell surface proteins were iodinated with 1 mCi (37 MBq) Na[I125] (Amersham, Arlington Heights, IL) using lactoperoxidase (Sigma, St Louis, MO).27 After 3 washes in cold phosphate buffered saline (PBS), hOSCAR-transfected U937 or mono-DCs were lysed in 1% Nonidet P-40 (NP-40, Sigma), 10 mM Tris [tris(hydroxymethyl)aminomethane]-HCl, pH 8, 150 mM NaCl (Tris buffered saline [TBS]), 2 mM EDTA (ethylenediaminetetraacetic acid), 20 mM iodoacetamide, and protease inhibitors (cOmplete Mini; Roche, Meylan, France). Insoluble material was pelleted by centrifugation. Lysate was precleared with protein G-sepharose (Amersham Pharmacia, Uppsala, Sweden) and then incubated overnight at 4° C with either a mix of the anti-hOSCAR mAbs or isotype control (Sigma) immobilized on sepharose 4B beads (Amersham Pharmacia). Precipitates were washed 4 times with TBS, 0.1% NP-40, and 4 times with 10 mM Tris-HCl pH 8, 0.1% NP-40. After separation by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), the gels were dried and exposed to Phosphoscreen (Kodak, Rochester, NY). Scanning was performed with a PhosphorImager (Bio-Rad). For analysis of glycosylation, immunoprecipitates were untreated or digested overnight with N-glycosidase F, O-glycosidase, neuraminidase (Roche) as described.24
Coimmunoprecipitation and immunoblotting
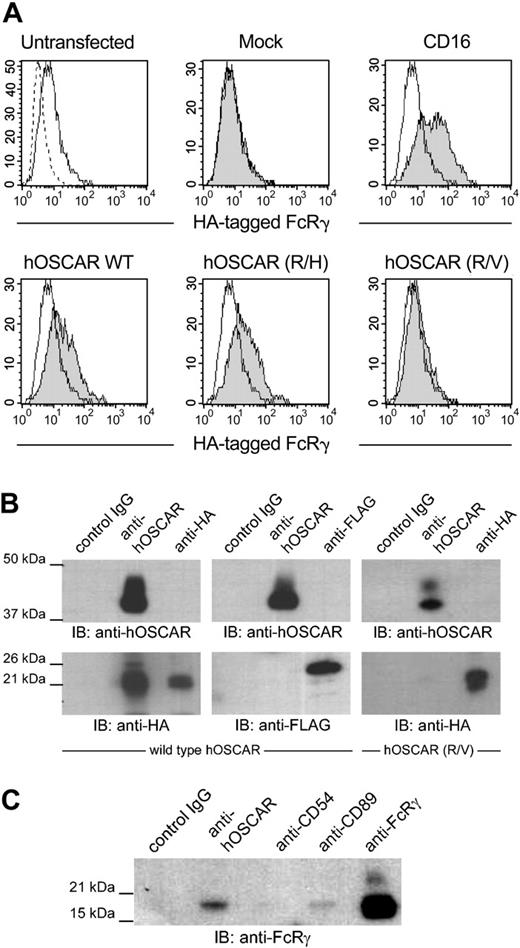
hOSCAR or hOSCAR (R/V) was cotransfected with HA-tagged FcRγ or FLAG-tagged DAP12 in HEK293T cells. After lysis in 1% digitonin (Wako, Richmond, VA), hOSCAR or hOSCAR (R/V) was immunoprecipitated. The HA-tagged FcRγ and FLAG-tagged DAP12 were precipitated with anti-HA (Roche) and anti–FLAG M2 (Sigma) mAbs. Coimmunoprecipitations also were performed on mono-DCs using MOPC21 (Sigma), anti-hOSCAR, anti-CD89, anti-CD54 (both from Immunotech), and anti-FcRγ (Upstate Biotechnology, Lake Placid, NY). Antibodies were immobilized on sepharose 4B beads (Amersham Pharmacia). Immunoprecipitates were separated by SDS-PAGE and then transferred to Immobilon membranes (Millipore, Bedford, MA). Blots were blocked in PBS, 5% milk, 0.2% Tween20, incubated with horse radish peroxidase (HRP)–conjugated anti-HA (Roche) or anti–FLAG M2 (Sigma), and biotinylated anti-hOSCAR followed by Extravidin-HRP (Sigma). For coimmunoprecipitations from mono-DCs, the FcRγ was detected using anti-FcRγ rabbit polyclonal IgG (1 μg/mL, Upstate Biotechnology) and donkey anti–rabbit conjugate (Vector, Burlingame, CA). Proteins were detected by enhanced chemiluminescence.
Cellular activation
Determination of intracellular calcium (Ca2+) flux was as described.24 Briefly, monocytes and mono-DCs were loaded with 5 μM Indo-1 am and 5 μM Pluronic F-27 (Molecular Probes, Eugene, OR). Ten micrograms per milliliter of mAbs were added to human serum–saturated cells and then cross-linked with 20 μg/mL of a goat F(ab′)2 anti-mouse (GAM) (Fc fragment specific) (Jackson ImmunoResearch, West Grove, PA). Anti-CD89 mAb (Immunotech) and recombinant human MIP1α (R&D Systems) were used as positive controls; anti-CD44 and anti–HLA-A, -B, -C mAbs (both from Immunotech) were negative controls.
In vitro stimulation of mono-DCs was performed as described elsewhere.10 Briefly, mono-DCs were incubated with anti-hOSCAR or irrelevant mAb MOPC21 (Sigma) coated onto the culture plates at a final concentration of 30 μg/mL. LPS was used at a final concentration of 10 ng/mL. Supernatants were collected after 24 hours and tested by ELISA for production of IL-8, IL-12p40, and tumor necrosis factor α (TNFα) (OptEIA kits; BD Biosciences Pharmingen). All mAbs used for tissue culture were shown to be endotoxin free, as determined by limulus-amebocyte assay (BioWhittaker, Walkersville, MD).
Internalization assays
Internalization assays were performed on mono-DCs as described,28,29 using anti-hOSCAR 11.1CN5 and F(ab′)2, anti-CD13 mAb (Immunotech), mAb DCGM1 (anti–mannose receptor28 ). The cells were first incubated with 2% human serum. As negative controls, the cells were either kept at 4° C or fixed with paraformaldehyde (PFA) before incubation at 37° C. Internalization is measured by the percentage decrease of cell surface mean fluorescence intensity (MFI) as compared to the MFI of cells incubated with isotype control (MOPC21; Sigma).
Confocal microscopy and endocytosis
Mono-DCs were allowed to adhere to glass slides coated by poly-l-lysine (Sigma), saturated with 2% human serum, and then incubated with anti-hOSCAR F(ab′)2 or isotype control (Sigma) at 10 μg/mL. The mAbs were subsequently cross-linked with an Alexa 594–coupled GAM F(ab′)2 (Molecular Probes) at 5 μg/mL. After washing, the cells were incubated in complete medium at 37° C or kept on ice. Cells were fixed in 4% PFA in PBS for 30 minutes at 4° C and permeabilized in PBS, 0.5% bovine serum albumin (BSA), 0.02% saponin. Intracellular stainings were performed after serum saturation with the following Ab: rabbit polyclonal Ab specific for Rab4 and Rab5a (Santa Cruz Biotechnology, Santa Cruz, CA), followed by Alexa 488–coupled donkey anti–rabbit Ab (Molecular Probes) or FITC-conjugated anti–Lamp-1 (BD Biosciences Pharmingen, Erembodegen, Belgium), or biotinylated anti–HLA-DR (Becton Dickinson), followed by Alexa 488–coupled streptavidin (Molecular Probes). Rabbit serum (Dako) and FITC- or biotin-conjugated isotype-matched mAbs (Dako) were used as negative controls. All Ab dilutions were done in PBS, 0.5% BSA, 0.02% saponin buffer. Slides were mounted with Fluoromount (Southern Biotechnology Associates, Birmingham, AL) and analyzed with a confocal laser microscope (Axiovert 100 M) using a 63× Plan-Apochromat objective (Carl Zeiss, Jena, Germany).
Antigen presentation assay
The Hd7 T cell clone (8.104 cells), which recognizes an epitope of mouse IgG1 in the context of HLA-DR0101/DQw1,30 was used in this assay. Cells were cocultured with serial dilutions of immature mono-DCs from an appropriate donor, irradiated by 30 Gy (3000 rad), prelabeled with 1 μg/mL mAbs, and cross-linked with GAM (Jackson ImmunoResearch). mAbs (all of IgG1 isotype) used in this assay were the following: MOPC21, anti-CD13, anti-hOSCAR, or anti–mannose receptor (Immunotech). After 48 hours, the cultures were pulsed with [3H]thymidine (1 μCi [0.037 MBq]/well), and radioactivity incorporated was measured after 16 hours.
Statistical analyses
Statistical analyses were performed in Microsoft Excel 5.0 (Microsoft, Redmond, WA) using Student 2-tailed t tests. A P value less than .05 was considered statistically significant. Unless otherwise indicated, means and standard deviation are shown.
Results
hOSCAR is a member of the leukocyte receptor complex
We searched the Human Genome Science expressed sequence tag (EST) databases using a tBLASTn algorithm31 to find novel members of the immunoglobulin superfamily (IgSF) expressed by DCs. Two ESTs (sequence identity HDPBT77R and HDPKD37R), coding for the same protein, were isolated from a mono-DC library. The amino acid sequence showed homology to members of the KIR family, with 2 recognizable C2-type Ig domains.32 Complete sequencing of the clones showed a transmembrane domain that contains a positively charged arginine residue and a short intracytoplasmic domain with no recognizable signaling motif. The cDNA sequence for this protein has been registered in GenBank by Kim and colleagues as the human homolog of mouse OSCAR (Genbank accession no. AF391162).19 By using the Stanford G3 radiation hybrid panel, we localized the hOSCAR gene to chromosome 19q13 proximal to CD89/FcαR and KIR family genes (data not shown). Homologous sequences were detected on human BAC clone no. AC009968, which contains part of the LRC.21 These data are consistent with the considerable homology between hOSCAR and other LRC members, particularly to CD89 and ILT family members (data not shown).
Myeloid cells express hOSCAR mRNA
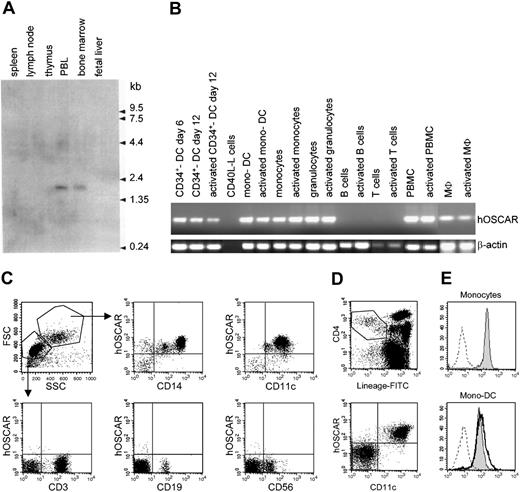
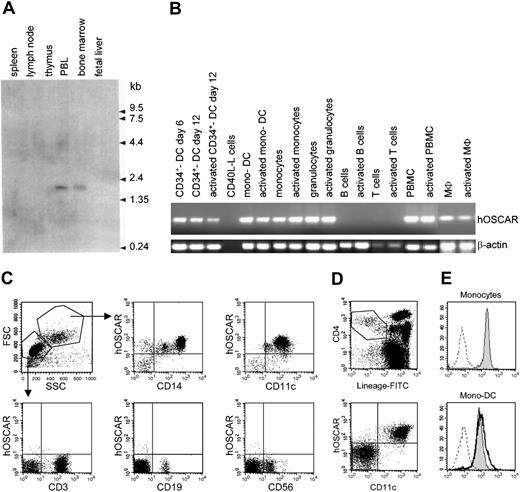
Northern blot analysis (Figure 1A) with a 1266-bp probe specific for hOSCAR showed a single band at approximately 1.8 kb only detected in peripheral blood leukocytes and in bone marrow (Figure 1A). No expression was detected in nonimmune tissues other than a faint expression in lung (data not shown).
hOSCAR is expressed in myeloid cells. (A) Northern blot analysis. A hOSCAR-specific single band at approximately 1.8 kb was detected in peripheral blood leukocyte (PBL) and bone marrow. (B) RT-PCR analysis. Macrophages (MΦ) and granulocytes were derived from cord blood CD34+ progenitors. DCs were generated from cord blood CD34+ progenitors (CD34+ DCs) or from blood monocytes (mono-DCs). PBMCs, monocytes, blood T cells, and tonsillar B cells were freshly isolated. Activations were by CD40-ligand transfected L cells (for in vitro–derived DCs and B cells), LPS (for monocytes), PMA/ionomycin (for granulocytes, PBMCs, and MΦ), or anti-CD3, anti-CD28 antibodies (for T cells). hOSCAR and β-actin were amplified for 30 and 25 cycles, respectively. (C) Two-color flow cytometry analysis of PBMCs. Cells were labeled with biotin-conjugated anti-hOSCAR, followed by incubation with streptavidin-PE and FITC-conjugated mAbs against the indicated cellular markers. Acquisition was performed by gating on the different cell populations, depending on the forward and right-angle scatter parameters. (D) Four-color flow cytometry analysis of PBMCs. Cells were stained with FITC-lineage cocktail, PE-Cy5–anti-CD4, APC–anti-CD11c, and biotin-conjugated anti-hOSCAR, followed by streptavidin-PE. Primary DCs are included within lineage–/CD4+ cells; CD11c+ cells correspond to myeloid DCs, whereas CD11c– cells correspond to plasmacytoid DCs. (E) Cell surface expression of hOSCAR in monocytes and mono-DCs. Untreated monocytes (shaded histogram) and mono-DCs treated with LPS for 24 hours (solid bold) or untreated (shaded histogram) were analyzed by FACS for cell surface expression of hOSCAR, using anti-hOSCAR mAbs and GAM-PE. Dashed profiles indicate background staining with a control IgG1 mAb.
hOSCAR is expressed in myeloid cells. (A) Northern blot analysis. A hOSCAR-specific single band at approximately 1.8 kb was detected in peripheral blood leukocyte (PBL) and bone marrow. (B) RT-PCR analysis. Macrophages (MΦ) and granulocytes were derived from cord blood CD34+ progenitors. DCs were generated from cord blood CD34+ progenitors (CD34+ DCs) or from blood monocytes (mono-DCs). PBMCs, monocytes, blood T cells, and tonsillar B cells were freshly isolated. Activations were by CD40-ligand transfected L cells (for in vitro–derived DCs and B cells), LPS (for monocytes), PMA/ionomycin (for granulocytes, PBMCs, and MΦ), or anti-CD3, anti-CD28 antibodies (for T cells). hOSCAR and β-actin were amplified for 30 and 25 cycles, respectively. (C) Two-color flow cytometry analysis of PBMCs. Cells were labeled with biotin-conjugated anti-hOSCAR, followed by incubation with streptavidin-PE and FITC-conjugated mAbs against the indicated cellular markers. Acquisition was performed by gating on the different cell populations, depending on the forward and right-angle scatter parameters. (D) Four-color flow cytometry analysis of PBMCs. Cells were stained with FITC-lineage cocktail, PE-Cy5–anti-CD4, APC–anti-CD11c, and biotin-conjugated anti-hOSCAR, followed by streptavidin-PE. Primary DCs are included within lineage–/CD4+ cells; CD11c+ cells correspond to myeloid DCs, whereas CD11c– cells correspond to plasmacytoid DCs. (E) Cell surface expression of hOSCAR in monocytes and mono-DCs. Untreated monocytes (shaded histogram) and mono-DCs treated with LPS for 24 hours (solid bold) or untreated (shaded histogram) were analyzed by FACS for cell surface expression of hOSCAR, using anti-hOSCAR mAbs and GAM-PE. Dashed profiles indicate background staining with a control IgG1 mAb.
As hOSCAR mRNA was mainly observed in peripheral blood leukocytes, we further investigated its distribution on hematopoietic cells by RT-PCR. As shown in Figure 1B, hOSCAR cDNA was strongly detected in PBMCs and circulating blood monocytes, while no signal was obtained from T and B cells. Moreover, cDNAs also could be amplified from CD34+-derived granulocytes and macrophages. A strong signal was also detected in DCs derived from monocytes and from cord blood CD34+ progenitors. No modulation of the level of hOSCAR transcripts was detected upon activation using LPS for monocytes, CD40L for DCs, and phorbol myristate acetate (PMA)/ionomycin for PBMCs and macrophages.
hOSCAR is a cell surface protein expressed by myeloid cells
To investigate the protein distribution of hOSCAR, 3 monoclonal antibodies (mAbs) were generated, specifically recognizing hOSCAR-transfected U937 cells. Flow cytometry analysis (Figure 1C) on peripheral blood mononuclear cells demonstrated the surface expression of hOSCAR by all CD11c+ cells and CD14+ monocytes, but not by T cells, B cells, nor natural killer (NK) cells. hOSCAR is expressed at the same level by ex vivo monocytes and blood myeloid DCs, as shown by 4-color flow cytometry analysis detecting hOSCAR on lineage-negative CD4+, CD11c+ cells. No staining was observed on plasmacytoid DCs (lineage-negative, CD4+, CD11c– cells) (Figure 1D). hOSCAR expression was maintained during monocyte differentiation into mono-DCs (Figure 1E) and still detected after LPS stimulation. These data show that hOSCAR is a cell surface protein expressed by cells of myeloid origin, including CD11c+ DCs.
hOSCAR is an N-glycosylated monomer of approximately 45 kDa
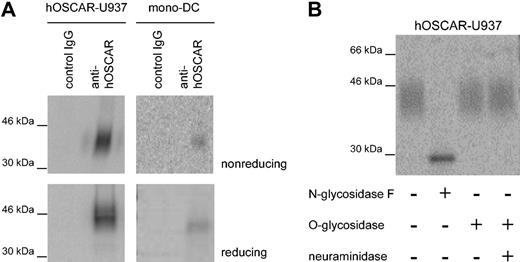
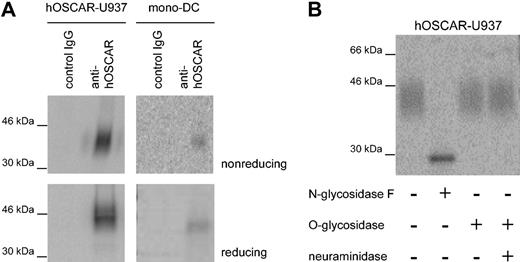
IgSF family proteins, such as CD28 or CD152, may form covalently linked homodimers or heterodimers. We performed immunoprecipitation on hOSCAR-transfected U937 and mono-DCs using a mix of the 3 anti-hOSCAR mAbs immobilized on sepharose beads. Analysis of the 125I-labeled immunoprecipitates revealed a protein of approximately 45 kDa under reducing and slightly less under nonreducing condition (Figure 2A); hOSCAR is thus present as a monomer not covalently linked to any other chain. The sequence of the hOSCAR extracellular domain showed the presence of 3 potential NH2-linked glycosylation sites,19 and the wide band observed upon immunoprecipitation suggested the presence of several glycosylated forms of the protein. Incubation of the U937 precipitate with N-glycosidase F resulted in a thin band, showing a reduced molecular mass of approximately 30 kDa, while neuraminidase and/or O-glycosidase treatment did not affect the apparent molecular mass (Figure 2B). These observations are in agreement with the computed molecular mass of hOSCAR at 29 kDa.
hOSCAR is a cell-surface monomeric glycoprotein. (A) SDS-PAGE analysis of hOSCAR immunoprecipitated from 125I-labeled hOSCAR-transfected human U937 cells (left panels) and 125I-labeled mono-DCs (right panels). The cell lysate was immunoprecipitated as indicated with either isotype-matched control mAb (control IgG) or anti-hOSCAR mAb. Upon gel exposure on phosphorscreen and phosphoImager detection, hOSCAR appears as an approximately 45-kDa band under both nonreducing and reducing conditions. (B) Glycosylation of anti-hOSCAR precipitates from hOSCAR-transfected 125I-labeled human U937 cells. Deglycosylation was performed with N-glycosidase F or with neuraminidase and/or O-glycosidase, before SDS-PAGE and detection as above. Upon digestion of the N-linked sugars, hOSCAR appears as a thin band of approximately 30 kDa, corresponding to its totally unglycosylated form. The data are representative of 3 independent experiments.
hOSCAR is a cell-surface monomeric glycoprotein. (A) SDS-PAGE analysis of hOSCAR immunoprecipitated from 125I-labeled hOSCAR-transfected human U937 cells (left panels) and 125I-labeled mono-DCs (right panels). The cell lysate was immunoprecipitated as indicated with either isotype-matched control mAb (control IgG) or anti-hOSCAR mAb. Upon gel exposure on phosphorscreen and phosphoImager detection, hOSCAR appears as an approximately 45-kDa band under both nonreducing and reducing conditions. (B) Glycosylation of anti-hOSCAR precipitates from hOSCAR-transfected 125I-labeled human U937 cells. Deglycosylation was performed with N-glycosidase F or with neuraminidase and/or O-glycosidase, before SDS-PAGE and detection as above. Upon digestion of the N-linked sugars, hOSCAR appears as a thin band of approximately 30 kDa, corresponding to its totally unglycosylated form. The data are representative of 3 independent experiments.
The ITAM-bearing adapter FcRγ associates with hOSCAR
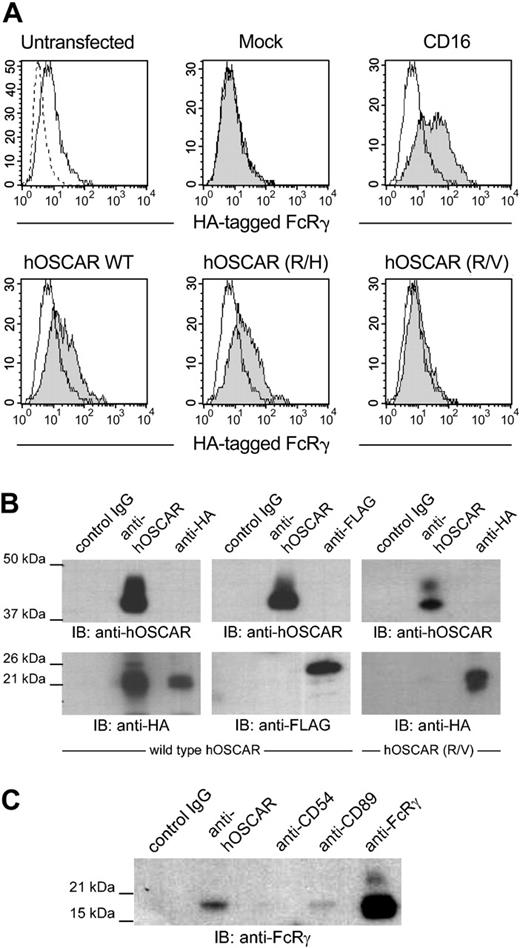
The transmembrane domain of hOSCAR contains a positively charged arginine residue, characteristic of receptors associated with FcRγ or CD3ζ, such as FcγRI, FcαR, or NKp46.20 Since hOSCAR is exclusively expressed in myeloid cells, its potential association with CD3ζ, restricted to lymphoid cells, was not investigated. We observed that transfection of HEK293T or U937 with hOSCAR alone gave rise to cell surface expression of the protein (data not shown). As HEK293T cells do not express any ITAM-bearing adapter, this observation suggests that the stability of hOSCAR in the extracellular membrane does not require any other molecule, as already reported for some receptors of this family, such as FcαR and MAIR-II.33,34 Conversely, transfection of FcRγ alone gave very little cell surface expression of this adapter. Cotransfection of hOSCAR with FcRγ in HEK293T did not alter the surface expression level of hOSCAR, while an increased cell surface expression of FcRγ was observed (Figure 3A), suggesting a translocation of this chain from the cytoplasm to the cell surface due to an association with hOSCAR, as observed with CD16.35
hOSCAR is associated with the ITAM-bearing adaptor FcRγ chain. (A) Stable HEK293T transfectants expressing an HA-tagged FcRγ were transfected with empty plasmid (mock) or plasmid-expressing CD16, hOSCAR wild-type (WT), or transmembrane mutant hOSCAR (R/H) and hOSCAR (R/V) substituting histidine and valine, respectively, for arginine. The HA-tagged FcRγ was detected by cell surface staining of the transfectants with anti-HA mAbs (shaded histograms) compared to in untransfected cells (open histograms). Dashed profiles indicate background staining with a control IgG1 mAb. (B) hOSCAR-transfected HEK293T with HA-tagged FcRγ (left panel) or FLAG-tagged DAP12 (center panel) and hOSCAR (R/V)-transfected HEK293T with HA-tagged FcRγ (right panel) were lysed in 1% digitonin buffer and immunoprecipitated with isotype-matched control mAbs (control IgG), anti-hOSCAR, or anti-tag mAbs. Western blot with biotin-conjugated anti-hOSCAR and ExtrAvidin-HRP allowed the detection of the receptor, while the presence of the adapters was revealed with HRP-conjugated anti-HA and anti-FLAG mAbs. HA-tagged FcRγ, but not FLAG-tagged DAP12, coimmunoprecipitates with hOSCAR. This association is lost with the R/V mutant. (C) Digitonin lysates from mono-DCs were incubated with isotype-matched control mAbs (control IgG), anti-hOSCAR, anti-CD54, anti-CD89 mAbs, or anti-FcRγ polyclonal Abs. The endogenous FcRγ in the immunoprecipitates was detected by Western blot with anti-FcRγ polyclonal Abs and HRP-conjugated donkey anti-rabbit Abs. The FcRγ coimmunoprecipitates with hOSCAR and CD89 in mono-DCs. The data are representative of 3 independent experiments.
hOSCAR is associated with the ITAM-bearing adaptor FcRγ chain. (A) Stable HEK293T transfectants expressing an HA-tagged FcRγ were transfected with empty plasmid (mock) or plasmid-expressing CD16, hOSCAR wild-type (WT), or transmembrane mutant hOSCAR (R/H) and hOSCAR (R/V) substituting histidine and valine, respectively, for arginine. The HA-tagged FcRγ was detected by cell surface staining of the transfectants with anti-HA mAbs (shaded histograms) compared to in untransfected cells (open histograms). Dashed profiles indicate background staining with a control IgG1 mAb. (B) hOSCAR-transfected HEK293T with HA-tagged FcRγ (left panel) or FLAG-tagged DAP12 (center panel) and hOSCAR (R/V)-transfected HEK293T with HA-tagged FcRγ (right panel) were lysed in 1% digitonin buffer and immunoprecipitated with isotype-matched control mAbs (control IgG), anti-hOSCAR, or anti-tag mAbs. Western blot with biotin-conjugated anti-hOSCAR and ExtrAvidin-HRP allowed the detection of the receptor, while the presence of the adapters was revealed with HRP-conjugated anti-HA and anti-FLAG mAbs. HA-tagged FcRγ, but not FLAG-tagged DAP12, coimmunoprecipitates with hOSCAR. This association is lost with the R/V mutant. (C) Digitonin lysates from mono-DCs were incubated with isotype-matched control mAbs (control IgG), anti-hOSCAR, anti-CD54, anti-CD89 mAbs, or anti-FcRγ polyclonal Abs. The endogenous FcRγ in the immunoprecipitates was detected by Western blot with anti-FcRγ polyclonal Abs and HRP-conjugated donkey anti-rabbit Abs. The FcRγ coimmunoprecipitates with hOSCAR and CD89 in mono-DCs. The data are representative of 3 independent experiments.
We next performed coimmunoprecipitation with digitonin-lysed HEK293T double transfectants. Immunoblotting after hOSCAR immunoprecipitation demonstrated that hOSCAR associates with HA-tagged FcRγ but not with FLAG-tagged DAP12 (Figure 3B). The specificity of this association was assessed by cotransfection of FcRγ with hOSCAR mutants, in which arginine 231 was mutated to noncharged valine (R/V) or to positively charged histidine (R/H). Wild-type and mutants of hOSCAR were expressed at the same levels as confirmed after labeling of the cells with anti-hOSCAR and flow cytometer analysis (data not shown). The presence of a neutral residue clearly abrogated the translocation of FcRγ (Figure 3A), while the similarly charged mutant maintained association. Equally, coimmunoprecipitation experiments demonstrated that hOSCAR (R/V) mutant was not able to associate with FcRγ (Figure 3B).
Finally, coimmunoprecipitation of mono-DCs (Figure 3C) allowed us to demonstrate a physical association between hOSCAR and the endogenous FcRγ. No coimmunoprecipitation was observed with the negative controls MOPC21 or anti-CD54, the latter targeting a cell surface receptor not associated with FcRγ. These results demonstrate a specific association between hOSCAR and FcRγ, an ITAM-bearing transduction subunit previously shown to mediate activating signals and to play a role in antigen presentation.
hOSCAR ligation triggers intracellular Ca2+ flux and cytokine secretion
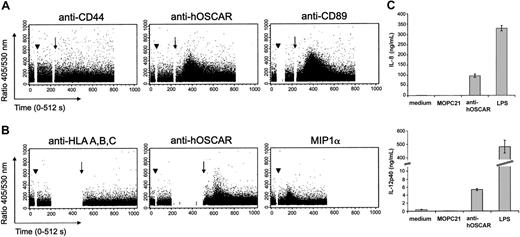
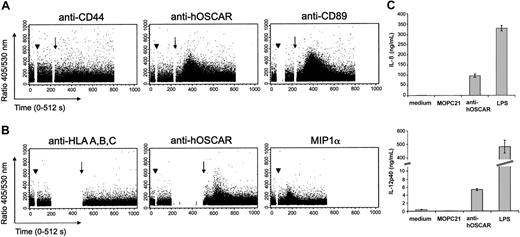
Early activation events via ITAM-linked receptors can be visualized by intracellular Ca2+ flux. We thus stimulated human serum-saturated monocytes (Figure 4A) and mono-DCs (Figure 4B) with hOSCAR-specific mAbs and measured the cytosolic Ca2+ concentrations ([Ca2+]i). Ligation of hOSCAR with a specific mAb did not by itself cause an increase of intracellular [Ca2+]i. However, when followed by a secondary cross-linking Ab, hOSCAR triggering elicited a rapid and transient increase of intracellular [Ca2+]i in Indo-1–loaded monocytes. Similar results were obtained with mono-DCs. Cross-linking of CD89 on monocytes and the addition of recombinant MIP1α to mono-DCs also triggered Ca2+ flux. In the same experiment, no [Ca2+]i rise was observed when the cells were stimulated either by anti-CD44 or anti–HLA-A, -B, -C mAb, demonstrating that the activation observed was not mediated by FcR engagement. The rapid rise of intracellular Ca2+ by hOSCAR aggregation indicates the ability of this receptor to trigger early activation events in monocytes and mono-DCs.
Cross-linking of hOSCAR triggers calcium flux mobilization in myeloid cells. Human serum–saturated monocytes (A) or mono-DCs (B) were loaded with Indo-1 am, and the rise in intracellular Ca2+ was analyzed on FACStarPlus flow cytometer, by recording in real-time the 405/530-nm fluorescence ratio. Monocytes were incubated (arrowhead) at 37° C with anti-CD44 and anti-CD89 mAbs, as negative and positive controls, respectively. Mono-DCs were incubated (arrowhead) at 37° C with anti–HLA-A, -B, -C mAbs and recombinant human MIPα, as negative and positive controls, respectively. The 405/530-nm ratio was recorded during the primary mAb binding. At set time points (arrow) goat F(ab′)2 anti-mouse was added to allow receptor cross-linking. Data shown are representative of 3 experiments. (C) Ligation of hOSCAR induces secretion of IL-8 and IL-12p40 by mono-DCs. Immature mono-DCs were stimulated by coated mAbs or LPS, as described in “Materials and methods.” After 24 hours, the levels of secreted IL-8 and IL-12p40 were tested by ELISA. Data shown are representative of 3 experiments and display the mean and standard deviation of 3 independent samples.
Cross-linking of hOSCAR triggers calcium flux mobilization in myeloid cells. Human serum–saturated monocytes (A) or mono-DCs (B) were loaded with Indo-1 am, and the rise in intracellular Ca2+ was analyzed on FACStarPlus flow cytometer, by recording in real-time the 405/530-nm fluorescence ratio. Monocytes were incubated (arrowhead) at 37° C with anti-CD44 and anti-CD89 mAbs, as negative and positive controls, respectively. Mono-DCs were incubated (arrowhead) at 37° C with anti–HLA-A, -B, -C mAbs and recombinant human MIPα, as negative and positive controls, respectively. The 405/530-nm ratio was recorded during the primary mAb binding. At set time points (arrow) goat F(ab′)2 anti-mouse was added to allow receptor cross-linking. Data shown are representative of 3 experiments. (C) Ligation of hOSCAR induces secretion of IL-8 and IL-12p40 by mono-DCs. Immature mono-DCs were stimulated by coated mAbs or LPS, as described in “Materials and methods.” After 24 hours, the levels of secreted IL-8 and IL-12p40 were tested by ELISA. Data shown are representative of 3 experiments and display the mean and standard deviation of 3 independent samples.
Mono-DCs then were stimulated by LPS or by mAbs coated onto culture plates, an efficient way of cross-linking the receptor associated to the ITAM-adapters. Cell supernatants were tested for cytokine secretion, an indication of physiological cellular activation. Both LPS and coated anti-hOSCAR mAb efficiently induced IL-8 and IL-12p40 secretion (Figure 4C), but anti-hOSCAR–stimulated mono-DCs were unable to produce TNFα (data not shown). The isotype-matched (IgG1) irrelevant mAb MOPC21 had no significant effect on cytokine secretion.
hOSCAR is an endocytic receptor on mono-DCs
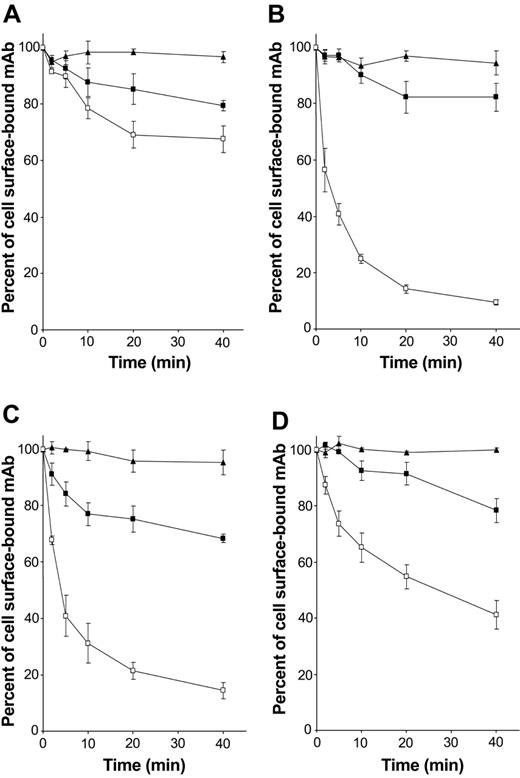
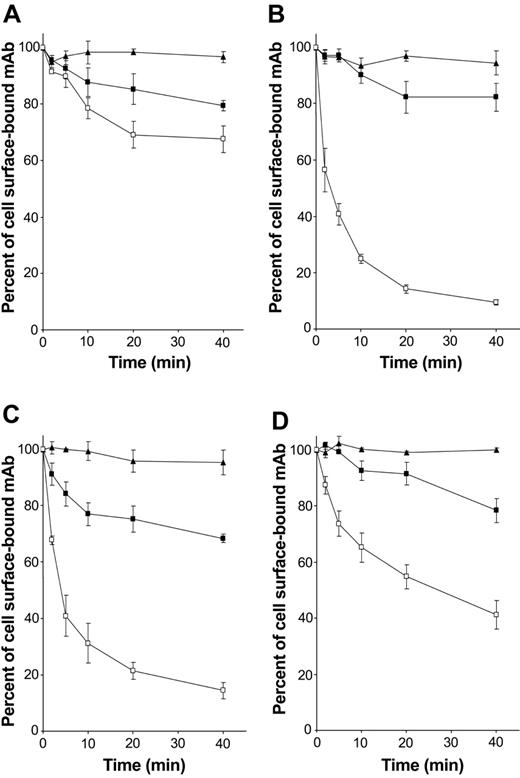
As FcRγ-associated receptors expressed by DCs are known to be involved in antigen capture and presentation, the role of hOSCAR in endocytosis was addressed. The ability of the receptor to internalize the anti-hOSCAR mAb was studied in mono-DCs. Anti-CD13 mAb showed no significant decrease at the cell surface, indicating that no active endocytosis takes place (Figure 5A). Upon cross-linking, the whole anti-hOSCAR mAb was rapidly endocytosed (Figure 5C) with kinetics similar to that of the anti–mannose receptor mAb (Figure 5B). This endocytosis also was observed with anti-hOSCAR F(ab′)2 (Figure 5D), although the internalization kinetics of anti-hOSCAR F(ab′)2 were slower than that of whole mAb (50% of internalized receptors after 20 minutes instead of 5 minutes). The requirement for cross-linking could explain this difference, cross-linking of a F(ab′)2 being less efficient than that of the whole mAb.
hOSCAR is an endocytic receptor of mono-DCs. Human serum–saturated mono-DCs were incubated with the following primary antibodies at 4° C for 30 minutes: (A) anti-CD13 mAb (negative control), (B) DC-GM1 mAb specific for mannose receptor (positive control), and (C) whole mAb and (D) F(ab′)2 specific for hOSCAR. After incubation with biotin-conjugated goat F(ab′)2 anti-mouse, cells were transferred at 37° C to allow endocytosis (□). At the indicated times, the cells were stored at 4° C, and the remaining surface receptors were detected by incubation with PE-conjugated streptavidin. PFA-fixed cells (▪) and cells kept at 4° C during the entire time period (▴) were used as negative controls of active endocytosis. Data shown are representative of 5 independent experiments and display the mean and standard deviation of 3 independent samples.
hOSCAR is an endocytic receptor of mono-DCs. Human serum–saturated mono-DCs were incubated with the following primary antibodies at 4° C for 30 minutes: (A) anti-CD13 mAb (negative control), (B) DC-GM1 mAb specific for mannose receptor (positive control), and (C) whole mAb and (D) F(ab′)2 specific for hOSCAR. After incubation with biotin-conjugated goat F(ab′)2 anti-mouse, cells were transferred at 37° C to allow endocytosis (□). At the indicated times, the cells were stored at 4° C, and the remaining surface receptors were detected by incubation with PE-conjugated streptavidin. PFA-fixed cells (▪) and cells kept at 4° C during the entire time period (▴) were used as negative controls of active endocytosis. Data shown are representative of 5 independent experiments and display the mean and standard deviation of 3 independent samples.
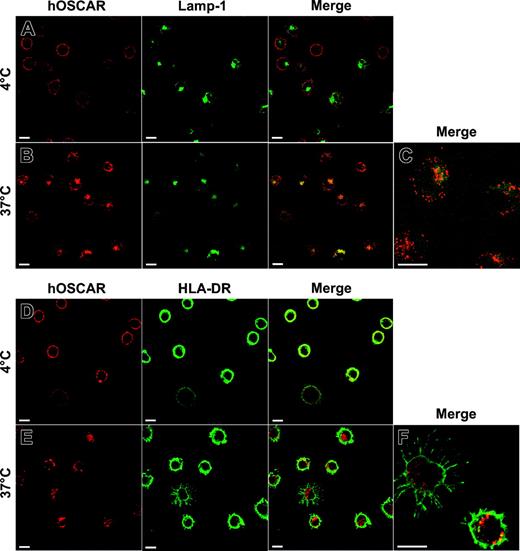
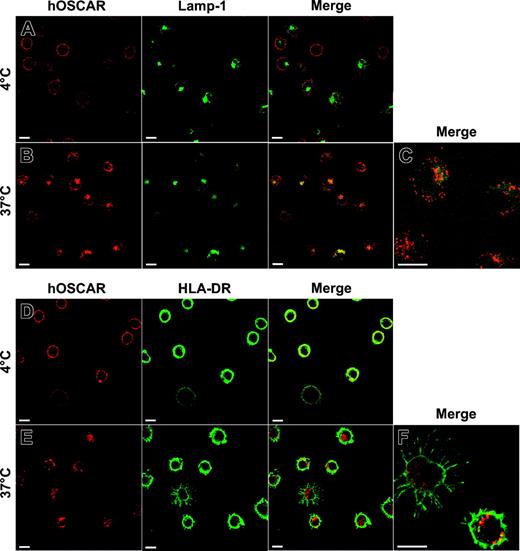
The fate of hOSCAR ligands upon internalization then was studied by confocal microscopy (Figure 6). Mono-DCs were labeled with anti-hOSCAR F(ab′)2 mAb. After cross-linking with Alexa 594–coupled GAM F(ab′)2, cells were either kept at 4° C or incubated at 37° C before fixing. To identify the nature of the vesicles containing material endocytosed via hOSCAR, the cells were then labeled with mAbs specific for Rab4, Rab5a, HLA-DR, and Lamp-1 (CD107a) and analyzed by confocal microscopy. After 5 minutes, the material internalized by hOSCAR was localized in early endocytic compartments, characterized by the expression of Rab4 and Rab5a markers (not shown). After 20 minutes, intracellular vesicles that contained Alexa 594–labeled complexes were positive for the lysosomal marker Lamp-1 (Figure 6B-C). Moreover, some internalized material also was shown to colocalize with intracellular HLA-DR molecules (Figure 6E-F), but to a lesser extent than with Lamp-1.
Colocalization of hOSCAR-internalized material with Lamp-1 and HLA-DR after 20 minutes of endocytosis. Mono-DCs were allowed to adhere onto poly-l-Lysine slides and incubated with hOSCAR-specific F(ab′)2 in the presence of 2% human serum. These primary mAbs then were cross-linked with Alexa 594–conjugated GAM F(ab′)2, and the cells were moved to 37° C (B-CE-F) to allow endocytosis or kept on ice (AD). After 20 minutes, the cells were moved to 4° C, fixed, and permeabilized to allow labeling of intracellular markers (A-C: anti–Lamp-1–FITC; D-F: anti–HLA-DR biotin and Alexa 488–conjugated streptavidin). To analyze the relative localization of the material internalized by hOSCAR (in red) and the intracellular markers (in green), the pictures were merged, and double-labeled areas appeared in yellow. Upon 20 minutes endocytosis, the intracellular compartments accessed by hOSCAR-internalized material were Lamp-1 (B-C) and HLA-DR positive (E-F). Bars: 10 μm. The data are representative of 2 experiments.
Colocalization of hOSCAR-internalized material with Lamp-1 and HLA-DR after 20 minutes of endocytosis. Mono-DCs were allowed to adhere onto poly-l-Lysine slides and incubated with hOSCAR-specific F(ab′)2 in the presence of 2% human serum. These primary mAbs then were cross-linked with Alexa 594–conjugated GAM F(ab′)2, and the cells were moved to 37° C (B-CE-F) to allow endocytosis or kept on ice (AD). After 20 minutes, the cells were moved to 4° C, fixed, and permeabilized to allow labeling of intracellular markers (A-C: anti–Lamp-1–FITC; D-F: anti–HLA-DR biotin and Alexa 488–conjugated streptavidin). To analyze the relative localization of the material internalized by hOSCAR (in red) and the intracellular markers (in green), the pictures were merged, and double-labeled areas appeared in yellow. Upon 20 minutes endocytosis, the intracellular compartments accessed by hOSCAR-internalized material were Lamp-1 (B-C) and HLA-DR positive (E-F). Bars: 10 μm. The data are representative of 2 experiments.
These data demonstrate that potential ligands internalized in mono-DCs via hOSCAR reach vesicles characteristic of the lysosomal/late endosomal MHC class II–loading compartments (MIIC).
Antigen internalized by hOSCAR is presented to CD4+ T cells
To further investigate the role of hOSCAR in antigen endocytosis and presentation, the ability of mono-DCs to process and present peptide derived from anti-hOSCAR mAb as a surrogate ligand was studied. In these experiments, we used a CD4+ MHC class II–restricted T-cell clone specific for an epitope of mouse IgG1.30,36 Immature mono-DCs from a donor with a compatible haplotype were labeled with primary mAb and then incubated with a cross-linker. [3H]-thymidine incorporation was measured as an indication of T-cell proliferation. Antigen presentation via anti-hOSCAR was compared to that of isotype-matched controls (MOPC21 and anti-CD13), which gave no significant T-cell proliferation. We found that mono-DCs presented anti-hOSCAR mAb to T cells in a similar manner to anti–mannose receptor (Figure 7).37 These data confirmed the observations obtained by confocal microscopy, showing that hOSCAR can efficiently deliver its ligand into antigen-processing compartments and mediate class II presentation.
Presentation of anti-hOSCAR mAb to a T-cell clone specific for mouse IgG1 by mono-DCs. The primary antibodies MOPC21 (▪; isotype control), anti-CD13 (▴), anti-hOSCAR (○), or anti–mannose receptor (•) were allowed to bind to mono-DCs. After cross-linking with GAM F(ab′)2, the irradiated mono-DCs were added to a T-cell clone specific for mouse IgG1. Antigen presentation was assessed by [3H]-thymidine incorporation corresponding to T-cell proliferation. Anti–mannose receptor and anti-hOSCAR were presented more efficiently than anti-CD13 mAbs or isotype control. Data shown are representative of 4 independent experiments using cells from 3 different donors and display the mean and standard deviation of quadruplets. Statistical significances of *P < .03; **P < .01 are given by comparison to values obtained with the negative control anti-CD13.
Presentation of anti-hOSCAR mAb to a T-cell clone specific for mouse IgG1 by mono-DCs. The primary antibodies MOPC21 (▪; isotype control), anti-CD13 (▴), anti-hOSCAR (○), or anti–mannose receptor (•) were allowed to bind to mono-DCs. After cross-linking with GAM F(ab′)2, the irradiated mono-DCs were added to a T-cell clone specific for mouse IgG1. Antigen presentation was assessed by [3H]-thymidine incorporation corresponding to T-cell proliferation. Anti–mannose receptor and anti-hOSCAR were presented more efficiently than anti-CD13 mAbs or isotype control. Data shown are representative of 4 independent experiments using cells from 3 different donors and display the mean and standard deviation of quadruplets. Statistical significances of *P < .03; **P < .01 are given by comparison to values obtained with the negative control anti-CD13.
Discussion
The hOSCAR gene maps to human chromosome 19q13 in the LRC. This region contains numerous genes coding for cell surface receptors involved in the initiation of immune responses, such as members of the KIR and ILT/LIR families, and CD89/FcαR.21 Some of these genes are not found in the syntenic region in mouse chromosome 7, indicating that the various duplications of genes in this cluster have occurred after the separation of the rodent and primate lineage. Conserved genes such as OSCAR,38 which have human and rodent homologs, probably represent the more ancient genes of the cluster and may conserve ancestral functions.
Murine OSCAR was recently shown to be an osteoclast-specific gene. Immunocytochemical staining of hOSCAR revealed that the receptor is also expressed by human osteoclasts generated from blood monocytes (E. M. and C. M.-C., unpublished data, December 2002), suggesting that hOSCAR could also play a role in human osteoclasts. We have shown in this study that hOSCAR has a broader expression pattern than the murine protein. A comparative analysis of the promoter regions of human and murine OSCAR shows significant variances in the potential transcription factor sites, and this may explain the different cellular expression patterns. Of note, the MITF and PU.1 sites present in the mouse promoter and recently shown to synergistically activate OSCAR gene expression39 are not present in humans (data not shown). We produced 3 mouse mAbs specifically recognizing hOSCAR. We were thus able to confirm cell surface expression of hOSCAR on circulating blood monocytes and CD11c+ DCs.
In myeloid cells, the activating receptors SIRP-β,20,40 MDL-1,35 and TREM-1 and -210,41 were shown to be associated with DAP12. In contrast, ILT-142 and PIR-A43 associate with FcRγ. A comparison of the transmembrane domain of DAP12-associated receptors with those of FcRγ-associated receptors showed that a positively charged residue is localized in the center of the transmembrane domain for the former and close to the extracellular domain for the latter.20 Like ILT-1, hOSCAR has a short cytoplasmic domain with no signaling motifs and an arginine at position 231. ITAM-bearing chains have been shown to require association with partner subunits for efficient transport to the cell surface.8 Cotransfection of FcRγ and hOSCAR in HEK293T cells allowed FcRγ expression at the cell surface, showing that hOSCAR associates with FcRγ. Similar experiments performed with a mutant hOSCAR, bearing a neutral residue instead of the positively charged arginine in the transmembrane, failed to translocate FcRγ. These data confirm the importance of oppositely charged residues in the transmembrane domains of the ITAM-bearing chains and receptors. hOSCAR associated with the ITAM-bearing FcRγ but not with DAP12, as shown by coimmunoprecipitation. This interaction was detected not only in transfected cells but also in mono-DCs, confirming that FcRγ is physiologically associated with the receptor. Recently, the juxtamembrane 6 amino acids of the cytosolic domain of GPVI also were shown to play a role in the association of this receptor with FcRγ.44 Interestingly, this region is highly conserved among the activating receptors of the LRC and is also present in the cytosolic domain of mouse and human OSCAR (residues 249 through 254).
Another myeloid receptor was recently shown to be important in bone modeling.45 TREM-2 was originally described as an activating receptor expressed by mono-DCs and associated with DAP12.10 TREM-2 was detected in osteoclasts and shown to be involved in osteoclast development,46 similarly to murine OSCAR.19 Patients with either mutated DAP12 or TREM-2 suffer from Nasu-Hakola disease,45,47 characterized by defects in osteoclast activity. Although no disease has yet been associated with OSCAR, this receptor will be of interest in the study of normal and diseased bone. TREM-2 and hOSCAR are myeloid receptors associated with alternative ITAM-bearing chains and involved in myeloid cell function and osteoclast differentiation. Differences in signalization by DAP12 and FcRγ-associated molecules in myeloid cells and osteoclasts could thus be addressed using these 2 molecules.
Signaling via FcRγ is achieved by phosphorylation of the ITAM by kinases of the src-family, recruiting Syk kinase.6 Consistent with the activation of tyrosine kinases by FcRγ, our results show that hOSCAR cross-linking triggers calcium release from monocyte and mono-DC intracellular stores, an early activation event. This is consistent with the hypothesis that the hOSCAR is able to transduce signal and to trigger activation of myeloid cells. In addition, we have shown that hOSCAR ligation on mono-DC triggers secretion of IL-8 and IL-12p40. Compared with the classical DC activation triggered by LPS, the level of cytokines secreted upon anti-hOSCAR stimulation is moderate but consistent, demonstrating that hOSCAR is an activating receptor on mono-DCs.
FcR coupled to FcRγ and expressed by DC promote antigen presentation via internalization of immune complexes.7 FcRγ contains a tyrosine-based motif that also has been implicated in ligand-induced internalization and in endosomal and lysosomal trafficking.15,48 Targeting of antibody-opsonized exogenous antigens to FcRγ-associated FcR results in presentation on MHC class II molecules.13,49,50 hOSCAR cross-linking induces endocytosis, directing the internalized material into class II loading compartments51-53 and allowing presentation to a specific CD4+ T-cell clone. Therefore, hOSCAR is able to mediate class II presentation by mono-DCs. FcR are poorly expressed by human DCs and not present on all subpopulations of myeloid DCs, and their expression is not maintained upon activation. hOSCAR represents the first FcRγ-associated endocytic receptor to be expressed on human myeloid DCs regardless of their differentiation and maturation stage, and it can be used to efficiently target circulating DCs for antigen presentation.
Activating receptors expressed by myeloid cells are as yet poorly understood in terms of ligand binding specificity. Despite amino acid sequence similarity between hOSCAR and LRC members, particularly CD89/FcαR, we did not detect any significant binding of immunoglobulins on hOSCAR-transfected cells (data not shown). We are continuing to define the relative contribution of hOSCAR in pathogen-derived antigen presentation as well as the physiological relevance of ligation of this molecule. hOSCAR could recognize pathogen components, similar to other pattern-recognition receptors, or be involved in the sensing of self- or stress-induced molecules. These hypotheses are not mutually exclusive in that the binding of endogenous molecules to trigger certain activities (and particularly cell activation), as well as pathogen-derived products, is observed for other dual receptors such as DC-SIGN or Dectin-1.54
In conclusion, we report the biochemical and functional characterization of hOSCAR, a novel IgSF member widely expressed by human myeloid cells. Its physical association with FcRγ confers it with signal transduction capacity. Interestingly, hOSCAR also is an endocytic receptor able to mediate class II presentation and potentially plays a role in the adaptive immune response. This finding adds to a growing wealth of literature on the receptors controlling the early immune response. Cell surface molecules of this type may have endogenous and/or exogenous ligands and be involved in the fine-tuning of the immune response on various cell types. The fact that hOSCAR not only triggers early activation events in human DCs but also can stimulate antigen presentation leads us to postulate a physiological role in the initiation of the adaptive response.
Prepublished online as Blood First Edition Paper, May 20, 2004; DOI 10.1182/blood-2004-03-0850.
E. M. is a recipient of a grant from the Fondation Marcel Mérieux (Lyon, France).
F. M.-J. is employed by Immunotech, whose potential product was studied in the present work.
Preliminary results were presented at the International Cytokine Society in Dublin, Ireland, in September 2003.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors wish to thank Profs Marc Daeron and Eric Vivier for helpful discussions at various stages of this work, Drs Christophe Caux and Francine Briere for useful comments on the manuscript, Ivan Perrot for technical help in 4-color labeling, and Bruno Salaun for invaluable advice on confocal slide preparation. Colleagues from EFS-Lyon, particularly Dr Dominique Rigal, provided us with typed blood. The CD4+ T-cell line, originally isolated in the laboratory of Antonio Lanzavecchia, was a generous gift from Drs Federica Sallusto and Anneke Engering. Aid with cell culture was provided by Chantal Guiet. Confocal microscopy was carried out at the ENS-Lyon, under the supervision of Catherine Koering.

![Figure 7. Presentation of anti-hOSCAR mAb to a T-cell clone specific for mouse IgG1 by mono-DCs. The primary antibodies MOPC21 (▪; isotype control), anti-CD13 (▴), anti-hOSCAR (○), or anti–mannose receptor (•) were allowed to bind to mono-DCs. After cross-linking with GAM F(ab′)2, the irradiated mono-DCs were added to a T-cell clone specific for mouse IgG1. Antigen presentation was assessed by [3H]-thymidine incorporation corresponding to T-cell proliferation. Anti–mannose receptor and anti-hOSCAR were presented more efficiently than anti-CD13 mAbs or isotype control. Data shown are representative of 4 independent experiments using cells from 3 different donors and display the mean and standard deviation of quadruplets. Statistical significances of *P < .03; **P < .01 are given by comparison to values obtained with the negative control anti-CD13.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/5/10.1182_blood-2004-03-0850/5/m_zh80170465720007.jpeg?Expires=1769124314&Signature=JLu3Go-9MCuxqKVq02XEnWJmmQobV4eLn9JyX-qDvg5aDBJtEwkNPZsEy-BQDzIYqSoMzwv~~WVbVwoB7oJiyRW1dfi69fzlGIksodsS2wbkvSiBOnXKBEZqq3we7Fu7CSf~tCBzlTiM94FDFXdefiZL0wJAb~BhI4xVD3D8tcTtTxmlQFc~F8T0IxMCG2iz-RUd0TH8r4uvxDXCtNIyT1mR2NSk7OYVTLorixcUROW6d8k-l1LVbJ4rQSd2oLgvlFvzispz2pCYJHCKJ-bHL-ffLiF4CyIn5azMMi4J2Uo3JMfcVd7RVQLsPLcV9pSC~ARMQjTvAFp03cgq9plq6Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 7. Presentation of anti-hOSCAR mAb to a T-cell clone specific for mouse IgG1 by mono-DCs. The primary antibodies MOPC21 (▪; isotype control), anti-CD13 (▴), anti-hOSCAR (○), or anti–mannose receptor (•) were allowed to bind to mono-DCs. After cross-linking with GAM F(ab′)2, the irradiated mono-DCs were added to a T-cell clone specific for mouse IgG1. Antigen presentation was assessed by [3H]-thymidine incorporation corresponding to T-cell proliferation. Anti–mannose receptor and anti-hOSCAR were presented more efficiently than anti-CD13 mAbs or isotype control. Data shown are representative of 4 independent experiments using cells from 3 different donors and display the mean and standard deviation of quadruplets. Statistical significances of *P < .03; **P < .01 are given by comparison to values obtained with the negative control anti-CD13.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/5/10.1182_blood-2004-03-0850/5/m_zh80170465720007.jpeg?Expires=1769219588&Signature=sRIGxHYSHp55Vf6Z0lIi93qS9h~fkFfIQCIlhb9e-n9-Oi85GLMI395Z8KiNBqHMP7k2Iu8JdZ5PDPXnfF-ZK4j1InpBs~SiKYyJVdcIxpMG4EubpGr-gJtuW9AUzfcy4ygrSHojiJ0ZRHSTdOmh2aKyXBDTdXOOFICJfSyg755vMzYcWk1x8F3OlXvtATm5IizmPZpzusCiDxRVoqtdqKfLtqyg2VogD-3Jf9lK0X58segoYLPPIuh3OFkrpk~D37~7aBfIv6bZQUN1V4rzPywuLSo43qlKubckSn91AL1FQhNZ3Gshg-uLxpWsi43xURHJPjwBkH3~W7wvxDmv6Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)