Abstract
The majority of cases of human hemophilia B are the result of missense mutations in the coagulation factor IX gene and defective circulating factor IX is detectable in most patients. The available mouse factor IX knockout models of hemophilia B (FIXKO mouse) reproduce the bleeding phenotype of human hemophilia B, but because the models produce no factor IX they fail to reproduce the dominant human phenotype. We have created a human factor IX mouse model of hemophilia B (R333Q-hFIX mouse) by homologous recombination in embryonic stem cells. The mouse expresses no mouse factor IX, but instead expresses a missense mutant human factor IX from the mouse FIX promoter. Mutant human factor IX mRNA transcript and circulating human factor IX are detectable throughout development, but factor IX activity is less than 1% and the mouse exhibits the hemophilic phenotype. When R333Q-hFIX mice were challenged by intramuscular injection of adeno-associated virus expressing human factor IX, factor IX expression without the development of antibodies was observed. In contrast, given the same treatment, FIXKO mice consistently develop antibodies. Our R333Q-hFIX mice strain will complement the FIXKO mice for studying factor IX circulating kinetics and gene therapy. (Blood. 2004;104:1733-1739)
Introduction
Hemophilia B is an X-linked recessive hemorrhagic diathesis resulting from lack of coagulation factor IX activity. Lack of factor IX activity may arise from a variety of molecular defects in the factor IX gene, including base substitutions, deletions, insertions, and gene inversions. The clinical definition of hemophilia B is based on the individual's factor IX activity level as mild (> 5%), moderate (1%-5%), or severe (< 1%). Of mutations resulting in clinical disease, 96% occur in the 2.2-kb sequence of the factor IX gene that consists of the promoter region, coding region, and splice junctions. We, and others, created mice deficient in factor IX by gene targeting1-3 that proved to be excellent models of hemophilia B. However, the mice deficient in factor IX were created by deletion of the promoter as well as a portion of the gene itself and produced no factor IX antigen.1 Therefore, because most patients with hemophilia B have circulating levels of defective factor IX (which is antigenically cross-reacting material, [CRM]) the hemophilia B mouse fails to mimic some important characteristics of the human disease. In particular, when the hemophilia B mice are treated with exogenous factor IX or by intramuscular gene therapy to correct their deficiency they usually develop neutralizing antibodies.4-7 This contrasts with patients with hemophilia B who rarely develop neutralizing antibodies.
Here we report the creation of a mouse hemophilia B model that more closely approximates the situation usually observed in patients with hemophilia B. This mouse expresses human factor IX R333Q. We chose R333Q because data on several patients with severe hemophilia B who exhibit this mutation have been reported.8 Moreover, these CRM+ patients have nearly normal levels of factor IX antigen and their clotting activity is usually less than 1%. To make the mutant mouse more useful we have expressed the human factor IX R333Q gene as the alanine form of the Ala148Thr dimorphism.9 Because the A-1 antibody binds well to factor IX with threonine at residue 148 but weakly to the alanine isoform, the effectiveness of gene therapy can be evaluated by expressing the threonine isoform and detecting its presence with the A-1 antibody. We anticipate that this mouse model will be an important tool for gene therapy and for studying the function of mutant factor IX in vivo.
Materials and methods
Targeting vector for generating the R333Q-human factor IX mouse
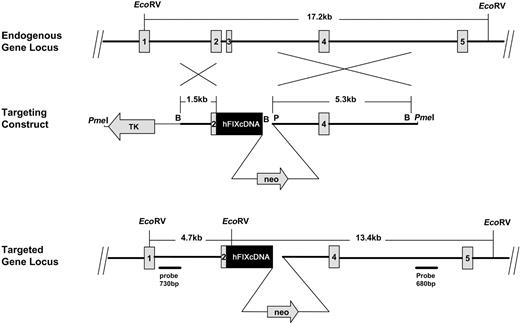
The targeting construct consisted of 1.5 kb of mouse factor IX (5′ homology), 2.6 kb of human factor IX cDNA, and a 5.3-kb PacI-BamHI fragment of mouse gDNA (3′ homology region; Figure 1). This construct was introduced into the osdupdeI plasmid vector (a gift from Dr Oliver Smithies's laboratory, the Pathology Department, University of North Carolina at Chapel Hill [UNCCH]) using standard recombinant DNA techniques.10 In addition to the 5′ and 3′ homology regions of mouse factor IX, the targeting construct included a positively selectable neomycin resistance gene (neo) controlled by the pMC promoter, and a herpes simplex virus thymidine kinase gene (HSV-tk), driven by the pGK promoter, as a negative selection marker. The 1.5-kb 5′ homology region contained part of the first intron of mouse factor IX and sequences coding for the propeptide and first 6 amino acids of the Gla domain of mouse factor IX. The human cDNA was joined to the mouse gene at codons 6 and 7 (human and mouse amino acid sequences are identical through amino acid 7 by creating an XhoI site without changing the amino acid sequence).
Strategy for targeting the mouse factor IX gene by homologous recombination. (Top) Endogenous gene locus. Exons 1 to 5 of the endogenous mouse factor IX gene are shown. (Middle) Targeting construct. The construct linearized with PmeI and used for homologous recombination is shown. Recombination between the 5′ (1.5 kb) and 3′ (5.3 kb) regions of homology is depicted. The PmeI site is within the plasmid vector, 29 nucleotides from the BamHI site. The human factor IX cDNA is depicted in black. (Bottom) Targeted gene locus. The mouse factor IX locus after homologous recombination is shown. The regions from which the 730-bp and 680-bp probes were generated are shown. After EcoRV cleavage of mouse gDNA, the 730-bp probe gives a 4.7-kb fragment and the 680-bp probe a 13.4-kb fragment when homologous recombination has occurred. Mouse gDNA is shown as a thick line and the inserted neo sequences as a thinner line. B indicates BamHI; P, PacI.
Strategy for targeting the mouse factor IX gene by homologous recombination. (Top) Endogenous gene locus. Exons 1 to 5 of the endogenous mouse factor IX gene are shown. (Middle) Targeting construct. The construct linearized with PmeI and used for homologous recombination is shown. Recombination between the 5′ (1.5 kb) and 3′ (5.3 kb) regions of homology is depicted. The PmeI site is within the plasmid vector, 29 nucleotides from the BamHI site. The human factor IX cDNA is depicted in black. (Bottom) Targeted gene locus. The mouse factor IX locus after homologous recombination is shown. The regions from which the 730-bp and 680-bp probes were generated are shown. After EcoRV cleavage of mouse gDNA, the 730-bp probe gives a 4.7-kb fragment and the 680-bp probe a 13.4-kb fragment when homologous recombination has occurred. Mouse gDNA is shown as a thick line and the inserted neo sequences as a thinner line. B indicates BamHI; P, PacI.
Site-directed mutagenesis
Two site-specific point mutations, T148A and R333Q, were created in the human factor IX cDNA by megaprimer polymerase chain reaction (PCR).11,12 The entire coding region of the human factor IX sequence was determined by the University of North Carolina-Chapel Hill (UNC-CH) Sequencing Facility to confirm that the sequence was as expected and no extra mutations were introduced.
Cell culture and electroporation
TC-1, an embryonic stem (ES) cell line derived from mouse strain 129/SvEvTac FBR,13 was cultured on feeder layers prepared from primary embryonic fibroblasts, in ES cell medium (Dulbecco modified Eagle medium-H medium from Gibco BRL [Gaithersburg, MD], with 15% fetal calf serum, 0.1 mM 2-mercaptoethanol, 0.1 mM l-glutamine). The targeting vector was linearized at its unique PmeI site (Figure 1) and electroporated into TC-1 cells. Electroporated cells were cultured in ES cell medium with G418 (200 μg/mL) and ganciclovir (2 μM). Nine to 11 days after electroporation, colonies resistant to G418 and ganciclovir were selected, expanded, and screened for homologous recombination.
Detecting homologous recombinants
Successful homologous recombination was determined by PCR and confirmed by Southern blotting. The Clontech Advantage PCR kit (BD Biosciences Clontech, Palo Alto, CA) was used for analysis; the forward primer, 5′-GCACACCCTCACTGTGCTATAACACTC-3′, was complementary to intron 1 of mouse factor IX, whereas the reverse primer, 5′-GGATTGGACTCACACTGGTCACCATC-3′, was complementary to human factor IX cDNA. After an initial incubation at 95°C for 1 minute, 35 cycles of 95°C for 30 seconds, 68°C for 3 minutes, and 68°C for 3 minutes were used. The primer pair gave the expected 1.8-kb PCR product from targeted DNA. For Southern blot analysis, gDNA from the ES cell clones was digested with EcoRV. After EcoRV digestion, DNA was fractionated by electrophoresis on an 0.8% agarose gel and transferred to a Nytran Super Charge membrane (Schleicher & Schuell BioScience, Keene, NH). The DNA probes were designed to hybridize with a 730-bp DNA fragment of intron 1 or a 680-bp DNA fragment of intron 4 of mouse factor IX (Figure 1). Wild-type cells showed a 17.2-kb fragment with either the 730-bp or 680-bp probe, whereas the targeted cells showed a 13.4-kb fragment with the 680-bp probe and a 4.7-kb fragment with the 730-bp probe.
Genotype analysis
PCR and Southern blotting were used for genotyping of tail DNA from R333Q-human factor IX (R333Q-hFIX) candidate mice. The primer pair (see “Detecting homologous recombinants”) was used to screen for correctly targeted mice. All mice carrying the R333Q human factor IX on one or both X chromosomes exhibited a 1.8-kb PCR band. Throughout this report homozygous refers to females carrying 2 R333Q mutant alleles (H/H, hemophilic female), hemizygous to males with one mutant allele (H/Y, hemophilic male), and heterozygous to females with one copy of the normal mouse factor IX gene and one copy of R333Q human factor IX (H/+, nonhemophilic carrier female). Mice with murine wild-type factor IX sequence, including nonhemophilic wild-type females (+/+), nonhemophilic males (+/Y), and heterozygous females (as defined), exhibit a 640-bp PCR fragment from the primer pair, 5′-CACACTGGAAACAGCCCAGCCAGAGG-3′ (forward, at intron 1), 5′-GGAGTCACCTCTCTAGTTCCACACTCC-3′ (reverse, at intron 2). The 730-bp probe described for Southern blotting hybridizes to a 17-kb fragment when wild-type murine factor IX gDNA is present. The same probe reveals a 4.7-kb fragment when human factor IX cDNA has undergone homologous recombination.
Generation of R333Q-hFIX mice
Targeted ES cells were microinjected into the blastocoele of C57BL/6 blastocysts and implanted into a pseudopregnant mouse. Resulting chimeric males were bred to C57BL/6 females to produce heterozygous (H/+) offspring. These mice were crossed with C57BL/6 males to generate hemizygous (H/Y) mice expressing human factor IX.
RT-PCR
Total RNA was extracted from liver tissue of hemizygous mice using TRIzol reagent (Invitrogen, Carlsbad, CA). First-strand cDNA was synthesized from 5 μg total liver RNA using oligo (dT) primers and 200 units SuperScript II reverse transcriptase (Invitrogen). The reverse transcription-PCR (RT-PCR) products from hemizygotes were sequenced to ensure that human factor IX had no inadvertent mutations introduced.
Quantitation of R333Q-hFIX mRNA by PCR
R333Q-hFIX mRNA levels were determined by quantitative real-time PCR (qPCR). First-strand cDNA of 6-week-old hemizygous, homozygous, heterozygous, and wild-type mice was synthesized by RT-PCR. The primers used, 5′-GGAAGCAGTATGTTGATGG-3′ (forward) and 5′-TGGTTCACAGGACTTCTGGT-3′ (reverse), were complementary to both human and mouse factor IX. Primers 5′-CACAGTGTTGTCTGGTGGTA-3′ (forward) and 5′-GACTCATCGTACTCCTGCTT-3′ (reverse) for the mouse β-actin gene were used as a control. Mouse factor IX cDNA was diluted in series to construct a standard curve. The DyNAmo SYBR Green qPCR Kit (MJ Research, Boston, MA) was used for qPCR, which was performed using the DNA Engine Opticon 2 System (MJ Research) and analyzed according to the manufacturer's instructions. Conditions for qPCR were 95°C for 5 minutes, followed by 45 cycles (95°C for 10 seconds, 60°C for 20 seconds, 72°C for 20 seconds). For each cycle, fluorescence signals were measured at 72°C, 78°C, and 84°C. For Tm (-dl/dT max) measurements, the initial temperature was 55°C and the final temperature was 90°C. A temperature increment of 0.2°C and a hold time of 10 seconds were set during the melting curve step. Interestingly, there was sufficient difference in the GC composition of the mouse and human factor IX cDNAs that differences in Tm were readily observed.
Additional animal care and experiments
C57BL/6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME); factor IX knockout mice (FIXKO) were described by our group1 and were bred in house. FIXKO mice are maintained in colonies of homozygous knockout (hemophilic) females and hemizygous hemophilic male mice. The founder mice for the colony were originally backcrossed 4 to 5 generations from the 129 strain background into the C57BL/6 background, so are in a mixed 129/C57BL/6 background. R333Q-hFIX missense mutant mice were generated as described (see “Generation of R333Q-hFIX mice”). R333Q-hFIX mice that received gene therapy were F3-F4 generation. All mice were maintained according to the guidelines of the UNC-CH Animal Care and Use Committee. Mice were anesthetized using inhaled isoflurane for all procedures. For gene therapy, 100 μL phosphate-buffered saline (PBS) containing 1 × 1011rAAV2/hFIX dot blot genomes were injected into the gastrocnemius muscle (50 μL for each hind limb) in 6- to 8-week-old FIXKO mice or 8- to 10-week-old R333Q-hFIX mice. Mice weighed 20 to 25 g at the time of treatment. Scheduled blood samples were collected from the retro-orbital plexus and the plasma was stored at -80°C for future enzyme-linked immunosorbent (ELISA) and activated partial thromboplastin time (APTT) assays.
Determination of hFIX and R333Q-hFIX concentration by ELISA
Plasma samples were collected from (1) 6-week-old untreated homozygous, hemizygous, heterozygous, and wild-type mice, or from (2) mice following gene therapy with a factor IX expression vector at the indicated time points. Initial characterization of the R333Q-hFIX mouse used a sandwich ELISA using a pair of polyclonal antibodies, as described.14 The capture antibody, rabbit anti-human factor IX polyclonal antibody (Dako, Carpinteria, CA) was diluted at 1:1200 with carbonate buffer. The detecting antibody was a sheep anti-human factor IX polyclonal antibody SAFIX-APHRP (Affinity Biologicals, Ancaster ON, Canada), which detects factor IX carrying either of the common polymorphisms at amino acid 148 (Thr148 or Ala148).
To preferentially detect the Thr148 variant of human factor IX, a mouse anti-human factor IX monoclonal antibody, A-1,15,16 conjugated with horseradish peroxidase (HRP) was used as the detecting antibody in the ELISA. The A-1 antibody was originally prepared and characterized by K. J. Smith with the author (D.W.S.)15,16 and was kindly supplied for the current studies by A. R. Thomson (University of Washington, Seattle, WA). The A-1 antibody was conjugated with HRP using an EZ-link Plus activated peroxidase kit (Pierce, Rockford, IL). The HRP-conjugated A-1 (A1-HRP, 2 mg/mL) was diluted 1:10 000. Factor IX antigen levels were calculated for each of the 2 ELISA systems using a human factor IX standard curve generated from purified recombinant human factor IX. The recombinant Thr148 factor IX17 was produced in human embryonic kidney 293 cells and purified using batch adsorption to Q Sepharose as previously described 18,19
Factor IX-specific APTT
Clotting activities of C57BL/6 wild-type, FIXKO, and R333Q-hFIX mice were measured using citrated plasma collected from the retro-orbital venous plexus. Untreated mice were sampled at 6 to 8 weeks of age. The START 4 Coagulation Analyzer (Diagnostica Stago, Asnières, France) was used to measure clotting times. Pooled normal human reference plasma (FACT, George King Biomedical, Overland Park, KS) was serially diluted with human factor IX-deficient plasma to create APTT standard curves. A uniform amount of mouse factor IX-deficient plasma was added to each of the standards to approximate the mouse plasma protein present in the samples. Each standard and each mouse plasma sample was further diluted 10-fold with diluting buffer (Owen-Koller, Diagnostica Stago). The mixture of diluted mouse plasma (50 μL), human factor IX-deficient plasma (50 μL; George King Biomedical), and cephalin/silica activator (50 μL; STAPTT Automate 5, Diagnostica Stago) was incubated at 37°C for 3 minutes. Then 50 μL 25 mM CaCl2 was added, the clotting time recorded, and the percentage human factor IX activity calculated from the APTT standard curve.
In vivo pharmacokinetic studies
Four micrograms recombinant wild-type human factor IX (Thr148 isotype) was injected into the left jugular vein of wild-type, FIXKO, and R333Q-hFIX mice (body weight, 25 g; age, 8-10 weeks). Then, 15 μL blood from a tail clip was collected into heparinized capillary tubes at 0.5, 1, 2, 4, 6, 8, 10, and 15 minutes after injection. The exogenous human factor IX level in the plasma was measured by the monoclonal antibody A-1 ELISA with a recombinant human factor IX standard.
AAV2 vector construction and rAAV2 production
The plasmid recombinant adeno-associated virus 2 (rAAV2)-CBA-hFIX was generated using standard recombinant DNA techniques10 and contains a PCR-amplified 1417-bp human FIX cDNA without introns or 5′and 3′ end untranslated region sequence. Inserted immediately upstream of the human FIX cDNA is the cytomegalovirus/chicken β-actin (CBA) promoter, digested as an 1869-bp BglII/EcoRI fragment from the plasmid CMV-β-actin-hAAT-AAV (a gift from Terence R. Flotte, Biochemistry Department, UNC-CH).20,21 A human β-globin/IgG chimeric intron from pCL-neo vector (Promega, Madison, WI) and a 180-bp murine α-globin polyadenylation signal from PHIH-polyA (a gift from W. Marzluff)22 were added downstream of the hFIX cDNA. Finally, a 630-bp fragment of noncoding λ phage DNA (New England BioLabs, Beverly, MA) was inserted between the polyadenylation signal and the downstream AAV terminal repeat to yield an AAV2 expression cassette of the approximate size of the wild-type AAV genome. The expression cassette is flanked by AAV serotype-2 inverted terminal repeats (ITRs). Recombinant AAV2-CBA-hFIX was produced, purified, and titered by dot blot assay for vector genome copy number at the UNC Virus Vector Core Facility using previously described methods.23
FIX Bethesda inhibitor assay
Inhibitor antibodies against human factor IX were measured by Bethesda assay. All mouse plasma samples were heated at 55°C for 30 minutes to inactivate endogenous mouse clotting factor or high levels of human factor IX. The inactivated plasma samples (undiluted or diluted to 1:32 in heat-treated FIXKO mouse plasma) were incubated with an equal volume of pooled normal human plasma (George King Biomedical) at 37°C for 2 hours and clotting activity assayed by APTT (see “Factor IX-specific APTT”). Each Bethesda unit corresponds to the neutralization of 50% of the factor IX clotting activity in standard normal plasma.
Results
Generation of R333Q-hFIX mice
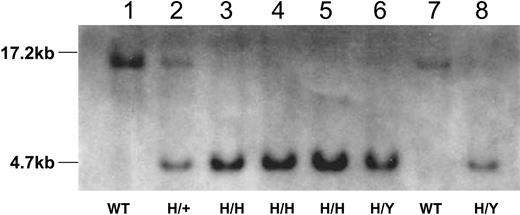
The targeting construct illustrated in Figure 1 was used to replace the mouse factor IX gene with human factor IX R333Q cDNA by homologous recombination. The construct used the signal sequence, first intron, and propeptide of mouse factor IX. Approximately 600 colonies, resistant to both G418 and ganciclovir, were screened by PCR. One clone, positive by PCR, was confirmed by Southern blot. The positive clone was microinjected into blastocysts and 8 chimeras (6 male, 2 female) were generated. Two of the 6 male chimeric mice transmitted the ES cell genome to their F1 offspring (indicated by their agouti coat color). The agouti F1 mice are heterozygous for the targeted R333Q-hFIX gene (and the normal mouse gene) because the human factor IX gene is located on the X chromosome and the TC-1 ES cells used for electroporation were derived from male mice. Hemizygous R333Q-hFIX mice were created by backcrossing heterozygous mice to C57BL/6 male mice. Mice homozygous for human R333Q factor IX were generated by crossing the F2 heterozygous mice with hemizygous mice. The genotypes of all mice were confirmed by both PCR (data not shown) and Southern blot (Figure 2) analysis.
Southern blot analysis of tail DNA from wild-type mice and mice homozygous (H/H), hemizygous (H/Y), heterozygous (H/+) for the R333Q-hFIX mutation. Tail DNA was digested with EcoRV and hybridized to the 730-bp probe. Lanes 1 and 7 show a 17.2-kb band from a wild-type female (WT nonhemophilic) and male (WT nonhemophilic) mouse, respectively. Lanes 3 to 5 show a 4.7-kb band from female R333Q-hFIX mice (H/H, homozygous hemophilic females) and lanes 6 and 8 also show the 4.7-kb band from male mice that are hemizygous for the mutation (H/Y, hemizygous hemophilic males). Lane 2 shows 4.7-kb and 17.2-kb bands from the heterozygous mouse (H/+ nonhemophilic carrier female).
Southern blot analysis of tail DNA from wild-type mice and mice homozygous (H/H), hemizygous (H/Y), heterozygous (H/+) for the R333Q-hFIX mutation. Tail DNA was digested with EcoRV and hybridized to the 730-bp probe. Lanes 1 and 7 show a 17.2-kb band from a wild-type female (WT nonhemophilic) and male (WT nonhemophilic) mouse, respectively. Lanes 3 to 5 show a 4.7-kb band from female R333Q-hFIX mice (H/H, homozygous hemophilic females) and lanes 6 and 8 also show the 4.7-kb band from male mice that are hemizygous for the mutation (H/Y, hemizygous hemophilic males). Lane 2 shows 4.7-kb and 17.2-kb bands from the heterozygous mouse (H/+ nonhemophilic carrier female).
Phenotype of R333Q-hFIX mice
The R333Q-hFIX mice are not morphologically different from wild-type mice. They survive well if no injury occurs. Litters have 4 to 8 pups with a sex ratio of approximately 1:1. When tails of the R333Q-hFIX mice are cut, they bleed and die unless the wound is cauterized.
Clearance of human factor IX from wild-type, FIXKO, and R333Q-hFIX mice
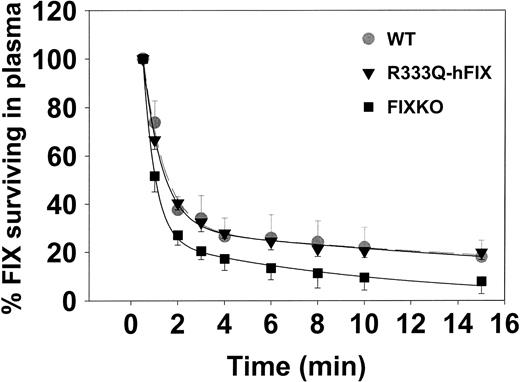
The initial clearance of hFIX from wild-type, FIXKO, and R333Q-hFIX mice is illustrated in Figure 3. Wild-type mice and mice expressing R333Q-hFIX showed essentially identical clearance in the first 15 minutes after injection but infused FIX disappears more rapidly from the knockout mouse.
Clearance of infused human factor IX. Mice (n = 3) producing wild-type factor IX (▾, gray broken line, error bars up only), R333Q-hFIX ( , solid line, error bars down only), or no factor IX (knockout; ▪, error bars down) were injected at zero time with 4 μg recombinant human factor IX via the jugular vein. Samples were collected from the tail vein at serial time points for 15 minutes after infusion, and the survival of the infused Thr148 isoform factor IX was determined by A-1 antibody ELISA. The concentration at the first time point (30 seconds) is defined as 100%.
, solid line, error bars down only), or no factor IX (knockout; ▪, error bars down) were injected at zero time with 4 μg recombinant human factor IX via the jugular vein. Samples were collected from the tail vein at serial time points for 15 minutes after infusion, and the survival of the infused Thr148 isoform factor IX was determined by A-1 antibody ELISA. The concentration at the first time point (30 seconds) is defined as 100%.
Clearance of infused human factor IX. Mice (n = 3) producing wild-type factor IX (▾, gray broken line, error bars up only), R333Q-hFIX ( , solid line, error bars down only), or no factor IX (knockout; ▪, error bars down) were injected at zero time with 4 μg recombinant human factor IX via the jugular vein. Samples were collected from the tail vein at serial time points for 15 minutes after infusion, and the survival of the infused Thr148 isoform factor IX was determined by A-1 antibody ELISA. The concentration at the first time point (30 seconds) is defined as 100%.
, solid line, error bars down only), or no factor IX (knockout; ▪, error bars down) were injected at zero time with 4 μg recombinant human factor IX via the jugular vein. Samples were collected from the tail vein at serial time points for 15 minutes after infusion, and the survival of the infused Thr148 isoform factor IX was determined by A-1 antibody ELISA. The concentration at the first time point (30 seconds) is defined as 100%.
mRNA and antigen levels of R333Q-hFIX in heterozygous, hemizygous, and homozygous mice
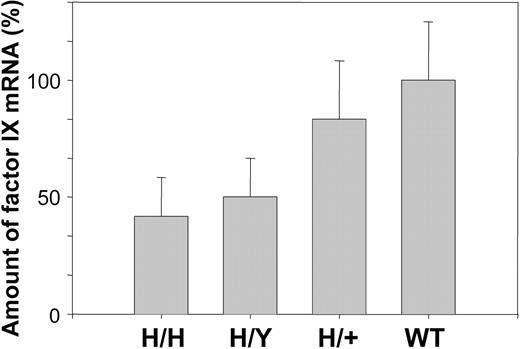
The amount of mRNA was determined by quantitative PCR as described in “Materials and methods.” The level of human factor IX mRNA in hemizygous and homozygous mice is 2- and 2.5-fold lower than that of mouse factor IX in wild-type mice, whereas the combined level of human and mouse factor IX mRNA in heterozygous mice is similar to that of factor IX in wild-type mice (Figure 4).
Comparison of mRNA levels of R333Q-hFIX and wild-type mice. mRNA level was measured by qPCR. The results are given as percentage of wild-type mouse mRNA. Homozygotes (H/H) represent 39%, hemizygotes (H/Y) 48%, and heterozygotes (H/+) 87% of wild-type (WT) mRNA levels. Homozygous refers to the R333Q mouse and heterozygous refers to a mouse with copies of human R333Q and wild-type mouse factor IX. Data represent the mean ± SD.
Comparison of mRNA levels of R333Q-hFIX and wild-type mice. mRNA level was measured by qPCR. The results are given as percentage of wild-type mouse mRNA. Homozygotes (H/H) represent 39%, hemizygotes (H/Y) 48%, and heterozygotes (H/+) 87% of wild-type (WT) mRNA levels. Homozygous refers to the R333Q mouse and heterozygous refers to a mouse with copies of human R333Q and wild-type mouse factor IX. Data represent the mean ± SD.
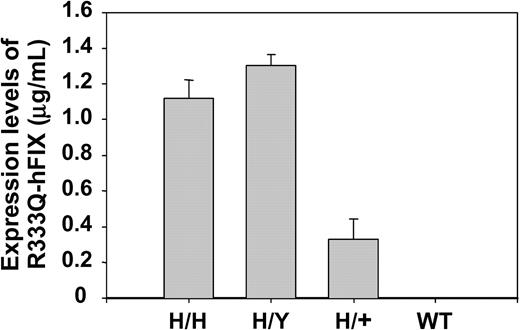
The antigen level of circulating R333Q-hFIX in mice that were homozygous, hemizygous, and heterozygous for the R333Q-hFIX mutation as well as in wild-type (nonhemophilic) mice was measured by ELISA using paired anti-hFIX polyclonal antibodies. These antibodies do not cross-react with the endogenous mouse factor IX because the ELISA detects no antigen in wild-type mice (Figure 5). Figure 5 also shows that the levels of R333Q-hFIX vary depending on the genotype. The levels are homozygous, 1.1 ± 0.1 μg/mL; hemizygous, 1.3 ± 0.1 μg/mL; and heterozygous 0.3 ± 0.1 μg/mL. Factor IX levels are about 0.8 μg/mL in the juvenile R333Q-hFIX mouse and rise to a stable level of approximately 1.2 μg/mL in adulthood (Figure 6).
Expression of R333Q-hFIX. The concentrations of R333Q-hFIX in homozygous (H/H), hemizygous (H/Y), heterozygous (H/+), and wild-type (WT) mice are shown in μg/mL as assayed by ELISA using paired anti-hFIX polyclonal antibodies. The levels are homozygous, 1.1 ± 0.1 μg/mL (n = 4); hemizygous, 1.3 ± 0.1 μg/mL (n = 3); heterozygous, 0.3 ± 0.1 μg/mL (n = 6). Wild-type mouse factor IX is not detectable with this antibody. Data represent the mean ± SD.
Expression of R333Q-hFIX. The concentrations of R333Q-hFIX in homozygous (H/H), hemizygous (H/Y), heterozygous (H/+), and wild-type (WT) mice are shown in μg/mL as assayed by ELISA using paired anti-hFIX polyclonal antibodies. The levels are homozygous, 1.1 ± 0.1 μg/mL (n = 4); hemizygous, 1.3 ± 0.1 μg/mL (n = 3); heterozygous, 0.3 ± 0.1 μg/mL (n = 6). Wild-type mouse factor IX is not detectable with this antibody. Data represent the mean ± SD.
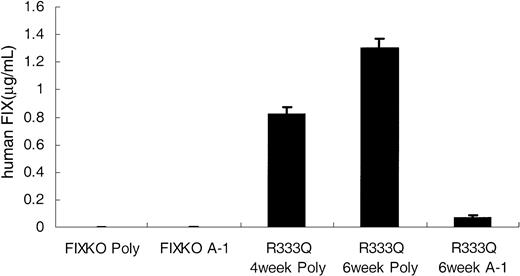
The alanine polymorphism is weakly detected by monoclonal antibody A-1. All mice in the figure are naive (untreated) mice. The left side of the figure shows that neither the polyclonal antibody (poly) nor monoclonal antibody A-1 demonstrates background factor IX reactivity in the knockout mouse. In the right side of the figure the first bar shows the level of factor IX detected by the polyclonal antibody in juvenile R333Q-hFIX mice (age 4 weeks). The second bar shows the level detected by the polyclonal antibody in adult mice (age 6 weeks). The third bar demonstrates the low cross-reactivity of A-1 for the alanine polymorphism of the factor IX expressed in the R333Q-hFIX mice (age 6 weeks). Data represent the mean ± SD.
The alanine polymorphism is weakly detected by monoclonal antibody A-1. All mice in the figure are naive (untreated) mice. The left side of the figure shows that neither the polyclonal antibody (poly) nor monoclonal antibody A-1 demonstrates background factor IX reactivity in the knockout mouse. In the right side of the figure the first bar shows the level of factor IX detected by the polyclonal antibody in juvenile R333Q-hFIX mice (age 4 weeks). The second bar shows the level detected by the polyclonal antibody in adult mice (age 6 weeks). The third bar demonstrates the low cross-reactivity of A-1 for the alanine polymorphism of the factor IX expressed in the R333Q-hFIX mice (age 6 weeks). Data represent the mean ± SD.
A well-characterized Ala/Thr polymorphism occurs at residue 148 within the activation peptide of human factor IX.9 The polyclonal antibodies used above recognize both the alanine and threonine forms. However, the monoclonal antibody A-1 recognizes the threonine isoform preferentially.24 In the original description of A-1 antibody immunoassay of normal human clinical samples the factor IX levels (background) of the Ala148 isoform were 1% to 10% of the levels assayed with a factor IX polyclonal antibody.15 Our values are consistent with these earlier measurements. Figure 6 shows that the low degree of cross-reactivity between the A-1 antibody and the alanine isoform of factor IX carried by untreated R333Q-hFIX mice is consistent with the experience from human samples.
Functional clotting activity of R333Q-hFIX mouse
In clinical practice, severe hemophilia B is defined by a factor IX activity level of less than 1%, which is considered the lower limit of reproducible detection of the APTT assay. We modified the factor IX-specific APTT to detect human factor IX activity in mouse plasma samples using human factor IX-deficient plasma. Human plasma standards were diluted in mouse factor IX-deficient plasma to provide mouse plasma proteins in both the standards and the samples. The clotting activity of wild-type C57BL/6 mice was less than 100% in this assay system, suggesting that mouse factor IX is less active in the human coagulation system than is human factor IX. The measured activity levels of 0.2% in FIXKO mice were below the level of reliable quantification (< 1%). It should be noted that we originally described an APTT factor IX activity level of 8% for the FIXKO mouse.1 Since that time it has become obvious that the relative amount of mouse plasma—even plasma that is completely deficient in factor IX protein—strongly affects the clotting of the human factor IX-deficient plasma in the APTT assay. The normal APTT of the mouse is markedly shorter than that in humans.25,26 Including mouse factor IX-deficient plasma in the standards reduces this artifact and confirms that the plasma of FIXKO mice does not shorten the clotting time of human factor IX-deficient plasma. R333Q-hFIX mice had slightly shortened APTT clotting times when compared to FIXKO mice (122 ± 9 seconds for FIXKO versus 104± 8 seconds for R333Q-hFIX mice); each value corresponds to a factor IX activity of less than 1% (Table 1).
R333Q-hFIX mouse model for hemophilia B gene therapy: lack of anti-hFIX inhibitor antibody response following hFIX expression
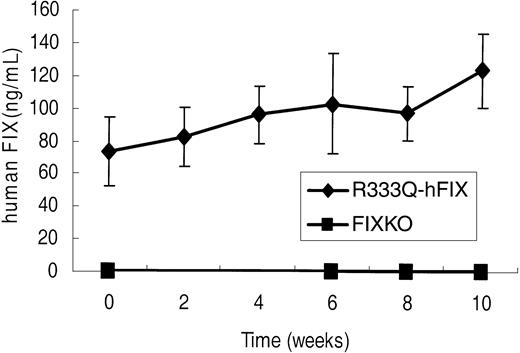
The value of the R333Q-hFIX mouse as a model for gene therapy of human CRM+ factor IX deficiency was assessed by intramuscular injection of an adeno-associated virus serotype 2 vector expressing the human factor IX cDNA. A relatively low dose of 1 × 1011 vector genomes was chosen for 2 reasons. First, doses in this range have been associated with the development of antibodies against the human factor IX xenoprotein in several muscle-directed AAV2 vector studies in wild-type C57BL/6,7,27 wild-type BALB/c,7 wild-type Fv129,7 and FIXKO mice (C57BL/6/129 background).5,28 Second, it was desirable to determine whether even relatively small degrees of expression from a gene therapy vector could be detected in the background of this novel CRM+ model. Following intramuscular injection of the rAAV2-CBA-hFIX vector, no human factor IX could be detected in FIXKO mice before or after infection (Figure 7 lower line). Nevertheless, human factor IX was clearly made in response to gene therapy in the FIXKO mice, because inhibitor antibodies against human factor IX protein were detectable by 4 weeks after treatment (Table 2). These inhibitor antibody titers continued to increase for 3 months following intramuscular gene therapy, and in 4 of 5 FIXKO mice, the titer rose to more than 5 Bethesda inhibitor units. A Bethesda inhibitor titer of more than 5 defines a clinically “high titer” inhibitor antibody.29
hFIX expression following gene therapy. Human factor IX in FIXKO (n = 5) and R333Q-hFIX (n = 6) mice before and after intramuscular rAAV2-CBA-hFIX gene therapy, as detected by immunoassay with monoclonal antibody A-1 (specific for the factor IX Thr148 polymorphism). The Thr148 factor IX is incorporated in the gene therapy vector but is not present in the genome of either strain of mice. Data represent the mean ± SD.
hFIX expression following gene therapy. Human factor IX in FIXKO (n = 5) and R333Q-hFIX (n = 6) mice before and after intramuscular rAAV2-CBA-hFIX gene therapy, as detected by immunoassay with monoclonal antibody A-1 (specific for the factor IX Thr148 polymorphism). The Thr148 factor IX is incorporated in the gene therapy vector but is not present in the genome of either strain of mice. Data represent the mean ± SD.
Gene therapy of R333Q-hFIX mice treated in exactly the same manner as the FIXKO mice, however, led to an increase in both the levels of FIX antigen (Figure 7 upper line) and activity (Table 1). The initial time point of the upper line in Figure 7 shows a relatively high background only because of the low level of cross-reactivity of the factor IX threonine-specific A-1 antibody with the factor IX Ala148 isoform of human factor IX that is expressed in R333Q-hFIX mice. The A-1 antibody assay detects a concentration of human factor IX of 73 ± 12 ng/mL in the R333Q-hFIX mice, whereas the actual factor IX level, assayed by a polyclonal antibody, was 1.2 μg/mL. That is, the background antigen in these experiments was 6%, whereas the background activity was less than 1%. Furthermore, unlike the knockout mouse, and consistent with our hypothesis that mice with human factor IX carrying a missense mutation would exhibit tolerance to factor IX gene therapy, R333Q-hFIX mice did not develop antifactor IX inhibitors following intramuscular injection of rAAV2-CBA-hFIX (Table 2).
Discussion
The goal of this study was to produce a mouse model that more accurately reflects the condition usually observed in human hemophilia B than do the currently available factor IX knockout mice. In human factor IX deficiency, lack of expressed factor IX correlates with an increased risk of inhibitor antibody development. For example, 17 patients with gross deletions and 12 with early stop mutations are known to have developed inhibitor antibodies. Of the 41 patients with inhibitory antibodies whose mutations are reported in the human hemophilia B database, (http://www.kcl.ac.uk/ip/petergreen/haemBdatabase.html), only 2 have missense mutations. Missense mutations, however, represent two thirds of the mutations recorded in the human hemophilia B database,8 and in accordance with this, the risk of inhibitor antibody formation to human factor IX is low, about 2% to 5%. A similar situation appears to hold true for canine hemophilia B. Dogs that express no antigen develop inhibitor antibodies in response to infused purified canine factor IX or to canine factor IX delivered by intramuscular gene therapy.30 In contrast, canine factor IX gene therapy is generally tolerated in dogs with a missense mutation.4,31,32 With specific exceptions, for example, liver-directed therapy,7,32 the FIXKO mice develop antibodies subsequent to therapy.5,7,33-35 Because mice are less expensive to maintain, have shorter gestational periods, and are easier to breed than dogs, a mouse model that more nearly mimics the human disease is desirable. Therefore, because 52 individuals with this mutation have been documented in the Hemophilia B Mutation Database, we made a mouse model expressing human factor IX with the missense mutation R333Q. Twenty-three of 43 patients with this mutation have reported factor IX activity levels less than or equal to 1%, and all but one have reported activity of less than 5% (the higher values probably represent variability in assay reproducibility among clinical laboratories). Moreover, all have factor IX antigen levels more than 30%. Again, most patients have normal levels and it is likely that the lower reported levels are the results of laboratory variability.
We confirmed by Southern blot analysis and mRNA and human factor IX protein determination that human R333Q hFIX replaced mouse factor IX. Our primers for qPCR were designed to recognize both mouse and human mRNA. Using these primers, wild-type and heterozygous females (chimeric for the murine and human sequences) had similar amounts of factor IX mRNA. However, the level of factor IX mRNA was reduced by half in homozygous R333Q-hFIX females and hemizygous males when compared to wild-type C57BL/6 mice. The R333Q-hFIX gene includes mouse intron 1 followed by the cDNA coding for the R333Q-hFIX, and mRNA processing appears to be less efficient than wild-type processing. The presence of human factor IX in the plasma of the R333Q-hFIX mouse was confirmed with a variety of monoclonal and polyclonal anti-FIX antibodies, all specific for the human protein. R333Q-hFIX antigen in the hemostatically normal heterozygous female is lower than in the hemizygous or homozygous mouse. This decrease in protein correlates with the reduction of human mRNA in the heterozygous mice. Wild-type mouse factor IX is not detectable by the anti-human factor IX antibodies used in this study.
The antigen level, expressed as μg/mL, of human R333Q factor IX is lower in the mouse than in humans with this mutation. As mentioned, one explanation may be that the human mRNA with only one intron is not as efficiently processed as the gene with its normal complement of introns. Alternatively, our finding of lower factor IX in the R333Q-hFIX mouse is consistent with the report of Mingozzi et al that the normal level of factor IX in mouse plasma is 2.5 μg/mL, which is half the normal level of human factor IX.36 It is possible that less mRNA is made from the mouse FIX promoter than from the human promoter. Therefore, expression of the R333Q-hFIX under the control of normal mouse transcriptional regulatory factors may result in a circulating factor IX level that is most physiologically relevant for a mouse model of hemophilia. In any case, the hFIX activity level measured in the R333Q mouse is less than 1% and, therefore, consistent with that expected from the human phenotype.
Although factor IX has a relatively long half-life in the circulation, exogenous factor IX undergoes significant clearance immediately after infusion in patients with hemophilia B.37 Rapid distribution from the circulation involves binding to vascular endothelial cell collagen IV, among other sites, and we have shown that mutant factor IX molecules lacking collagen IV binding have decreased initial clearance from circulation of hemophilia B knockout mice.14,38 It has been reported that the recovery of infused factor IX in patients with CRM+ hemophilia B is higher than in those who are CRM-.39 Consistent with this observation, the initial clearance of infused human factor IX in the CRM+ R333Q-hFIX mice is identical to that of wild-type mice with normal circulating mouse factor IX levels. On the other hand, clearance from the FIXKO mice that have no circulating factor IX antigen is faster. However, although there is a measurable difference in clearance, it is small. Thus, either the number of collagen IV-binding sites is very large or there are other significant binding sites for factor IX.
The development of inhibitor antibodies is an infrequent but important complication of current human factor IX protein replacement therapy and frequently complicates animal studies of hemophilia gene therapy. Our hypothesis is that gene therapy with human factor IX in the mouse expressing defective human factor IX will not elicit inhibitor antibodies. To test this hypothesis, we challenged the R333Q-hFIX mouse with an AAV vector expressing functional human factor IX. We chose a vector dose and route of injection that numerous studies had shown were most likely to stimulate the production of inhibitory antibodies in FIXKO mice.5,7,34 Subsequent to gene therapy of R333Q-hFIX mice with the threonine isoform of normal human factor IX an increase in both antigen and activity levels of factor IX are observed. The increase measured by APTT is 6%, whereas the increase measured by immunoassay is equivalent to 1% of the normal human factor IX (and 2% of the normal mouse FIX level). Our experience has been that results from the APTT can appear artifactually high when assaying factor IX activity of one species mixed with plasma proteins of another species (P.E.M., T.G., and J. R. Elia, manuscript in preparation). Nevertheless, for the purposes of characterizing human factor IX tolerance in the R333Q-hFIX mouse, the rAAV dose chosen led to low levels of factor IX produced by gene therapy. The expression of human factor IX resulted in inhibitory antibody formation in only the factor IX knockout mice and not in mice expressing R333Q-hFIX. This observation is consistent with the outcomes of 2 human clinical trials of AAV human factor IX gene therapy, in which none of the 14 patients with hemophilia B (all expressing missense mutations) developed inhibitory antibodies.40,41
The R333Q-hFIX will be a valuable research resource, especially for studies in which large numbers of animals are required (making the use of missense hemophilic dogs impractical). Nevertheless, the use of any animal model to predict antibody responses in human therapeutic approaches requires caution. Other factors in addition to gene defect clearly contribute to the development of inhibitor antibodies in the setting of coagulation protein replacement. Among these are the individual's immune background (eg, HLA type) and local or systemic inflammation/illness concomitant with factor VIII or IX challenge.
In summary, this study describes the generation of a CRM+ mouse model of human hemophilia B arising from the expression of a missense mutant human factor IX gene (R333Q-hFIX). As expected, these mice express antigenically detectable but functionally deficient human factor IX protein. Furthermore, when infected with AAV expressing human factor IX, mice expressing defective human factor IX, as contrasted with FIXKO mice, did not produce neutralizing antibodies. This suggests that the presence of a functionally inactive human factor IX present throughout the development of the mouse results in relative immunologic nonresponsiveness to a human factor IX challenge in the adult R333Q mouse. Therefore, the R333Q-hFIX model will complement the available FIXKO model for preclinical studies of factor IX.
Supported by National Institutes of Health grant PO1 HL 66973.
An Inside Blood analysis of this article appears in the front of this issue.
Prepublished online as Blood First Edition Paper, June 3, 2004; DOI 10.1182/blood-2004-01-0138.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors acknowledge Tomoko Hatada, Hui-Ju Lee, and the Smithies-Maeda Laboratory for their generous help and discussions and David Straight for reading and discussing the manuscript.