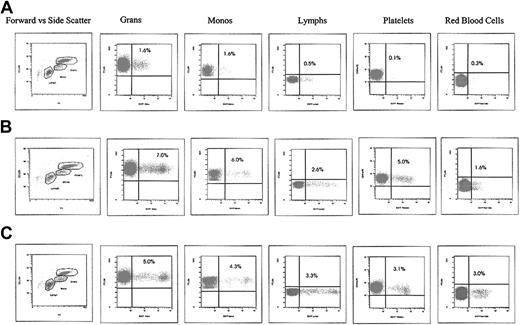
We read with great interest the article by Morris et al demonstrating the appearance of cytotoxic T lymphocytes specific to a transgene protein in baboons following myeloablative conditioning and transplantation of cytokine mobilized bone marrow (BM)–derived immunoselected CD34+ cells transduced with a human lentiviral vector.1 Morris et al find no long-term marking and conclude that induction of the transgene-specific immune responses after transplantation may be due to the use of lentiviral vectors as opposed to oncoretroviral vectors. We believe this interpretation is overly simplistic. Our studies with lentiviral vectors in rhesus macaques demonstrate long-term marking without immune responses to the transgene. Contrary to the experience of Morris et al, our animals that underwent transplantation continue to maintain enhanced green fluorescent protein (EGFP) expression levels for nearly 6 years to date at the same levels as originally detected. For example, we have seen no change in expression in an animal who had undergone transplantation more than 5 years ago with a first-generation lentiviral vector (Figure 1A) and have several animals over 1 year after transplantation at the 5% level (Figure 1B-C) using a third-generation human self-inactivating lentiviral vector (An et al2,3 ; and R.E.D. and I.S.Y.C., unpublished data, 2004). Not only do we not see evidence of any immune response, but we are also unable to elicit an immune response to the transgene product following active immunization, indicating a state of tolerance.4
EGFP expression in circulating hematopoietic cells following HIV lentiviral gene transfer and transplantation of rhesus cytokine-mobilized peripheral blood CD34+ cells. (A) RC505. First-generation lentiviral vector at 5 years, 8 months. (B) 95E132. Self-inactivating lentiviral vector at 4 years, 6 months. (C) 2RC003. Self-inactivating lentiviral vector at 14 months.
EGFP expression in circulating hematopoietic cells following HIV lentiviral gene transfer and transplantation of rhesus cytokine-mobilized peripheral blood CD34+ cells. (A) RC505. First-generation lentiviral vector at 5 years, 8 months. (B) 95E132. Self-inactivating lentiviral vector at 4 years, 6 months. (C) 2RC003. Self-inactivating lentiviral vector at 14 months.
Although not discussed in the paper by Morris et al, the discrepancy with our findings likely reflects a difference in source of CD34+ cells. They use cytokine-mobilized BM CD34+ cells, whereas we use cytokine-mobilized peripheral blood (PB) CD34+ cells. Indeed, we observed that cytokine-mobilized BM CD34+ cells contributed little to long-term engraftment,3 consistent with their results. On the other hand, even with a lower transduction efficiency (15%-20% evaluated immediately following transduction),3,5 cytokine-mobilized PB CD34+ cells contributed far more reliably to long-term engraftment.3 This observation is consistent with earlier murine studies evaluating cytokine-mobilized stem cell populations.6 As CD34+ cells are a heterogenous population, one reason for the immune response observed by these authors is that the BM-derived CD34+ cells may be a better source of antigen-presenting cells and that the cytokine cocktail used in their study (interleukin-3 [IL-3], IL-6, stem cell factor, megakaryocyte growth and development factor, and FMS-like tyrosine kinase 3-ligand) may lead to differentiation to antigen-presenting cells. Indeed, a combination of 4 of these cytokines can differentiate CD34+ cells to dendritic cell precursors.7 Other differences in protocols include our use of a lower multiplicity of infection and few to no cytokines during the transduction period.
Morris et al argue that the immune response may be responsible for the lack of reconstitution of lentiviral-transduced CD34+ cells. An alternative explanation, however, is that their transplantation protocol resulted in inefficient engraftment precluding long-term engraftment and the tolerizing effect of transgene expression. That, along with a concomitant antigen presentation, primed an immune response directed toward the transgene. Based on our observations, lentiviral vectors continue to hold tremendous promise in gene therapy.
Cytotoxic T-lymphocyte responses to enhanced green and yellow fluorescent proteins after myeloablative conditioning
We appreciate the opportunity to respond to the letter from Donahue and Chen regarding our article in the January 15, 2004, issue of Blood in which we describe the development of transgene-specific immune responses after transplantation of lentivirally transduced hematopoietic cells in a myeloablative setting.1 Donahue and Chen cite their successful transplantation of lentivirally transduced cells without immune responses in the rhesus macaque model.2-4 They contend that the transplantation protocol used, not the use of lentiviral vectors, explains the difference between our results. While we agree that protocol variables might affect the development of immune responses, we believe that the vector used is one variable contributing to the induction of transgene-specific immune responses.
The major contention of Donahue and Chen is our use of cytokine-primed bone marrow as the stem cell source. They observed little contribution to long-term repopulation by marrow CD34+ cells in the rhesus.3 In contrast, we have transplanted marrow CD34+ cells transduced by enhanced green fluorescent protein (EGFP)–encoding oncoretroviral vectors in 19 baboons, achieving long-term engraftment of EGFP+ cells in all 19 animals at levels up to 30%.5,6 Additionally, we observed efficient gene transfer to canine marrow cells with both lentiviral and oncoretroviral vectors.7-9 Similar cytokine combinations were used in these animals and those receiving lentivirally transduced cells in our study.1 Thus, it is unlikely that stem cell source or growth factor cocktail contributed to the development of immune responses.
In our article1 we suggested explanations for why lentivirally transduced cells evoked immune responses; one factor not addressed is the level of immunosuppression. Total body irradiation (TBI; 10.2 Gy) was sufficient when transplanting oncoretrovirally transduced cells in our baboons, but others showed rejection of oncoretrovirally transduced cells in rhesus macaques after 4 Gy TBI.10 In our experience, macaques are more sensitive to TBI than baboons,11 and it is possible that the 10-Gy TBI used by Donahue and Chen in rhesus macaques provided more potent immunosuppression than in our baboons, allowing for EGFP tolerance. As further evidence of the importance of immunosuppression, using only myeloablative TBI in the dog led to rejection of lentivirally transduced cells in the majority of cases; however, adding cyclosporine has allowed for the induction of EGFP tolerance.9 Therefore, we believe that differential immunosuppression resulting from inherent species differences is a more likely explanation for the difference between our1 results and those of Donahue and Chen.
We agree that lentiviral vectors have tremendous potential for gene therapy. Note that we achieved long-term, multilineage marking in several baboons receiving lentivirally transduced grafts,12 and we have achieved especially promising results with lentiviral vectors in the dog.9 We believe each transplantation scenario will require a specific level of immunosuppression for tolerance induction. This level will depend on the summation of many variables including those discussed by us1 and Donahue and Chen as well as factors such as the expression level and immunogenicity of the transgene. These observations demonstrate the complexity of these experiments and underscore the importance of evaluating gene transfer protocols in relevant animal models.
Correspondence: Hans-Peter Kiem, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D1-100, Seattle, WA 98109; e-mail: hkiem@fhcrc.org.