Abstract
The importance of genetic factors in etiology of chronic lymphocytic leukemia (CLL) is suggested by family and population studies. However, the spectrum of malignancies sharing common genetic factors with CLL and the effects of sex and age on familial risk are unknown. We used the Swedish Family-Cancer Database to test for increased familial risks of CLL and other lymphoproliferative tumors. Cancer diagnoses from 1958 to 1998 were assessed in 14 336 first-degree relatives of 5918 CLL cases and in 28 876 first-degree relatives of 11 778 controls. Cancer risks in relatives of cases were compared with those in relatives of controls using marginal survival models. Relatives of cases were at significantly increased risk for CLL (relative risk [RR] = 7.52; 95% confidence interval [CI], 3.63-15.56), for non-Hodgkin lymphoma (RR = 1.45; 95% CI, 0.98-2.16), and for Hodgkin lymphoma (RR = 2.35; 95% CI, 1.08-5.08). CLL risks were similar in parents, siblings, and offspring of cases, in male and female relatives, and were not affected by the case's age at diagnosis. Anticipation was not significant when analyzed using life table methods. We conclude that the familial component of CLL is shared with other lymphoproliferative malignances, suggesting common genetic pathways. However, because clinically diagnosed CLL is uncommon, absolute excess risk to relatives is small.
Introduction
B-cell chronic lymphocytic leukemia (B-CLL) is a neoplastic disease characterized by the accumulation of small, mature-appearing lymphocytes in the blood, bone marrow, and lymphoid tissues. CLL accounts for 30% of all leukemia and is the most common form of leukemia among older adults in Western countries. Data from the United States Surveillance, Epidemiology, and End Results (SEER) Registry estimate the U.S. incidence in the period from 1996 to 2000 to be 3.7 per 100 000 with a median age at diagnosis of 72 years (http://seer.cancer.gov/csr/1975_2000/).1 Incidence rates in men are nearly twice as high as in women. Although advanced age, white ancestry, and family history of hematologic malignancies are recognized risk factors, the etiology of CLL is unknown. Case-control studies have evaluated diverse environmental and occupational exposures such as radiation, magnetic fields, viruses, and pesticides but have not found consistent associations,2 with the exception of a small increase in risk related to farming exposures.3 Family history of CLL or other hematolymphoproliferative (HLP) cancers, on the other hand, has consistently been identified as a risk factor for CLL.4-9 Clinical descriptions of CLL families have appeared in the literature over a number of years,10 including a recently described series of 32 CLL families seen at the National Cancer Institute.11 However, there is no consistent pattern of illness in families that would suggest a common mode of genetic transmission, although age at onset in the familial cases is generally reported to be earlier than in sporadic CLL cases. In families with affected individuals in 2 generations, decreasing age at onset of illness between generations (anticipation) has been observed.12-14
A number of somatic chromosome abnormalities as well as mutations in single genes (ATM and p53) have been described in CLL tumor cells,15,16 but no germ-line changes have been found that could account for familial CLL.
Despite the evidence suggesting a familial risk component in CLL, questions remain regarding the spectrum of other tumors associated with CLL and the effects of sex and age at diagnosis of the cases on familial risk. The availability of a very large familial cancer database in Sweden17 allowed us to quantify the degree of familial aggregation of CLL and related lymphoproliferative (LP) malignancies using population-based data. The present study is unique in several ways compared with earlier epidemiologic studies. First, previous investigations often considered all leukemias and lymphomas combined because of small sample sizes. Earlier analyses of the Swedish Family-Cancer Database have computed familial risks for all leukemia without reference to specific subtypes.18 Second, earlier studies did not compute risks separately for subgroups (such as sex or age) of CLL cases or relatives. Third, none of the previous studies evaluated the risks of familial aggregation simultaneously for the entire spectrum of LP malignancies. Taking advantage of the large population, we assessed risk not only for CLL but for other types of LP tumors separately in relatives of CLL cases. In contrast to procedures that rely on external population rates, we used a case-control design with a survival model that compares risks in first-degree relatives of CLL case patients with risks in first-degree relatives of matched controls, taking advantage of the age of onset information in relatives. Our novel analytic approach accounted for correlation among related individuals, truncation in the data due to start dates of cancer registrations, and complete ascertainment of all CLL cases in the population. We also incorporated heterogeneity in aggregation by sex, type of relative, and age at onset of the CLL case proband. In addition to detailed risk estimates, the availability of 40 years of cancer diagnoses also allowed us to evaluate anticipation in age at diagnosis in an unbiased way.
For CLL patients and clinical practitioners, our risks derive from large numbers and are population based so they are more accurate than are estimates derived from small clinical samples or epidemiologic studies in highly selected populations. Our findings regarding the spectrum of LP malignancies that aggregate together in families also inform strategies for mapping susceptibility genes in high-risk families and testing candidate genes in families and populations.
Patients, materials, and methods
Swedish Family-Cancer Database
The Swedish Family-Cancer Database has been described in detail.17 Briefly, Sweden maintains a multigeneration register consisting of individuals born in 1932 and later (referred to as “offspring”) with links to their parents. The multigeneration registry has been merged with the Swedish Cancer Registry (containing all cancers registered from 1958 to 1998) to create the Family-Cancer Database. Demographic and vital status information was obtained by linking this database to the nationwide census and death notification databases, respectively. The current version of the database from which we drew our data contains 10.2 million individuals and includes 75% of all tumors registered in the Swedish cancer registry. About half of offspring who died before 1991 (and 12% of offspring with cancer) do not have links to parents. All offspring who died before 1960 are missing from the database. We used a case-control design and sampled from the Family-Cancer Database all individuals with a primary diagnosis of chronic lymphocytic leukemia (ICD7: 204.1). For each case, 2 cancer-free controls were chosen from the Family-Cancer Database and matched by sex, year of birth, and county of residence. County of residence was used as a matching criterion to allow for regional variability over time in reporting of cancers to the central registry. For each case and control, referred to as “probands,” all first-degree relatives were included in the data set. Although the CLL probands were selected only if they had a first primary diagnosis of CLL, first-degree relatives were classified as affected or not based on considering up to 3 primary cancer registrations. In this study, we report on analyses of 14 336 first-degree relatives of 5918 CLL probands and 28 876 first-degree relatives of 11 778 control probands.
Statistical analysis
The statistical approach is based on a model proposed by Liang19 and described in detail elsewhere.20 Briefly, we applied a marginal survival model, where tij denotes the age at onset of disease or the age at censoring for member j in family i. The outcome tij is modeled by a marginal proportional hazards model, λ(tij Xij|Zij) =λ0 (tij) exp(β Xij + γ Zij). λ0 is the arbitrary baseline hazard function, Xij denotes measured covariates for that individual (in our analysis, sex, type of relative, and age at onset of the proband), and Zij is an indicator of the proband's disease status (Zij = 1 if the proband of family i is a case and 0 otherwise). Testing for familial aggregation corresponds to testing the null hypothesis H0: γ= 0 (ie, hazard ratio = 1). The parameters β and γ are estimated under a working independence assumption (PROC PHREG, SAS v8.02; SAS, Cary, NC). The robust sandwich covariance matrix accounts for the dependence of the family members.21 We use the term “relative risk” to denote the hazard ratio defined in the equations above.
An individual entered the risk period at his or her age at the start of cancer registrations (1958) or at date of birth (or immigration) if later. Censoring events were death, emigration, or the end of the data acquisition period (1998). Individuals were not censored if they developed a cancer other than the LP tumor being tested because they will still be at risk for developing LP as a subsequent tumor. We tested separately for increased risk of CLL, non-Hodgkin lymphoma (NHL), Hodgkin lymphoma (HL), and multiple myeloma (MM) in relatives and also tested for increased risk of developing any one of the 4 tumors considered as a combined entity. We also considered other factors affecting risk by including sex, type of relative, and age of disease onset in the case proband in the same model. We compared the risk in siblings with that in parents and offspring, because a recessive gene would predict a higher risk in siblings compared with parents and offspring whereas a dominant gene would predict equal risks in siblings, parents, and offspring. For many complex diseases, early age at diagnosis is a feature of strong genetic susceptibility. While familial CLL is thought to be associated with earlier age at diagnosis, there is no obvious threshold to divide the CLL probands into early and late onset of disease. In this study, we classified the probands as early versus late using age at diagnosis of 65 years and under as early, following SEER reporting standards.1 To test for anticipation, we compared the average age at diagnosis of CLL in parents and offspring of cases. In addition, we computed Kaplan-Meier estimates of risk of CLL by age and tested for homogeneity of parent and offspring strata using nonparametric tests (PROC LIFETEST, SAS v8.02; SAS).
As an exploratory analysis, we tested whether other cancer sites (including leukemias other than CLL) were more common in CLL relatives than in control relatives by standard χ2 2 × 2 table comparisons.
Results
Familial aggregation
There were 5918 CLL probands and 11 778 matched control probands in the sample. The male to female ratio among probands was 1.86, which is consistent with the approximately 2-fold higher incidence rates in men in SEER.1 The probands were born from 1875 to 1973, with about 90% born before 1932 (ie, arising from the “parental” generation). Table 1 shows the distribution of numbers and types of first-degree relatives, offspring being the largest group of relatives. The median age at diagnosis of CLL patients was 69 years (mean, 67.6 years; SD, 10.7 years).
The numbers and proportions of relatives of cases and controls diagnosed with CLL, NHL, HL, MM, and any of the 4 are shown in Table 2. Relatives of CLL cases were at higher risks than relatives of controls for each LP tumor except for MM. The relative risks for familial effects based on survival analyses are shown in Table 3. The relative risk estimate for CLL associated with being a relative of a CLL proband was 7.52 and highly significant. The risk of HL was also significantly increased in case relatives compared with control relatives (relative risk [RR] = 2.35, P = .03), and the risk of NHL was increased (RR = 1.45, P = .06). We tested whether sex, type of relative, and age at diagnosis of proband between case and control relatives were significant predictors in the model. None of these effects were significant. To compare with other studies, Table 3 shows risk estimates stratified by these factors. The familial risks of CLL and NHL were slightly higher in women, whereas the risks of the other tumors were higher in men. The risk of CLL was roughly the same in parents, offspring, and siblings, whereas the risk of NHL appeared to be somewhat higher in parents. For HL and MM, there were not enough cases to assess risk by type of relative. The CLL and NHL familial risks were somewhat higher among the relatives of younger probands, whereas HL and MM had higher risks among relatives of the older age group probands. These results do not support the hypothesis that probands with a younger age at onset are more likely to have a genetic subtype of CLL. It is possible that stronger genetic factors are associated with very young-onset CLL (50 years or younger), but there were not enough cases in this category in our data to allow for meaningful comparisons in the relatives.
Age at onset of familial and sporadic cases
Our sample contained a total of 44 familial CLL cases, 3 of whom had CLL as a second primary tumor and were not themselves probands. The mean age at diagnosis of the 44 familial CLL cases was 60.4 years, which was significantly lower than the mean age (67.7 years) for the remaining 5877 sporadic cases. However, the 11 CLL cases in relatives of controls had an average age at diagnosis of 62.7 years, which was not significantly different from that in the 44 familial cases using the nonparametric Wilcoxon test (Table 4). Ages at diagnosis for the other LP tumors show similar trends (Table 4). For example, mean age at diagnosis for the 42 relatives of CLL cases who had NHL was 56.0 years and was not significantly different from the mean age at diagnosis (53.4 years) in the 58 relatives of control probands who had NHL. Both of these were lower than the mean age at diagnosis of NHL (67.6 years) in the sample of 19 651 cases from the Family-Cancer Database. Thus, our data do not support previous findings of earlier ages of onset for familial CLL cases.
Anticipation
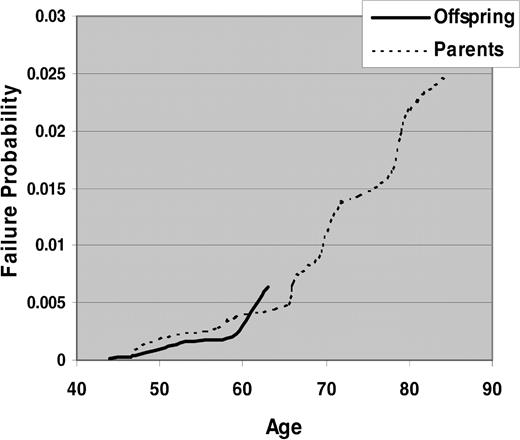
To test for anticipation, we studied 20 parent-offspring pairs in our data. Consistent with published reports, the average age at diagnosis of parents was 69.1 years and of offspring was 52.6 years. However, parents with an early age at onset of CLL would not appear in the database if they were diagnosed before cancer registrations took place (before 1958), which would lead to ascertainment bias. Similarly, at the end of case selection in 1998, many offspring were not old enough to develop CLL. Figure 1 shows the survival curves for CLL in parents and offspring separately. There was no statistically significant difference between the 2 strata using nonparametric tests. Thus, the data do not support the anticipation hypothesis.
Kaplan-Meier estimates of risk of CLL by age in parents versus offspring of CLL cases.
Kaplan-Meier estimates of risk of CLL by age in parents versus offspring of CLL cases.
Other cancer sites in relatives
Leukemias other than CLL (ALL, AML, and CML) were all rare outcomes and did not aggregate in case relatives. Analyses of other solid tumor sites found none that were significantly more common in case than in control relatives.
Discussion
This study shows a significant familial aggregation of CLL and other related LP tumors. Due to the large sample size, we were able to detect not only a significantly elevated relative risk of CLL in first-degree relatives of cases but also elevated risks of NHL (RR = 1.45, P = .06) and HL (RR = 2.35, P = .03). There was no increased risk of MM, although there was a nonsignificant excess in male relatives. While several other studies showed increased risk of CLL associated with family history of leukemia, lymphoma, or all HLP cancers,4-8 none of them had sufficient power to test more specific outcomes. We found no consistent sex differences in familial risk of the 4 LP tumors when treated as separate outcomes. Table 3 shows a slightly higher familial risk of CLL in women that was not significant. This contrasts with the findings from the Utah registries of a higher familial risk of CLL in female compared with male cases9 and the study by Linet et al,4 which found a higher risk of HLP in female relatives of CLL cases than in male relatives.
We found similar risks in parents, offspring, and siblings, which argues in favor of dominant or codominant gene effects, because a recessive gene(s) would predict higher risks in siblings. However, given the rarity of CLL in the population, such genes would likely be less common than the single genes found for breast cancer, melanoma, and colon cancer. In contrast, a smaller study by Pottern et al6 found that a history of leukemia or lymphoma in siblings but not parents was a significant predictor of CLL. The study by Linet et al4 found a significant increase of leukemia and all HLP cancers in both parents and siblings.
This study also provides evidence of shared genetic etiology among LP tumors. In addition to being at high risk for CLL, first-degree relatives of cases were also at higher risk for NHL and HL but not for MM. Linet et al4 also noted an increased risk of HL in parents of CLL cases. Other data imply common etiology of these tumors. Jaffe et al22 have described patients who develop composite HL/NHL or HL/CLL where the 2 tumors coexist within the same biopsy sample and are shown to be clonally related. CLL cases are also at an increased risk for developing HL and NHL (Richter syndrome), and the second malignancies are thought to derive from clonal evolution.23 Notably, MM cases do not consistently develop second LP malignancies, and we also found no increased familial risk of MM in the relatives of CLL patients.
Consistent with the literature, familial CLL cases in our study had an earlier age at diagnosis than did sporadic cases. However, CLL cases occurring in relatives of controls were similar to the familial cases in age at diagnosis. Because cases and control probands were matched by age, the distribution of ages of first-degree relatives was similar, making this a better comparison than simply dividing the CLL cases from the Family-Cancer Database into familial and sporadic. There were no significant differences in the relative risks of relatives of early- versus late-onset cases when probands were stratified at age at diagnosis of less than 65 years compared with 65 or greater. In contrast, the Utah study found increased risk only in late-onset (more than 65) probands.9 Thus, our data do not support an association of familial risk and age at diagnosis.
Evidence for anticipation where offspring have significantly lower age at onset of CLL than do their parents has been reported in several studies of familial samples.12-14 Our data seem consistent with this hypothesis, where 18 of 20 parent-offspring pairs had a lower age at onset in the offspring than in the parent. However, biases due to censoring of observed ages in offspring may give rise to observed anticipation and have been described previously.24-26 In our study, an additional bias is truncation of parents who were diagnosed before the start year of the cancer registry in 1958. Applying the test statistics proposed by Rabinowitz and Yang26 also showed no difference between parent-offspring pairs (results not shown), and survival analysis as well showed no parent-offspring difference (Figure 1).
Other case-control studies that have addressed associations of other solid tumors with CLL in families found increased risk of kidney and breast4 and stomach and prostate cancers.6 In our data, there was no significant increased occurrence of other solid tumors in case relatives.
To eliminate possible ascertainment bias, some studies start the follow-up period of the relatives at the date at birth or diagnosis of the case.27 In our study, case and control probands were matched, and we assumed that any bias was similar in case and control relatives. However, because many individuals born before 1991 are missing from the Swedish database,17 our estimates could be subject to survival bias. Relative risks based only on outcomes from 1991 and later, where we know that the database is most complete, were very close to those computed when all of the data are included, indicating that the familial aggregation we see is not a result of survival bias.
Because CLL is often diagnosed asymptomatically, the published population rates are likely to be underestimates of true rates. However, this does affect our estimates of familial aggregation, because they are based on case-control comparisons. It could be argued that close relatives of CLL cases are under increased surveillance for CLL due to their having an affected relative. While this is certainly the case in studies of “heavily loaded” CLL families (similar to studies of families with multiple cases of breast cancer) this bias is not likely to be operating in the general population, especially over the long time period we have examined in the Swedish study. In addition, we did not find any families with more than 2 cases, and the average lag in year of diagnosis between the 2 cases was 14 years. The knowledge of the familial component for CLL is a recent phenomenon, because relevant population-based studies have appeared only since the late 1980s.4-9 If there were a surveillance bias, this should be more evident during the later years of the Swedish database. When we divided the 40-year risk period of our study (1958 to 1998) into 2 equal halves, there was no significant difference in relative risk of CLL in first-degree relatives between the 2 periods (results not shown).
The strengths of this study were the large sample size and unbiased assessment of cancer status in relatives. On the other hand, we did not have information about any risk factors for LP malignancies, which may be correlated within families. There was no clinical information related to the outcome (stage at diagnosis, course of illness) or other biologic markers (cytogenetics, VH somatic mutations) that might be important predictors of familial risk.
Importantly, even though the risk to relatives of CLL cases is significantly increased compared with the risks in relatives of controls, the absolute excess risk of CLL is small. From SEER data,1 the lifetime risk of developing CLL is 0.41% (0.51% in men and 0.31% in women). Even if we apply the relative risk of 7.52 (6.93 in men, 8.59 in women), the risk to all first-degree relatives is increased to 3.05% (3.53% in men and 2.69% in women). However, as stated above, clinically diagnosed CLL may represent only a proportion of CLL-related phenotypes. Recently, it has been shown that 3.5% of adults over age 40 years28 and 5% or more of the elderly28,29 have a monoclonal B-lymphocyte expansion in the presence of normal blood counts. This subclinical “CLL-like” phenotype is found at a significantly higher rate (13.5% to 18%) among unaffected first-degree relatives of CLL cases in families selected for the presence of 2 or more CLL cases.30,31 This adds further support to the hypothesis that not only clinically diagnosed CLL aggregates in families but that a subclinical phenotype also shares common susceptibility factors with CLL.
Several candidate genes (ATM, HLA) have been ruled out as common causes of familial CLL.32-35 In a genomewide scan of 18 CLL families,36 we did not identify any significant linkage regions segregating in families, although a few regions had elevated linkage statistics, including regions on chromosomes 12, 13, 6, and 17 that overlap with cytogenetic abnormalities found in CLL. Linkage studies with larger sample sizes are clearly needed. The significant familial aggregation shown here justifies the continued application of gene-mapping approaches in high-risk families and suggests that within families the same gene may lead to expression of a range of phenotypes.
Prepublished online as Blood First Edition Paper, May 25, 2004; DOI 10.1182/blood-2004-01-0341.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Emily Steplowski and Shannon Merkle, Information Management Services, Silver Spring, MD, for their assistance with data preparation and Drs Neil Caporaso and Margaret Tucker for their comments. The Swedish Family-Cancer Database was created by linking registers maintained at Statistics Sweden and the Swedish Cancer Registry.