Abstract
We report a transfusion trial of platelets photochemically treated for pathogen inactivation using the synthetic psoralen amotosalen HCl. Patients with thrombocytopenia were randomly assigned to receive either photochemically treated (PCT) or conventional (control) platelets for up to 28 days. The primary end point was the proportion of patients with World Health Organization (WHO) grade 2 bleeding during the period of platelet support. A total of 645 patients (318 PCT and 327 control) were evaluated. The primary end point, the incidence of grade 2 bleeding (58.5% PCT versus 57.5% control), and the secondary end point, the incidence of grade 3 or 4 bleeding (4.1% PCT versus 6.1% control), were equivalent between the 2 groups (P = .001 by noninferiority). The mean 1-hour posttransfusion platelet corrected count increment (CCI) (11.1 × 103 PCT versus 16.0 × 103 control), average number of days to next platelet transfusion (1.9 PCT versus 2.4 control), and number of platelet transfusions (8.4 PCT versus 6.2 control) were different (P < .001). Transfusion reactions were fewer following PCT platelets (3.0% PCT versus 4.4% control; P = .02). The incidence of grade 2 bleeding was equivalent for PCT and conventional platelets, although posttransfusion platelet count increments and days to next transfusion were decreased for PCT compared with conventional platelets.
Introduction
More stringent donor selection and increased laboratory testing have been extremely effective in improving the safety of the US blood supply.1-6 However, transmission of some infections still occurs because the present approach is limited to specific known pathogens, is not effective against bacterial contamination,7-9 does not test for all pathogens,10 fails to prevent transmission of cytomegalovirus (CMV) despite testing,11 and tests for new pathogens, such as West Nile virus,12 can only be implemented after the new agent is identified. With increasing globalization, previously localized transfusion-transmitted infections such as malaria, trypanosomiasis, or babesiosis are now becoming more widespread. Therefore, strategies have been developed to treat the blood components in a way that will inactivate viruses, bacteria, protozoa, and contaminating leukocytes but retain therapeutic efficacy of the components.13-17
Amotosalen HCl, formerly designated S-59, is a synthetic psoralen compound that intercalates into helical regions of DNA or RNA and on illumination with ultraviolet A (UVA) light reacts with pyrimidine bases to form internucleic and intranucleic acid strand cross-links. The photochemical treatment (PCT) inhibits replication of any DNA or RNA. This achieves reduction of a broad range of viruses, bacteria, and protozoa to levels below those likely to transmit infection (Table 1). Extensive toxicology, mutagenicity, carcinogenicity, phototoxicity, and pharmacologic studies established an adequate safety profile for PCT platelets.24,25 In vitro platelet function of PCT platelets was preserved following up to 7 days of storage.15,16 Recovery and survival of radiolabeled PCT platelets in healthy subjects were reduced compared with conventional untreated platelets but within acceptable therapeutic ranges.26 PCT and conventional untreated platelets resulted in comparable correction of prolonged bleeding times in patients with thrombocytopenia.27 A randomized, controlled, double-blind, parallel group phase 3 study in 103 patients with thrombocytopenia of PCT buffy coat platelets demonstrated that 1-hour platelet count increments were not different for PCT and conventional buffy coat platelets.28 We now report on a prospective, randomized, controlled, double-blind, parallel group phase 3 study to evaluate the efficacy, as determined by the prevention and treatment of significant bleeding, and safety of PCT apheresis platelets compared with conventional platelets.
Patients, materials, and methods
Patients
Patients were eligible for enrollment if they had thrombocytopenia requiring platelet transfusion support and were at least 6 years of age. Patients were excluded from study participation if they had any factors that could potentially interfere with assessment of the study end points. These exclusion criteria included positive lymphocytotoxic antibody (> 20% panel reactive antibody at screening) or history of clinical refractoriness, history of immune or thrombotic thrombocytopenic purpura or hemolytic uremic syndrome, diagnosis of acute promyelocytic leukemia, recent surgery or psoralen ultraviolet A (PUVA) therapy, interleukin-11 therapy, or participation in another study with pathogen-inactivated blood products. Patients who met all inclusion and exclusion criteria were randomly assigned in a 1:1 ratio to receive all of their platelet transfusions with either PCT or control platelet concentrates for up to 28 days or until transfusion independence (7 days without platelet transfusion) prior to day 28. On completion of the transfusion period, patients entered a 7-day surveillance period to monitor for additional adverse events. The study was approved by each site's institutional review board (IRB), and all patients gave informed consent to participate.
All individuals involved in clinical care and assessment of patients were blinded to study treatment assignment. These individuals included the principal investigator, clinical study coordinators and nurses making hemostatic assessments, clinicians and nurses caring for the patient, and the study sponsor. Blood bank and transfusion service personnel responsible for randomization, collection, processing, and issue of study platelets were not blinded.
End points
The primary efficacy end point was the proportion of patients with grade 2 bleeding, as assessed by using expanded World Health Organization (WHO) criteria (Table 2),29 on any day during the period of platelet support. Additional secondary efficacy end points included the proportion of patients with WHO grade 3 or 4 bleeding; number of days of WHO grade 2 bleeding; 1- and 24-hour platelet count increments (CIs) and corrected count increments (CCIs); number of days to next platelet transfusion; number of platelet transfusions; incidence of platelet refractoriness; and number of red blood cell (RBC) transfusions. Safety end points included number of platelet transfusion reactions, development of antibody to potential amotosalen neoantigens, and overall safety.
Platelet collection and photochemical treatment
Both PCT and control study platelet transfusions were collected on the Amicus Separator (Baxter Healthcare, Round Lake, IL), which includes process leukoreduction, to attain a targeted average platelet transfusion dose of 3.7 × 1011. PCT platelets were suspended in 30% to 45% plasma and 70% to 55% platelet additive solution (Intersol; Baxter Healthcare, Deerfield, IL), whereas control platelets were suspended in 100% plasma. Photochemical treatment15 was performed at each study site within 24 hours of platelet collection by adding 150 μM amotosalen, mixing, and exposing the platelets to 3 J/cm2 UVA light in an illumination device for 3 to 5 minutes with constant gentle agitation. Following illumination, platelets were transferred to a plastic container with a compound adsorption device (CAD) to reduce the concentration of residual amotosalen and free photoproducts. After adsorption for 6 to 8 hours, PCT platelets were transferred to another container and were stored for up to 5 days according to blood bank standards.30 All donors and platelet products underwent required blood bank testing.30 PCT and control platelet concentrates were issued for transfusion in identical plastic containers with identical labeling. Because PCT platelets were manufactured solely for the purpose of the trial, there were occasional inventory shortages that resulted in transfusion of non-PCT platelets to patients randomly assigned to the PCT group (“off-protocol” transfusion) or transfusion of low-dose PCT products that would not otherwise have been transfused to prevent an off-protocol transfusion. Control platelet transfusions not collected on the Amicus Separator were also off-protocol transfusions.
Transfusion strategies
Platelet transfusions were given according to each institution's guidelines either prophylactically to prevent bleeding or therapeutically to treat existing bleeding or prepare for an invasive procedure. The most common threshold for prophylactic transfusions was 10 × 109/L. Each institution's policies determined platelet ABO type, use of irradiation, volume reduction, and HLA matching or cross-matching for donor selection. Patients received conventional red cell products; more than 98% of red cell units were leukocyte reduced and 99% were gamma irradiated in both treatment groups.
Hemostatic assessments and laboratory evaluation
Hemostatic assessments of 8 potential bleeding sites were performed by trained observers blinded to the treatment assignment. At each assessment, each of the 8 potential bleeding sites was assigned a WHO bleeding grade (Table 2) ranging from 0 (no bleeding) to 4 (life-threatening bleeding). The first hemostatic assessment encompassed the 12 hours preceding the first study platelet transfusion. Subsequent hemostatic assessments were performed daily and for 3 days following the last study platelet transfusion. The overall bleeding grade for each assessment was the highest grade observed for any of the 8 sites assessed. If grade 2 bleeding was observed at any potential bleeding site on any assessment during the transfusion period, the patient met the primary end point. For example, a patient with a 2-inch ecchymosis on day 3 of the transfusion period but no other bleeding events during the transfusion period would have been classified as having experienced grade 2 bleeding and would have met the primary end point of the trial.
The daily platelet count obtained for routine care was used for the study pretransfusion platelet count. The 1-hour and 24-hour posttransfusion platelet counts were obtained 10 minutes to 4 hours and 10 to 24 hours, respectively, following each platelet transfusion. Lymphocytotoxic antibody (LCA) testing to determine study eligibility was performed locally, and patients whose serum reacted with more than 20% of panel cells (PRA) were excluded. Plasma samples for LCA and antibody to amotosalen neoantigen testing were drawn weekly; baseline and end-of-study samples were analyzed at central laboratories for LCA by using standard techniques31 and for antibodies to potential amotosalen neoantigens by using a validated enzyme-linked immunosorbent assay (ELISA; Cerus, Concord, CA).28 If the patient became platelet refractory, all samples from the patient were analyzed for LCA, antibody to amotosalen neoantigens, and platelet-specific alloantibodies32,33 in central laboratories. The CCI, a measure of the response to platelet transfusion that takes into account patient body size as well as transfused platelet dose, was calculated as the difference between the platelet count after transfusion and the platelet count before transfusion, multiplied by the body surface area (in meters squared) and divided by the number of platelets transfused (× 10-11). A patient was considered clinically refractory if the 1-hour CCI was less than 5 × 103 following each of 2 consecutive platelet transfusions. Immunologic refractoriness was defined as clinical refractoriness (2 consecutive CCIs < 5 × 103) in the presence of any of the following: LCA (> 20% PRA), platelet-specific alloantibodies, and/or antibody to amotosalen neoantigens.
Adverse events and transfusion reactions
Adverse events were collected from initiation of first study transfusion through the end of the 7-day surveillance period. Adverse event and transfusion reaction severity was assigned on the basis of the most severe symptom or sign present. Reactions to study platelet transfusions were assessed for the 6 hours following each transfusion.
Randomization and statistical methods
A sample size of 300 patients per group was estimated before the start of the study to provide more than 90% power to reject the null hypothesis of inferiority with respect to grade 2 bleeding at a significance level of 0.05. All patients who received at least one study platelet transfusion were included in the analyses. Randomization was stratified by study site.
The study was designed as a noninferiority trial. Differences between treatment groups for the primary end point (the proportion of patients with grade 2 bleeding) and one secondary end point (the proportion of patients with grade 3 or 4 bleeding) were analyzed using one-sided tests of noninferiority with prespecified noninferiority margins of 12.5% and 7%, respectively. All other secondary end points were analyzed for differences between treatment groups. For the primary end point, the test statistic was (PT - PR - 0.125)/(Var[PT - PR])1/2, where PT is the observed proportion of patients with grade 2 bleeding in the PCT group, PR is the observed proportion of patients with grade 2 bleeding in the control group, and Var(PT - PR) is the variance estimated by the maximum likelihood estimate.34 The one-sided 95% confidence interval for the treatment difference in the proportion used the same estimated variance.
Analysis of variance with treatment and study site in the model was used for continuous variables. Fisher exact test was used for comparison of adverse events. Time to grade 2 bleeding was compared by using the log-rank test. Longitudinal regression analysis was used to adjust platelet count increment and transfusion interval for platelet dose.35,36 Except for the tests of noninferiority, all other statistical tests were 2-sided with a significance level of 0.05.
Results
Of the 671 patients randomly assigned, 645 received at least one study platelet transfusion (318 PCT; 327 control) and composed the intention-to-treat (ITT) population. The 26 patients not included in the ITT analyses did not require platelet transfusions before recovery from thrombocytopenia. There were no differences between the groups for sex, age, ethnic origin, diagnosis, or receipt of stem cell transplant (Table 3) or in baseline hematology, chemistry, and coagulation laboratory studies (data not shown).
The proportion of patients completing the transfusion period (89%) and the surveillance period (81%), the mean duration of platelet support (11.8 days PCT versus 10.6 days control), and the proportion of patients achieving and maintaining platelet transfusion independence prior to day 28 (66% PCT versus 70% control) were not different between treatment groups (Table 4).
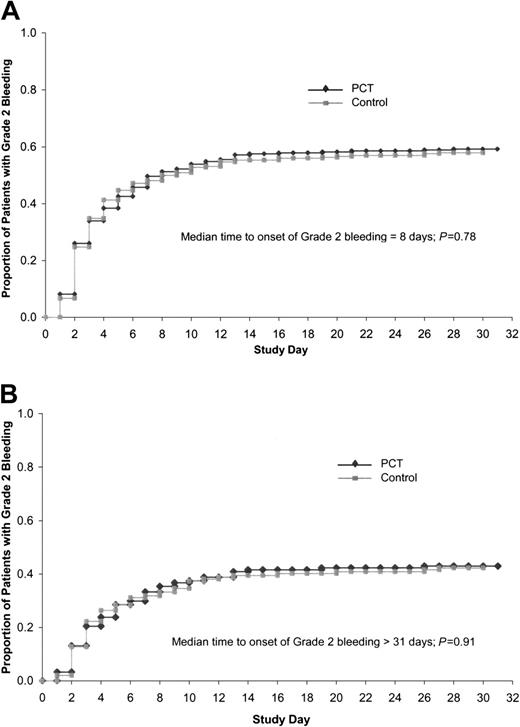
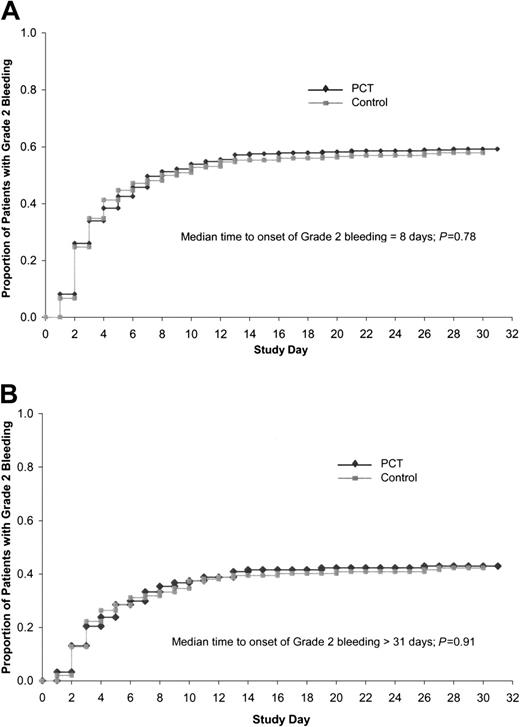
The primary end point of the trial, the proportion of patients with grade 2 bleeding, was equivalent for the PCT group and control group, both overall, as well as for any of the 8 potential bleeding sites (Table 5). Grade 2 bleeding occurred during the transfusion period in 58.5% of patients in the PCT group compared with 57.5% of patients in the control group. The time to onset of grade 2 bleeding after beginning the study was not significantly different between PCT and control patients, either for the ITT population (Figure 1A, P = .78) or for those patients without grade 2 bleeding at study entry (Figure 1B, P = .91). Grade 2 bleeding occurred on a mean of 3.2 days in the PCT group as compared with 2.5 days in the control group (P = .02) and on a median of 1 day for each group.
Time to onset of grade 2 bleeding. (A) Time to onset of grade 2 bleeding in ITT population (n = 645). Median time to onset of grade 2 bleeding was 8 days, log rank P = .78. (B) Time to onset of grade 2 bleeding in patients with no (grade 0) bleeding at baseline (n = 541). Median time to onset of bleeding more than 31 days, log-rank test P = .91.
Time to onset of grade 2 bleeding. (A) Time to onset of grade 2 bleeding in ITT population (n = 645). Median time to onset of grade 2 bleeding was 8 days, log rank P = .78. (B) Time to onset of grade 2 bleeding in patients with no (grade 0) bleeding at baseline (n = 541). Median time to onset of bleeding more than 31 days, log-rank test P = .91.
The maximum grade of bleeding at any potential bleeding site was grade 2 for most patients. Grade 3 or 4 bleeding occurred in only 4.1% of patients in the PCT group and 6.1% in the control group. There were no statistically significant differences between the groups in grade 3 or 4 bleeding overall or for any of the 8 potential bleeding sites. The most common site of grade 3 or 4 bleeding was the neurologic system (3 of 318, 0.9% PCT versus 6 of 327, 1.8% control).
The 645 patients in this study received a total of 4719 platelet transfusions (2678 PCT; 2041 control) (Table 6). Most units of platelets transfused (91.5% PCT and 95.2% control) were prepared according to study methods (“on-protocol transfusions”). During the study transfusion period, exclusively on-protocol transfusions were received by 68% of patients in the PCT group and 85% of patients in the control group (P < .01). Of patients who received any off-protocol transfusions, most (53% PCT and 59% control) received only one. The proportion of platelet transfusions that were HLA matched (1.5%), cross-match compatible (0.2%), volume reduced (7.5%), or irradiated (99.8%) were comparable between the 2 groups. Slightly more PCT transfusions were ABO-matched (with patient pretransplantation blood type) than control transfusions (78.5% versus 75.4%, P = .01). Mean platelet storage duration prior to transfusion was 3.4 days for PCT as compared with 3.6 days for control platelets (P < .01).
Patients in the PCT group received more platelet transfusions overall (8.4 PCT versus 6.2 control; P < .001; Table 6) and more platelet transfusions per day of platelet support (0.74 PCT versus 0.65 control; P < .001). These differences may be partially explained by the lower mean dose of platelets per transfusion in the PCT group compared with the control group (3.7 × 1011 PCT versus 4.0 × 1011 control; P < .001) and the greater proportion of PCT platelet doses that contained less than 3.0 × 1011 platelets (20% PCT versus 12% control; P < .01; Figure 2). Sixty percent of patients in the PCT group received at least one platelet dose less than 3.0 × 1011 compared with 36% of patients in the control group (P < .01). However, by using longitudinal linear regression to adjust for platelet dose, when equal doses of PCT and control platelets were given, the 1-hour posttransfusion platelet count was estimated to be 10.4 × 109/L lower for PCT than for control platelets (P < .001), and the time to the next transfusion was shorter by 0.4 days for PCT than for control platelets (P < .001). Other factors that can affect platelet recovery,37 such as splenomegaly, fever, sepsis, and amphotericin use were comparable between treatment groups. There was no difference between the groups in the mean number of red blood cell transfusions or the mean number of red blood cell transfusions per day of platelet support (Table 6).
Distribution of transfused platelet doses. A greater proportion of doses were less than 3.0 ×1011 in the PCT group compared with the control group (P < .01).
Distribution of transfused platelet doses. A greater proportion of doses were less than 3.0 ×1011 in the PCT group compared with the control group (P < .01).
Most transfusions were given for prophylaxis (93.5% PCT versus 90.1% control; P < .01); the others were considered to be therapeutic either to treat active bleeding or to prepare for an invasive procedure. Although mean pretransfusion platelet counts were similar for patients in both groups, the mean 1-hour posttransfusion platelet count was lower in the PCT group (36.5 × 109/L PCT versus 49.5 × 109/L control; P < .001), as were the mean 1-hour and 24-hour CI and CCI (Table 7).
Platelet clinical refractoriness occurred in 21.4% of PCT as compared with 7.0% of control patients (P < .001; Table 8). One-hour CCIs less than 5 × 103 were observed with 27.4% of all PCT transfusions and 12.7% of all control platelet transfusions (P < .001) and 33.4% of PCT as compared with 12.3% of control platelet transfusions with platelet doses less than 3.0 × 1011 (P < .001). Most refractory episodes were transient, involving only a single episode of 2 consecutive 1-hour CCIs less than 5 × 103 (57% PCT versus 65% control). Only 6% of refractory patients in the PCT group and 9% of refractory patients in the control group remained refractory through study completion. Alloimmunization to HLA, platelet-specific antigens, or amotosalen neoantigens as the basis for platelet refractoriness occurred 4.7% of PCT patients as compared with 3.1% of control patients in the ITT population (P = .31) and in 22% of PCT patients as compared with 44% of control patients in the refractory subset of patients (P = .06). Among refractory patients, LCA was more common in the control group (39%) compared with the PCT group (15%; P = .02). Platelet alloantibodies occurred with similar frequency among refractory patients (12% PCT compared with 10% control; P = 1.00).
Although there were fewer transfusion reactions following transfusion of PCT platelet units (3.0% PCT versus 4.4% control transfusions; P = .02), there was no difference in the proportion of patients who experienced a reaction (16.0% PCT versus 19.3% control; P = .30). Reactions were primarily fever, chills, urticaria, or rash. Almost all patients experienced one or more adverse events (Table 9). Adverse events were coded to 898 MedDRA Preferred Terms.39 The most common adverse events (reported in > 30% of patients in either treatment group), such as hematuria, diarrhea, hypokalemia, rigors, petechiae, epistaxis, fecal occult blood, contusion (bruising), and dermatitis, were consistent with those expected for the patient population enrolled in this study. As expected, with the large number of statistical comparisons performed, there were statistically significant differences between treatment groups for some types of adverse events, but these differences were not considered to be clinically relevant and will be reported in detail separately. Grade 3 or 4 adverse events, those considered by the investigator to be probably or possibly related to study platelet transfusion, and adverse events meeting US Food and Drug Administration (FDA) criteria for serious were not different between the PCT and control groups (Table 9). There were 28 deaths (3.5% PCT versus 5.2% control) during the study, mostly because of infectious or respiratory complications.
Discussion
Despite improvements in the safety of the US blood supply, the public wants transfusion risks to be as close to zero as possible, and political and health policy decisions reflect this goal. As new transfusion-transmitted infectious agents are identified, new tests for these agents may be implemented, but this approach will always have limitations. Inactivation of a broad spectrum of viruses, bacteria, and protozoa in blood products is a promising new strategy to improve blood safety.
The low prevalence of pathogens in blood components precludes a study of the prevention of transfusion-transmitted infection by PCT platelets. Therefore, we studied the effect of PCT on platelet transfusion hemostatic effectiveness rather than transfusion transmissible infections. The trial, the largest one of its kind, evaluated platelet hemostasis as the primary end point while also evaluating the quality and safety of PCT platelets. PCT and control platelets were hemostatically comparable overall and, for each of the 8 potential bleeding sites evaluated, established that PCT platelets were clinically effective. Patients who received PCT platelets had lower platelet count increments following transfusion, received more platelet transfusions, and had a shorter interval between transfusions compared with patients who received conventional apheresis platelets. The lower platelet count increment is partly explained by the lower mean platelet dose in the PCT group and the disproportionate number of transfusions containing doses less than 3.0 × 1011 (Figure 2). The greater proportion of low-dose platelets transfused to the PCT group may have resulted in the greater number of platelet transfusions in the PCT group.41 Reasons for lower platelet doses in the PCT group primarily reflected clinical trial requirements. These reasons included a clinical prototype of the device was used with a nonintegrated processing set and a prototype CAD; processing loss for PCT platelets was acknowledged; samples taken for amotosalen assay came from PCT but not control; to avoid off-protocol transfusions, low doses of PCT platelets were transfused when a higher dose unit was not available; and because PCT units were produced solely for the purpose of the clinical trial, control units were more readily available, resulting in higher platelet doses. During routine use, it is expected that doses of PCT platelets will be comparable to control platelets. Following completion of this trial, an integrated PCT processing set with an improved CAD was developed and evaluated in a small supplemental trial in Europe. That trial in 43 patients demonstrated no increase in the number of platelet transfusions required to manage patients transfused with PCT platelets for up to 28 days42 ; those results will require confirmation in a larger study.
Another factor accounting for the reduced platelet responses with PCT platelets was a decrease in platelet viability; ie, at equal platelet doses, there was a significant reduction in both platelet increment and days to next transfusion comparing PCT with control platelets. An effect of the PCT process on platelet viability was suggested in previous studies in healthy research subjects and patients.26,27 As a consequence of the lower platelet count increments in the PCT group, clinical platelet refractoriness occurred more frequently in patients receiving PCT platelets; however, it tended to be transient, persisting to the end of the study in only 6% of PCT and 9% of control refractory patients. Alloimmune platelet refractoriness and the need for HLA-matched platelets were uncommon and were similar in both groups. Among platelet refractory patients, the incidence of LCA was lower in the PCT group, but platelet-specific alloantibodies were similar. Despite the lower platelet count increments, the shorter intervals between platelet transfusions, and the resultant greater number of PCT platelets transfused, the PCT platelets were hemostatically equivalent to the control platelets; therefore, differences in these secondary end points appear to have little effect on product efficacy and patient benefit.
Overall, no unusual toxicities or adverse events were associated with the transfusion of PCT platelets. A companion safety analysis will be reported separately. Although the proportion of patients who experienced a transfusion reaction was similar in the 2 groups, fewer PCT platelet transfusions were associated with a reaction. This could be due to leukocyte inactivation, resulting in less cytokine production during storage of PCT platelets or the reduced volume of plasma in the PCT units.43 Other adverse events, including hemorrhagic adverse events and death, were not different between the 2 groups of patients.
Photochemically treated platelets were clinically effective in maintaining hemostasis, appear to be associated with an acceptable safety profile, and offer the potential to further reduce the infectious risks of blood transfusion, including those associated with emerging transfusion-transmitted infections.
Prepublished online as Blood First Edition Paper, May 11, 2004; DOI 10.1182/blood-2003-12-4443.
J.-S.L., L.L., P.M., D.H.B., L.C., and M.G.C. are employees of Baxter or Cerus Corporations. J.M., S.J.S., and S.M. are members of the Cerus Medical Advisory Committee, for which $2500 per year is received. J.M., E.S., and S.M. are consultants for Baxter or served on the Fenwal Medical Advisory Committee, for which less than $10 000 was received in the past year. J.M., R.J.B., A.P., T.R., E.S., S.M., and I.L.-P. participated in other clinical trials sponsored by Cerus or Baxter; they received funds only to support the cost of those studies.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the many nurses, technologists, and study coordinators who worked on this project at each study site and to the following: Cerus Corporation: Steven Anderson, MT; Tanya Baculik, MD; Marlene Bartram, RN, MT; Ruth Dye, RN; Anne Elliott, RN; and Lecia Shaffer. Baxter Healthcare Corporation: Janis Drerup, Julie Gavigan, Jaime Houghton, Alamdar Rizvi, and Lindsey Wood, PhD. Data Safety Monitoring Board: Margot Kruskall, MD, Chair; Marcella Contreras, MD; Paul Holland, MD; and Janet Wittes, PhD.