Abstract
We have investigated the potential role of CD1d-restricted natural killer T (NKT) cells in the development of atherosclerosis in mice. When fed an atherogenic diet (AD), NKT cell-deficient CD1d-/- mice had significantly smaller atherosclerotic lesions than AD-fed C57BL/6 (wild-type [WT]) mice. A significant reduction in atherosclerotic lesions was also demonstrated in AD-fed, low-density lipoprotein receptor-deficient (Ldlr-/-) mice reconstituted with CD1d-/- bone marrow cells compared with the lesions observed in Ldlr-/-mice reconstituted with WT marrow cells. In addition, repeated injections of α-GalCer or the related glycolipid OCH to apolipoprotein E knockout (apoE-/-) mice during the early phase of atherosclerosis significantly enlarged the lesion areas compared with mice injected with vehicle control. However, administering α-GalCer to apoE-/- mice with established lesions did not significantly increase the lesion area but considerably decreased the collagen content. Atherosclerosis development in either AD-fed WT or apoE-/- mice was associated with the presence of Vα14Jα18 transcripts in the atherosclerotic arterial walls, indicating that NKT cells were recruited to these lesions. Thioglycolate-elicited macrophages pulsed with oxidized low-density lipoproteins expressed enhanced CD1d levels and induced NKT cells to produce interferon-γ, a potentially proatherogenic T-helper 1 (TH1) cytokine. Collectively, we conclude that NKT cells are proatherogenic in mice. (Blood. 2004;104:2051-2059)
Introduction
Atherosclerosis is an inflammatory vascular disease that involves components of the innate and acquired immune systems.1-3 Several studies have suggested that lymphocytes, which are detected in atherosclerotic lesions in humans and mice,4,5 play a proatherogenic role.6-8 Recently, the role of distinct lymphocyte subsets in the development of atherosclerosis has been evaluated. For example, emerging evidence indicates that T-helper 1 (TH1) cells are proatherogenic,9 whereas TH2 cells are antiatherogenic.10,11 These observations are further supported by the finding that TH1 cytokines (eg, interferon-γ [IFN-γ and interleukin-12 [IL-12]) are important in the progression of atherosclerosis12-15 and that, among TH2 cytokines, IL-10 is antiatherogenic.16 On the other hand, recent studies have suggested that B cells play a protective role in atherogenesis.17,18
Natural killer T (NKT) cells are a unique subset of lymphocytes that have surface markers and functions of T cells and NK cells.19-23 Several characteristics of NKT cells suggest that they may play a role in the atherogenic process. Most NKT cells express an invariant Vα14Jα18 T-cell receptor (TCR)-Vα chain paired with a restricted set of TCR-Vβ chains. These classical NKT cells recognize lipid antigens presented by the major histocompatibility complex (MHC) class 1-like molecule CD1d, produce copious amounts of IFN-γ and IL-4 on activation,22 and constitutively express Fas-ligand.23 Moreover, NKT cells play a protective role in several autoimmune diseases, infections, and tumor progression/metastasis.20 Protective effects of NKT cells and their ligands in autoimmunity are largely attributed to their capacity to promote TH2 immune responses.24,25 However, in some situations, NKT cells can contribute to the development of TH1 immune responses as well.26 Therefore, it was difficult to predict whether NKT cells would play a proatherogenic or an antiatherogenic role.2
To date, few studies have investigated the role of CD1d and CD1d-dependent T cells in atherogenesis. CD1d-expressing cells are present in human atherosclerotic plaques,27 suggesting that NKT cells may be recruited to the lesions. Furthermore, treatment of apolipoprotein E knockout (apoE-/-) mice,28 a model of severe atherosclerosis, with lipopolysaccharide (LPS) resulted in NKT cell recruitment to the atherosclerotic plaques.29 However, whether NKT cells are directly involved in the development or the regulation of atherosclerosis remains to be investigated.
In the present study, we compared atherosclerotic lesions induced by an atherogenic diet (AD) between NKT cell-deficient CD1d-/-30 and wild-type C57BL/6 (WT) mice and between low-density lipoprotein receptor-deficient (Ldlr-/-) mice31 reconstituted with bone marrow (BM) cells from CD1d-/- mice and Ldlr-/- mice reconstituted with BM of WT mice. Moreover, we examined whether NKT cell ligands (α-galactosylceramide32 (α-GalCer) and OCH33 ) could modulate atherogenesis in apoE-/-mice. Our findings consistently demonstrated that NKT cells played a proatherogenic role. Possible mechanisms underlying the proatherogenic role of NKT cells are discussed.
Materials and methods
Glycolipids
α-GalCer (Pharmaceutical Research Laboratories, Kirin Brewery, Gunma, Japan) and OCH were dissolved in either 0.5% polysorbate-20 at 220 μg/mL or dimethyl sulfoxide at 100 μg/mL, respectively, and were further diluted with phosphate-buffered saline (PBS) before use.
Mice
Female WT (Japan SLC, Hamamatsu, Japan), CD1d-/-30 (Vanderbilt University, Nashville, TN), Jα18-/-34 (Chiba University, Chiba, Japan), Ldlr-/-, and apoE-/- (The Jackson Laboratory, Bar Harbor, ME) mice with the C57BL/6 genetic background were used throughout the study. WT and CD1d-/- mice were fed a regular chow diet or the atherogenic diet (AD) (15% fat, 1.25% cholesterol, and 0.5% cholic acid; Nihon-nohsan, Yokohama, Japan) from 10 to 30 weeks of age. All animal care and experimental procedures conformed to the regulations of the Committee of Hokkaido University on Animal Experimentation.
BMT
Bone marrow transplantation (BMT) was performed with lethally irradiated (9.5 Gy) Ldlr-/- mice as recipients, as previously described.35 Briefly, recipient mice were injected with T cell-depleted BM cells (5 × 106) from WT mice (Thy1.1 in BMT protocol, referred to as [WT→Ldlr-/-]), CD1d-/- mice ([CD1d-/-→Ldlr-/-]), or Ldlr-/- mice ([Ldlr-/-→Ldlr-/-]). Treated mice were administered oxytetracyclin (Pfizer Japan, Tokyo, Japan) in drinking water for 4 weeks and then placed on the AD for 5 weeks. Reconstitution was assessed by evaluating thymocyte expression of CD1d for CD1d-/- donors and both Thy1.1 (donor) and Thy1.2 (recipient) for WT donors using flow cytometry.
Induction of atherosclerotic lesions
Early-phase studies. ApoE-/- mice were divided into 4 groups (n = 10 each): 1 group received intraperitoneal (intraperitoneal) injections of 0.1 μg/g body weight (BW) α-GalCer; 1 group received its vehicle; and the remaining groups were administered 0.3 μg/g BW OCH or its vehicle, respectively. Injections were started at 8 weeks of age and repeated every 2 weeks. At 13 weeks of age, mice were killed and used for experiments. Blood samples were consecutively collected from the retro-orbital plexus at 0, 2, 5, 12, 24, 48, and 72 hours after injection of either α-GalCer or OCH, and the levels of IFN-γ and IL-4 were quantitated using enzyme-linked immunosorbent assay (ELISA; Biosource, Camarillo, CA).
Late-phase studies. Ten mice received intraperitoneal injections of 0.1 μg/g BW α-GalCer or vehicle every week starting from 8 weeks of age. One week after the eleventh injection, mice were killed and used for experiments.
Serum chemistry
Amounts of total cholesterol, high-density lipoprotein (HDL) cholesterol, and triglyceride concentrations in sera were determined with colorimetric assay kits (Kyowa Medex, Tokyo, and Serotekku, Sapporo, Japan). Individual serum alanine aminotransferase and total bilirubin were quantitated using the Fuji Drychem system (Fujifilm Medical, Osaka, Japan).
Quantitative analyses of atherosclerotic lesion areas
Atherosclerotic lesions were analyzed as previously described.35 In brief, the basal portion of the heart and proximal aortic root were excised and embedded in OCT compound and frozen in liquid nitrogen. Eight serial cryosections of 10-μm thickness at 80-μm intervals throughout the aortic sinus were stained with oil red O (Sigma, St Louis, MO) and hematoxylin. Lesion images were captured with an Olympus BX50 microscope (Tokyo, Japan) equipped with a Fujix HC-300Z/OL digital camera (Fijifilm, Kanagawa, Japan) and Photograb-300 SH-3 software (Fujifilm). Captured images were further analyzed with Scion Image software (Scion, Frederick, MD). For advanced lesions, the entire aorta was examined using the en face method, as described elsewhere.36
Characterization of atherosclerotic lesions
Immunohistochemistry was performed on 8-μm thick cryosections, as previously described.37 Rat monoclonal antibodies (mAbs) to mouse macrophages (MOMA-2; Serotec, Oxford, United Kingdom), hamster antimouse CD3 (BD Biosciences, San Jose, CA), anti-α-smooth muscle actin (DAKO, Glostrup, Denmark), rat antimouse IFN-γ (BioSource), rat antimouse IL-10 (Endogen, Woburn, MA), biotinylated secondary antibodies to the respective primary reagents, and streptavidin-horseradish peroxidase (DAKO) were used for detection. Signals were developed with DBA kits (Vector Laboratories, Burlingame, CA). The number of CD3+ cells per cross-section of lesion area was counted at × 400 magnification. Elastica-Masson staining was performed to analyze the composition of the lesion using 3 aortic cross-sections per animal from 10 animals. The percentage of collagen-rich matrix areas among the total lesion areas was defined as collagen contents. Total cell numbers per lesion were also counted.
RT-PCR
WT (fed the chow diet or the AD), apoE-/-, and Jα18-/- mice were killed after overnight fasting. After whole body perfusion with cold RNase-free PBS, aortae from the ascending portion to the end of the thoracic aorta were removed, dissected longitudinally, and washed meticulously in cold PBS to remove attached hematocytes and tissue fragments outside the aortae. RNA extraction and reverse transcription-polymerase chain reaction (RT-PCR) were performed as described previously.38
Flow cytometry
Splenocytes were prepared by lysing red blood cells with Tris-NH4Cl solution. Hepatic mononuclear cells (HMNCs) were isolated using 33% Percoll (Amersham Pharmacia Biotech, Piscataway, NJ), as previously reported.39 Cells were incubated with 2.4G2 mAb (anti-FcγR) to block nonspecific staining and were stained with a combination of the following mAb conjugates: for lymphocytes—biotinylated anti-Thy1.1 (OX7), fluorescein isothiocyanate (FITC) anti-Thy1.2 (Coulter, Miami, FL), anti-CD1d (1B1), anti-TCRβ (H57-597), and phycoerythrin (PE) anti-NK1.1 (PK136) (all from BD Biosciences, except Thy1.2); for macrophages—biotinylated anti-H-2Kb (AF6-88.5), anti-I-Ab (AF6-120.1), anti-CD40 (3/23), and antimouse (BALB/c) immunoglobulin G2aκ (IgG2aκ) (G155-178; BD Biosciences), FITC anti-CD1d (1B1) and -rat IgG2b (LODNP57; Immunotech, Marseille, France), and PE anti-Mac-1 (CL8941; Cedarlane, Hornby, Ontario, Canada). Streptavidin-allophycocyanin (APC) (BD Biosciences) was used for detection of biotinylated mAb. Mouse CD1d/α-GalCer tetramers were prepared as previously described.40 Cells were incubated with FITC anti-TCRβ and PE anti-NK1.1 and then with APC α-GalCer-loaded CD1d tetramers. Propidium iodide (Sigma) positive cells were electronically gated out from the analysis, and stained cells were analyzed using a FACSCalibur flow cytometer, as described elsewhere.38
In vitro culture of splenocytes from AD- or chow-fed WT mice treated with α-GalCer
Splenocytes were obtained from either AD- or chow-fed WT mice 2 to 12 hours after intravenous injection with 0.1 μg/g BW α-GalCer. Cells were suspended in RPMI 1640 supplemented with 10% fetal calf serum, 100 U/mL penicillin, 100 μg/mL streptomycin, and 5 × 10-5 M 2-mercaptoethanol (culture medium) and were cultured in 24-well plates at 5 × 106/mL for 1.5 hours without additional stimulation. Culture supernatants were harvested and quantitated for IL-4 levels with ELISA kits (Biosource) and for IFN-γ and IL-10 with Cytometric Bead Array kits (BD Biosciences) by flow cytometry, according to the manufacturer's instructions.
Response of HMNCs to oxidized low-density lipoprotein in vitro
Peritoneal cells were harvested from young WT or CD1d-/- mice 4 days after intraperitoneal injection of 4.05% thioglycolate. Cells were suspended at a concentration of 2 × 106/mL in culture medium, incubated at 37°C for 24 to 48 hours with LDL, oxidized LDL (OxLDL) (10 and 50 μg/mL; Biomedical Technologies, Stoughton, MA), or vehicle alone, and used for flow cytometric analysis. For cytokine analysis, the peritoneal cells (2 × 105/well) were cultured in 96-well plates at 37°C for 2 hours and were washed to remove nonadherent cells. Adherent cells were incubated at 37°C for 48 hours with LDL or OXLDL. After incubation, each well was washed 3 times, and the adherent macrophages were irradiated with 30 Gy x-rays. HMNCs isolated from WT mice (2 × 105/well) were cultured with these macrophages in the presence of recombinant human IL-2 (1000 U/mL; Takeda Chemical Industries, Osaka, Japan) for 24 hours. The supernatant was quantitated for IFN-γ and IL-4 levels with ELISA kits (Biosource).
Statistical analysis
Results were expressed as mean ± SE. Statistical analysis was performed using the Student t test or the Mann-Whitney U rank sum test. All data analyses were performed using Statview software (Abacus Concepts, Berkeley, CA). Values with P < .05 were considered statistically significant.
Results
Development of atherosclerotic lesions in CD1d-/- mice
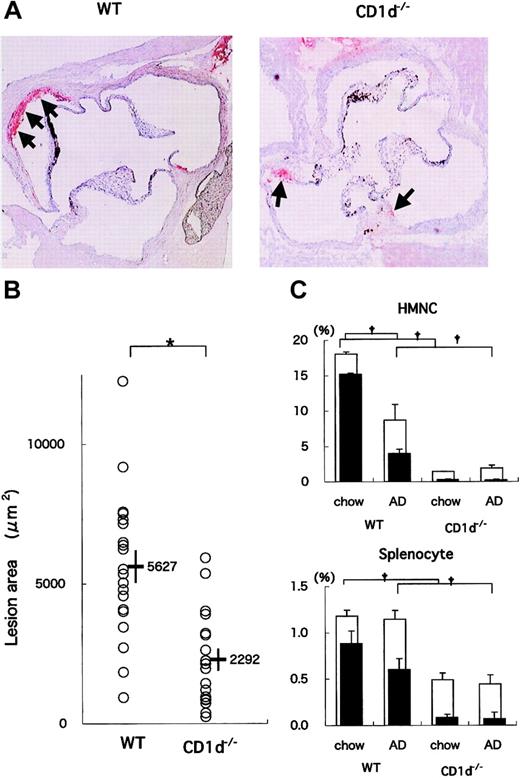
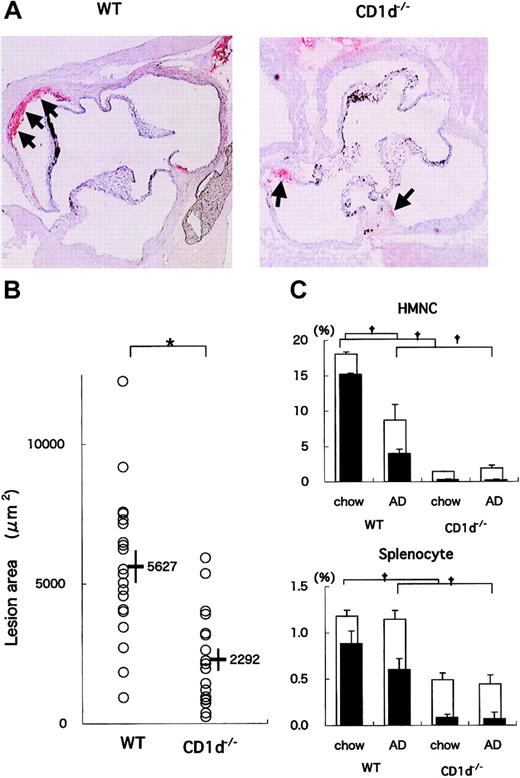
WT (n = 20) and CD1d-/- (n = 18) mice were fed on the AD for 20 weeks. All mice on the AD appeared generally to be in good health throughout the study except for the development of diet-induced liver steatosis and its consequential liver damage. When the sizes of atherosclerotic lesions in aortae were compared, the lesions in CD1d-/- mice were smaller than those in WT mice (Figure 1A). Mean lesion areas in CD1d-/- mice (2292 ± 397 μm2) were significantly smaller than those in WT mice (5627 ± 580 μm2) (P = .014) (Figure 1B). These findings demonstrate that CD1d deficiency reduces atherosclerotic lesions. Concerning serum lipid profiles, total cholesterol, HDL cholesterol, and triglyceride levels were not significantly different between WT (137.9 ± 8.0 mg/dL, 36.3 ± 1.6 mg/dL, and 57.5 ± 3.4 mg/dL, respectively) and CD1d-/- (140.2 ± 14.7 mg/dL, 38.1 ± 2.4 mg/dL, and 59.9 ± 4.4 mg/dL, respectively) mice. Histologic findings of liver sections stained with hematoxylin and eosin revealed typical steatosis to similar extents for WT and CD1d-/- mice fed on the AD (data not shown). Serum alanine aminotransferase and total bilirubin levels in WT and CD1d-/- mice also decreased within similar levels (WT, 111.3 ± 6.9 U/L, 0.6 ± 0.1 mg/dL; CD1d-/-, 108.2 ± 14.5 U/L, 0.6 ± 0.1 mg/dL).
Atherosclerotic lesion areas in WT and CD1d-/- mice fed on the AD. (A) Representative histologic sections of WT and CD1d-/- mice fed on the AD. Arrows represent the oil red O-positive atherosclerotic lesions typically observed within the internal elastic lamina (original magnification, × 40). (B) Mean lesion areas of WT and CD1d-/- mice. Each symbol represents the lesion area of an individual mouse. Horizontal bars and numbers represent the mean of all mice within each group, and vertical bars represent SEM. (C) Prevalence of NKT cells in WT and CD1d-/- mice. HMNCs and splenocytes were prepared and stained with FITC anti-TCRαβ, PE anti-NK1.1, and APC-CD1d/α-GalCer tetramer, as described in “Materials and methods.” Open columns represent the proportion of total NKT cells, and closed columns represent the proportion of CD1d/α-GalCer tetramer+ cells. Each value represents the mean ± SE calculated from more than 5 experiments. Statistical analyses were performed with the Mann-Whitney U test. †P < .01 (for closed columns and open columns); *P < .05.
Atherosclerotic lesion areas in WT and CD1d-/- mice fed on the AD. (A) Representative histologic sections of WT and CD1d-/- mice fed on the AD. Arrows represent the oil red O-positive atherosclerotic lesions typically observed within the internal elastic lamina (original magnification, × 40). (B) Mean lesion areas of WT and CD1d-/- mice. Each symbol represents the lesion area of an individual mouse. Horizontal bars and numbers represent the mean of all mice within each group, and vertical bars represent SEM. (C) Prevalence of NKT cells in WT and CD1d-/- mice. HMNCs and splenocytes were prepared and stained with FITC anti-TCRαβ, PE anti-NK1.1, and APC-CD1d/α-GalCer tetramer, as described in “Materials and methods.” Open columns represent the proportion of total NKT cells, and closed columns represent the proportion of CD1d/α-GalCer tetramer+ cells. Each value represents the mean ± SE calculated from more than 5 experiments. Statistical analyses were performed with the Mann-Whitney U test. †P < .01 (for closed columns and open columns); *P < .05.
Flow cytometric analyses of NKT cells
Using flow cytometry, we analyzed NKT cells in the liver, spleen, and peripheral blood of WT mice fed either the chow diet or the AD. NK1.1+TCRβint (ie, NKT) cells represented 18.1% ± 2.6% of the HMNCs of WT mice on the chow diet (Figure 1C, top panel). Among NK1.1+TCRβint cells, 84.2% ± 4.1% stained with α-GalCer-loaded CD1d tetramers. It should be noted that the mean proportion of total NK1.1+TCRβint cells in HMNCs of AD-fed WT mice (8.7% ± 2.3%) was significantly lower than that in chow-fed WT mice (P = .009). This was attributed to the considerable reduction of CD1d/α-GalCer tetramer+ cells in AD-fed mice. Proportions of CD1d/α-GalCer tetramer- cells remained unaltered among chow- and AD-fed animals. Similarly, a mild reduction in the prevalence of tetramer+ NKT cells among splenocytes of AD-fed mice was noted (Figure 1C, bottom panel; P = .07), but the proportion of total NK1.1+TCRβint cells was unchanged. In CD1d-/- mice, the proportion of NK1.1+TCRβint HMNCs was markedly smaller than that in WT mice, and tetramer+ cells were not detected. Of note, the proportion of NK1.1+TCRβint cells in CD1d-/- mice was unaffected by AD feeding (1.5% ± 0.3% on the chow diet compared with 1.9% ± 0.4% on the AD). Similar results were obtained with splenocytes of CD1d-/- mice. No significant changes were seen in conventional T-cell subsets (CD4+, CD8+), γδ T cells, and NK cells by AD feeding in WT and CD1d-/- mice (data not shown).
Production of cytokines by splenocytes from AD- or chow-fed WT mice treated with α-GalCer
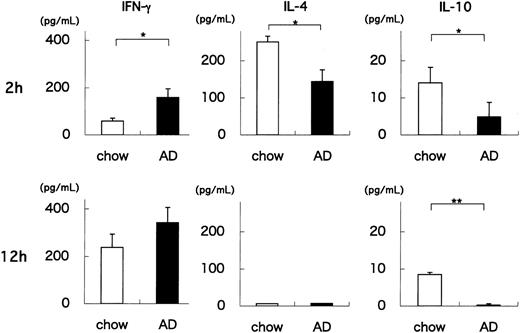
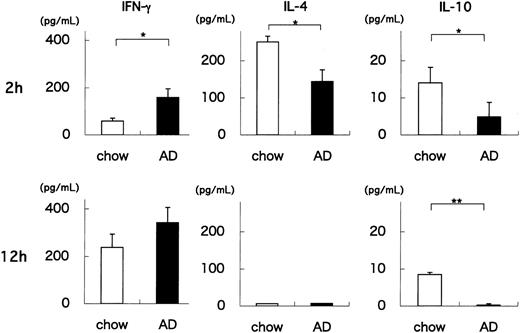
Our results indicate that AD feeding quantitatively and qualitatively alters the Vα14 NKT cell population of WT mice. One hallmark of NKT cells is their capacity to rapidly produce cytokines on TCR engagement.22,41 To examine whether AD feeding influences the functional status of NKT cells, we administered a synthetic glycolipid, α-GalCer, to AD- or chow-fed WT mice, and 2 or 12 hours later we measured the amounts of IFN-γ, IL-4, and IL-10 produced by splenocytes in vitro. Two hours after α-GalCer injection, IFN-γ levels were significantly higher in AD-fed WT splenocytes than in chow-fed WT splenocytes (P = .034) (Figure 2). In contrast, IL-4 and IL-10 levels were significantly lower in AD-fed WT splenocytes than in chow-fed WT splenocytes (P = .021, P = .047, respectively). At 12 hours, IFN-γ levels were slightly higher in AD-fed WT splenocytes (P = .094), amounts of IL-4 decreased to undetectable levels in both groups, and IL-10 levels were still significantly lower in AD-fed WT splenocytes (P = .009). Experiments using HMNCs from AD- and chow-fed WT mice showed similar results (data not shown). Because NKT cells (particularly CD1d/α-GalCer tetramer+ NKT cells) were decreased in AD-fed WT mice (Figure 1C), these findings indicate that NKT cells in AD-fed WT mice exhibit an enhanced capacity to produce cytokines, especially IFN-γ. It should be noted that AD feeding of WT mice shifted the cytokine production pattern in response to α-GalCer stimulation toward a TH1 profile. Importantly, it has been reported that TH1 responses are proatherogenic.10-14
Production of cytokines by splenocytes from AD- or chow-fed WT mice treated with α-GalCer. Splenocytes were obtained from either AD- or chow-fed WT mice 2 or 12 hours after intravenous injection with 0.1 μg/g BW α-GalCer. Cells were cultured for 1.5 hours without additional stimulation. Culture supernatants were harvested, and IFN-γ, IL-4, and IL-10 levels were quantitated. Values are mean ± SE. Statistical analyses were performed using the Mann-Whitney U test. *P < .05; **P < .01.
Production of cytokines by splenocytes from AD- or chow-fed WT mice treated with α-GalCer. Splenocytes were obtained from either AD- or chow-fed WT mice 2 or 12 hours after intravenous injection with 0.1 μg/g BW α-GalCer. Cells were cultured for 1.5 hours without additional stimulation. Culture supernatants were harvested, and IFN-γ, IL-4, and IL-10 levels were quantitated. Values are mean ± SE. Statistical analyses were performed using the Mann-Whitney U test. *P < .05; **P < .01.
Development of atherosclerosis in Ldlr-/- mice reconstituted with BM cells from CD1d-/- or WT mice
Next, to examine whether NKT cell deficiency is directly related to the reduction of atherosclerotic lesions, [WT→Ldlr-/-] and [CD1d-/-→Ldlr-/-] BM chimeric mice (n = 7 in each group) were prepared. Four weeks after BMT, almost all thymocytes from [CD1d-/-→Ldlr-/-] chimeras used in these experiments were CD1d- and, thus, of donor origin (Figure 3A). In addition, thymocytes from [WT→Ldlr-/-] chimeras were mostly Thy1.1+ (donor) (donor chimerism = 99.0% ± 0.81%). AD feeding for 5 weeks led to similar levels of hypercholesterolemia in both groups (total cholesterol or HDL cholesterol, [WT→Ldlr-/-]: 2147 ± 144 mg/dL or 15.0 ± 3.5 mg/dL; [CD1d-/-→Ldlr-/-]: 2207 ± 119 mg/dL or 15.9 ± 1.6 mg/dL, respectively). However, the atherosclerotic lesions in [CD1d-/-→Ldlr-/-] mice were significantly smaller than those in [WT→Ldlr-/-] mice (Figure 3C-D). Immunohistochemistry revealed that the main components of the lesions were MOMA-2+ macrophages in both groups (Figure 3E, upper). Notably, CD3+ cells were significantly more abundant in [WT→Ldlr-/-] mice than in [CD1d-/-→Ldlr-/-] mice (Figure 3E, middle, 3F; P = .006), and IFN-γ-positive cells, probably lymphocytes, were detected at more significant numbers in [WT→Ldlr-/-] chimeras than in [CD1d-/-→Ldlr-/-] chimeras (Figure 3E, lower). There were no overt differences in the staining patterns of α-smooth muscle actin and IL-10 between these 2 groups (data not shown). Mice reconstituted with syngeneic BMT ([Ldlr-/-→Ldlr-/-]) showed the same atherosclerotic lesions as those in [WT→Ldlr-/-] mice (data not shown).
Atherosclerotic lesions in Ldlr-/- mice reconstituted with BM cells from CD1d-/- or WT mice. (A) Representative CD1d expression pattern on thymocytes from [WT→Ldlr-/-] and [CD1d-/-→Ldlr-/-] mice. Red lines and filled histograms indicate CD1d staining and isotype control, respectively. (B) Thy1.1 and Thy1.2 expression on thymocytes from [WT→Ldlr-/-] and [CD1d-/-→Ldlr-/-] chimeras. Representative results of Thy1.1 and Thy1.2 stainings are shown in the right panels with their respective isotype controls (left panels). (C) Representative histologic sections of [WT→Ldlr-/-] and [CD1d-/-→Ldlr-/-] mice stained with oil red O (original magnification, × 40). Arrowheads represent oil red O-positive lesions. (D) Lesion area in [WT→Ldlr-/-] and [CD1d-/-→Ldlr-/-] mice. Each symbol represents the lesion area of an individual mouse. Horizontal bars and numbers represent the mean of all mice within each group. **P < .01 (E) Representative immunohistochemical section of [WT→Ldlr-/-] and [CD1d-/-→Ldlr-/-] mice. Sections were stained with anti-MOMA-2, anti-CD3, and anti-IFN-γ mAb (original magnification, × 200). Arrowheads represent respective mAb-positive cells. (F) Numbers of CD3+ cells per cross-section of lesion area. **P < .01.
Atherosclerotic lesions in Ldlr-/- mice reconstituted with BM cells from CD1d-/- or WT mice. (A) Representative CD1d expression pattern on thymocytes from [WT→Ldlr-/-] and [CD1d-/-→Ldlr-/-] mice. Red lines and filled histograms indicate CD1d staining and isotype control, respectively. (B) Thy1.1 and Thy1.2 expression on thymocytes from [WT→Ldlr-/-] and [CD1d-/-→Ldlr-/-] chimeras. Representative results of Thy1.1 and Thy1.2 stainings are shown in the right panels with their respective isotype controls (left panels). (C) Representative histologic sections of [WT→Ldlr-/-] and [CD1d-/-→Ldlr-/-] mice stained with oil red O (original magnification, × 40). Arrowheads represent oil red O-positive lesions. (D) Lesion area in [WT→Ldlr-/-] and [CD1d-/-→Ldlr-/-] mice. Each symbol represents the lesion area of an individual mouse. Horizontal bars and numbers represent the mean of all mice within each group. **P < .01 (E) Representative immunohistochemical section of [WT→Ldlr-/-] and [CD1d-/-→Ldlr-/-] mice. Sections were stained with anti-MOMA-2, anti-CD3, and anti-IFN-γ mAb (original magnification, × 200). Arrowheads represent respective mAb-positive cells. (F) Numbers of CD3+ cells per cross-section of lesion area. **P < .01.
Effects of NKT cell activation on the development of early atherosclerotic lesions in apoE-/- mice
To examine influences of NKT cell activation on the development of atherosclerosis, we administered α-GalCer or OCH to apoE-/- mice. ApoE-/- mice spontaneously develop severe atherosclerosis early in life.8,9,35 It has been reported that α-GalCer and OCH activate NKT cells with differential patterns of cytokine production.33
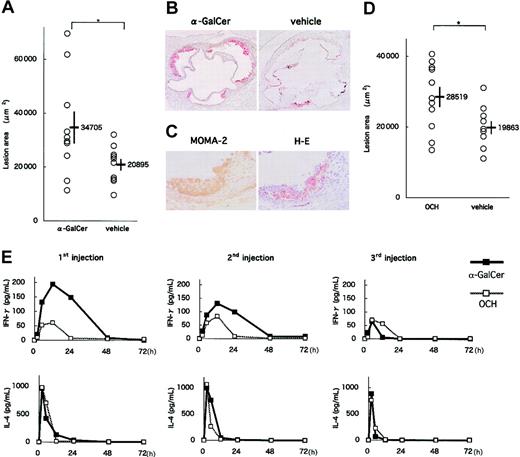
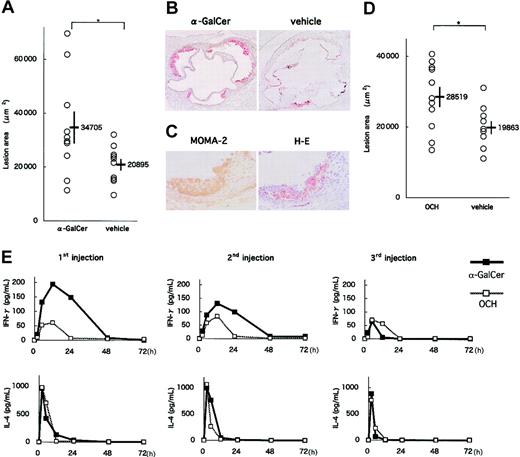
In an early-phase study, apoE-/- mice were intraperitoneally injected 3 times with either 0.1 μg/g BW α-GalCer, 0.3 μg/g BW OCH or the respective vehicle at 8, 10, and 12 weeks of age. At 13 weeks of age, the mice were killed and examined for atherosclerotic lesions. No significant differences in physiologic status or serum lipid profiles were observed between experimental and control groups (α-GalCer or OCH vs their vehicle; data not shown). α-GalCer administration increased atherosclerotic lesion areas of apoE-/- mice compared with the vehicle control group (34 705 ± 5908 μm2 vs 20 895 ± 2155 μm2; P = .039) (Figure 4A-B). Major components of the atherosclerotic lesions in α-GalCer-treated mice included MOMA-2+ macrophages (Figure 4C). OCH administration also increased atherosclerotic lesion areas compared with control (28 519 ± 2822 μm2 vs 19 863 ± 1813 μm2; P = .048) (Figure 4D). Lesion areas in the OCH-treated group, however, were relatively smaller than those in the α-GalCer-treated group. To determine a potential mechanism for the differences observed between the α-GalCer- and OCH-treated mice, we evaluated the sequential patterns of IFN-γ and IL-4 production in the serum after glycolipid injection. Both glycolipids induced robust cytokine production; however, though IL-4 production was similar, α-GalCer induced more IFN-γ than OCH, which is consistent with earlier findings (Figure 4E).33
Effects of α-GalCer and OCH on the early phase of atherosclerosis. (A) ApoE-/- mice were intraperitoneally injected 3 times with α-GalCer or the vehicle alone, as described in “Materials and methods.” Five weeks later, mice were examined for the development of atherosclerosis. Each symbol represents the lesion area of an individual mouse. Horizontal bars and numbers represent the mean of all mice within each group, and vertical bars represent SEM. (B) Representative histologic sections of the α-GalCer group and its control group stained with oil red O (original magnification, × 40). (C) Representative immunohistochemical section of the α-GalCer group stained with MOMA-2 and a serial section stained with hematoxylin and eosin (original magnification, × 200). (D) ApoE-/- mice were injected with OCH or vehicle. Mean lesion areas (OCH vs vehicle) are indicated as in Figure 4A. (E) Serum concentration of cytokines after administration of α-GalCer or OCH. Mean concentrations (n = 3) of IFN-γ (top) and IL-4 (bottom) in α-GalCer (▪) and OCH (□) groups are shown after the first injection (left), the second injection (middle) and the third injection (right). Statistical analyses were performed using the Mann-Whitney U test. *P < .05.
Effects of α-GalCer and OCH on the early phase of atherosclerosis. (A) ApoE-/- mice were intraperitoneally injected 3 times with α-GalCer or the vehicle alone, as described in “Materials and methods.” Five weeks later, mice were examined for the development of atherosclerosis. Each symbol represents the lesion area of an individual mouse. Horizontal bars and numbers represent the mean of all mice within each group, and vertical bars represent SEM. (B) Representative histologic sections of the α-GalCer group and its control group stained with oil red O (original magnification, × 40). (C) Representative immunohistochemical section of the α-GalCer group stained with MOMA-2 and a serial section stained with hematoxylin and eosin (original magnification, × 200). (D) ApoE-/- mice were injected with OCH or vehicle. Mean lesion areas (OCH vs vehicle) are indicated as in Figure 4A. (E) Serum concentration of cytokines after administration of α-GalCer or OCH. Mean concentrations (n = 3) of IFN-γ (top) and IL-4 (bottom) in α-GalCer (▪) and OCH (□) groups are shown after the first injection (left), the second injection (middle) and the third injection (right). Statistical analyses were performed using the Mann-Whitney U test. *P < .05.
Effects of long-term administration of α-GalCer on advanced atherosclerotic lesions in apoE-/- mice
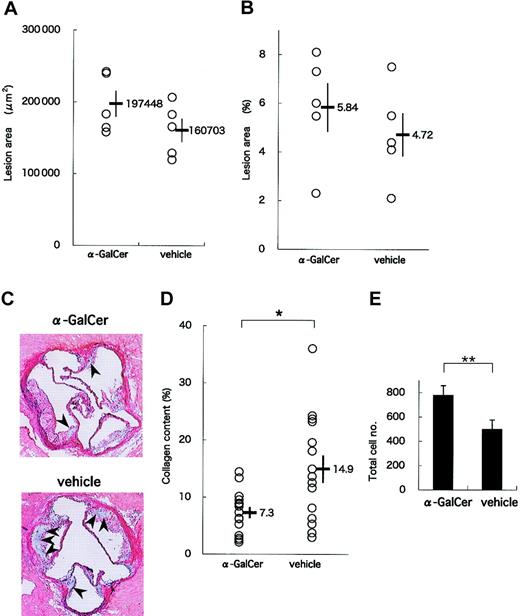
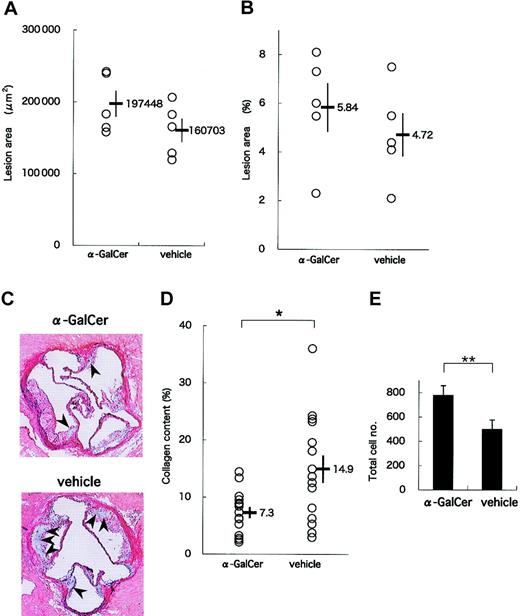
In a late-phase study, we analyzed lesions in 19-week-old apoE-/- mice that had received 11 intraperitoneal injections of either α-GalCer or its vehicle. Again, no significant differences were observed in the physiologic status and serum lipid profiles between α-GalCer- and vehicle-treated mice (data not shown). Mean areas of lesions in the aortic sinus were slightly larger in the α-GalCer group than in the control group (197 448 ± 18 259 μm2 vs 160 703 ± 16 320 μm2) (Figure 5A). In addition, assessing lesion areas by the en face method showed slightly enlarged lesion areas in the α-GalCer group (5.8% ± 1.0%) compared with the control group (4.7% ± 0.9%) (Figure 5B). These results suggest that activating NKT cells exerts only slight influences on the development of advanced atherosclerotic lesions in apoE-/- mice. These findings are consistent with a previous report suggesting that lymphocytes are mostly involved in the early phase of atherogenesis.6
Effect of intensive α-GalCer administration on the late phase of atherosclerosis. ApoE-/- mice were injected weekly with α-GalCer or vehicle for a period of 11 weeks and examined for atherosclerosis at 19 weeks of age. (A) Mean lesion areas of each group are indicated as in Figure 4A. (B) Proportions of the oil red O-positive area to the whole lumen of the entire aorta were assessed by the en face method. (C) Representative histology of aortic sections from the α-GalCer group (top) or the control group (bottom) (Elastica-Masson staining). The collagen content is stained as blue in the lesion. Note that the blue region (arrowheads) in the α-GalCer-treated mouse is smaller than that in the control mouse. Original magnification, × 40. (D) Morphometric analysis of collagen contents of the atherosclerotic lesion. Mean lesion areas staining blue were quantitated with 3 aortic cross-sections per animal from a total of 10 animals. Statistical analyses were performed with the Mann-Whitney U test. *P < .05. (E) Total cell numbers per cross-section of lesion area. Values are mean ± SE. **P < .01.
Effect of intensive α-GalCer administration on the late phase of atherosclerosis. ApoE-/- mice were injected weekly with α-GalCer or vehicle for a period of 11 weeks and examined for atherosclerosis at 19 weeks of age. (A) Mean lesion areas of each group are indicated as in Figure 4A. (B) Proportions of the oil red O-positive area to the whole lumen of the entire aorta were assessed by the en face method. (C) Representative histology of aortic sections from the α-GalCer group (top) or the control group (bottom) (Elastica-Masson staining). The collagen content is stained as blue in the lesion. Note that the blue region (arrowheads) in the α-GalCer-treated mouse is smaller than that in the control mouse. Original magnification, × 40. (D) Morphometric analysis of collagen contents of the atherosclerotic lesion. Mean lesion areas staining blue were quantitated with 3 aortic cross-sections per animal from a total of 10 animals. Statistical analyses were performed with the Mann-Whitney U test. *P < .05. (E) Total cell numbers per cross-section of lesion area. Values are mean ± SE. **P < .01.
Figure 5C shows representative histologic analyses of the atherosclerotic lesions of α-GalCer- and vehicle-treated animals. Of note, the collagen content stained with Elastica-Masson was smaller in the α-GalCer-treated group than in the control group. When mean collagen content was compared between these 2 groups, the collagen content in the α-GalCer-treated group was significantly smaller than in the control group (7.3% ± 1.1% and 14.9% ± 2.4%, respectively; P = .040) (Figure 5D). However, the total cell number within the lesion per slice was significantly larger in the α-GalCer-treated group than in the control group (Figure 5E; P = .009). These findings suggest that NKT cell activation in the late phase of atherosclerosis alters the quality of the lesion from one rich in collagen to one characterized by high cellularity.
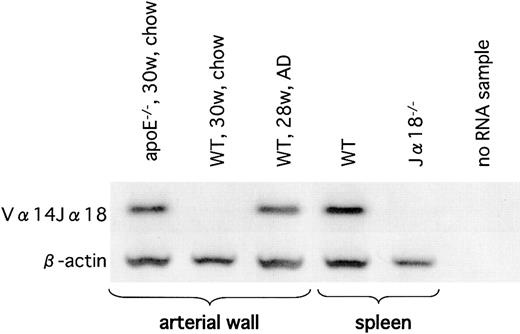
Vα14Jα18 TCR-α mRNA expression in atherosclerotic lesions of apoE-/- and AD-fed WT mice
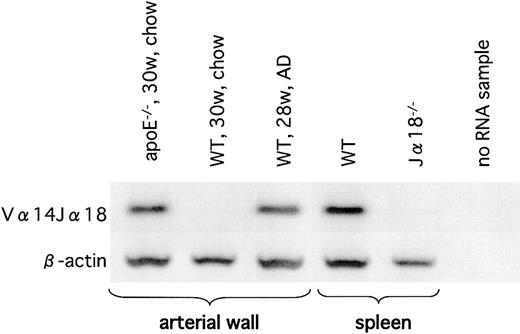
Next, we examined atherosclerotic lesions by nested RT-PCR for detection of the invariant Vα14Jα18 TCR-α rearrangement characteristic of classic NKT cells. We were able to amplify the Vα14Jα18 rearrangement in the atherosclerotic tissues of apoE-/- mice and of WT mice on the AD (Figure 6), but we were unable to detect this rearrangement in the aortae of WT mice on the chow diet. Although we were unable to quantify numbers of NKT cells in the lesion, our results clearly demonstrated that the presence of Vα14Jα18-positive cells was restricted to the atherosclerotic lesions.
Vα14Jα18 mRNA in the atherosclerotic lesions. Expression of Vα14Jα18 mRNA in the atherosclerotic lesion was examined using RT-PCR. A sample from WT spleen was used as a positive control, and a sample from Jα18-/- spleen was used as a negative control. Note that Vα14Jα18 expression is detected only in the atherosclerotic tissues of apoE-/- mice (on the chow diet) and in WT mice on the AD. Representative result from 3 separate experiments is shown.
Vα14Jα18 mRNA in the atherosclerotic lesions. Expression of Vα14Jα18 mRNA in the atherosclerotic lesion was examined using RT-PCR. A sample from WT spleen was used as a positive control, and a sample from Jα18-/- spleen was used as a negative control. Note that Vα14Jα18 expression is detected only in the atherosclerotic tissues of apoE-/- mice (on the chow diet) and in WT mice on the AD. Representative result from 3 separate experiments is shown.
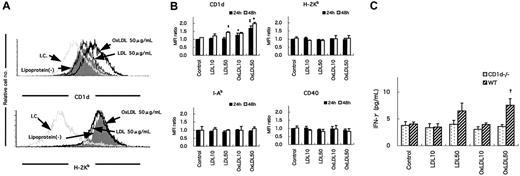
CD1d expression and IFN-γ production by WT peritoneal macrophages treated with LDL or OxLDL
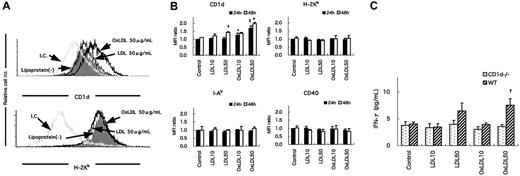
Classic NKT cells recognize glycolipid antigens in the context of CD1d.19,21,24-26,32,33 To investigate the mechanism by which NKT cells are activated and promote atherogenesis, peritoneal exudate macrophages were harvested from WT mice, treated with LDL, OxLDL, or medium alone for 24 or 48 hours, and examined for expression of several surface molecules. The expression of CD1d on WT macrophages was enhanced by incubation with OxLDL for 24 hours, but not with LDL or medium alone (Figure 7A, top). No increase in the expression of MHC class 1 (H-2Kb) molecules was induced on macrophages by OxLDL (Figure 7A, bottom). In addition, OxLDL specifically enhanced CD1d expression in a dose-dependent manner (Figure 7B). No enhancement of H-2Kb, I-Ab or CD40 expression was seen by treatment with LDL or OxLDL. When cultured for a longer time (48 hours) with OxLDL, CD1d expression was further augmented (Figure 7B). Of note, at a high dose (50 μg/mL) and after a long incubation period (48 hours), LDL enhanced CD1d levels on macrophages (Figure 7B).
CD1d expression and IFN-γ production by WT peritoneal macrophages treated with LDL or OxLDL. WT peritoneal macrophages were treated with LDL or OxLDL or without additional lipoproteins (control) for 24 hours. (A) Representative histograms of CD1d or H-2Kb expression on the macrophages. I.C. indicates each isotype control for either anti-CD1d or anti-H-2Kb mAb. Cells with PIlow and Mac-1high phenotypes were gated for analysis. (B) Mean fluorescence intensity (MFI) for CD1d, H-2Kb, I-Ab, or CD40 staining on WT peritoneal macrophages treated with LDL or OxLDL (10 or 50 μg/mL). Each column represents a ratio of MFI of a respective surface molecule to controls at either 24 hours (closed columns) or 48 hours (open columns). Values are mean ± SE of 3 independent experiments. *P < .05 vs control (24 hours). ‡P < .01 vs control, LDL10 or LDL50 (24 hours). §P < .05 vs control (48 hours). †P < .01 vs control, LDL10, LDL50, or OxLDL10 (48 hours). (C) Production of IFN-γ in the supernatant of the mixed culture of HMNCs with CD1d-/- or WT macrophages. HMNCs were cultured for 24 hours with peritoneal macrophages treated with LDL or OxLDL from CD1d-/- or WT mice. Then IFN-γ levels in the supernatant of the mixed culture were analyzed using ELISA. Values are mean ± SE of 3 independent experiments. †P < .05 vs control, LDL10, or OxLDL10 (WT). P < .01 vs OxLDL50 (CD1d-/-).
CD1d expression and IFN-γ production by WT peritoneal macrophages treated with LDL or OxLDL. WT peritoneal macrophages were treated with LDL or OxLDL or without additional lipoproteins (control) for 24 hours. (A) Representative histograms of CD1d or H-2Kb expression on the macrophages. I.C. indicates each isotype control for either anti-CD1d or anti-H-2Kb mAb. Cells with PIlow and Mac-1high phenotypes were gated for analysis. (B) Mean fluorescence intensity (MFI) for CD1d, H-2Kb, I-Ab, or CD40 staining on WT peritoneal macrophages treated with LDL or OxLDL (10 or 50 μg/mL). Each column represents a ratio of MFI of a respective surface molecule to controls at either 24 hours (closed columns) or 48 hours (open columns). Values are mean ± SE of 3 independent experiments. *P < .05 vs control (24 hours). ‡P < .01 vs control, LDL10 or LDL50 (24 hours). §P < .05 vs control (48 hours). †P < .01 vs control, LDL10, LDL50, or OxLDL10 (48 hours). (C) Production of IFN-γ in the supernatant of the mixed culture of HMNCs with CD1d-/- or WT macrophages. HMNCs were cultured for 24 hours with peritoneal macrophages treated with LDL or OxLDL from CD1d-/- or WT mice. Then IFN-γ levels in the supernatant of the mixed culture were analyzed using ELISA. Values are mean ± SE of 3 independent experiments. †P < .05 vs control, LDL10, or OxLDL10 (WT). P < .01 vs OxLDL50 (CD1d-/-).
We then examined whether the enhanced expression of CD1d on OxLDL-treated macrophages related to their capacity to stimulate NKT cells. We mixed HMNCs isolated from WT mice (containing 15%-30% NKT cells) with irradiated peritoneal macrophages from WT or CD1d-/- mice treated with LDL or OxLDL for 48 hours. After culture for 24 hours, IFN-γ and IL-4 levels in the supernatants were quantitated. NKT cells produced significantly higher amounts of IFN-γ in the cultures with OxLDL (50 μg/mL)-treated peritoneal exudate cells from WT mice compared with control cultures (Figure 7C). CD1d-/- macrophages treated in the same manner induced no enhancement of IFN-γ production. No IL-4 was detected in the supernatant in our culture conditions (data not shown).
Discussion
In this study we demonstrate, using 3 atherosclerosis models (apoE+/+ mice fed with AD, Ldlr-/- chimeras fed with AD, and apoE-/- mice fed with normal chow), that NKT cells play a significant role in the development of atherosclerosis. In addition, we show that NKT cell activation modulates the disease process. Atherosclerotic lesion areas in AD-fed CD1d-/- mice were significantly smaller than those in AD-fed WT mice (Figure 1B). Because the development of invariant NKT cells is markedly hampered in CD1d-/- mice,30 our findings suggest that NKT cell deficiency is related to the amelioration of atherosclerosis. It has been reported that AD induces inflammatory cytokines in the liver because of its high concentration of cholesterol and cholic acid42 and that it may alter physiologic conditions. In the present study, WT and CD1d-/- mice were subjected to AD feeding in an identical manner. These 2 groups of mice showed comparable degrees of liver steatosis and similar levels of serum alanine aminotransferase and total bilirubin. Thus, we conclude that the significant differences in the atherosclerotic lesions between WT and CD1d-/- mice are directly related to the presence and absence, respectively, of the intact CD1d-restricted T-cell population.
The prevalence of NKT cells (mainly the CD1d/α-GalCer tetramer+ fraction) among HMNCs of WT mice substantially decreased through the AD. A slight reduction of NKT cells was also observed in splenocytes of the AD-fed WT mice (Figure 1C). One characteristic of NKT cells is the prominent production of cytokines, IFN-γ, and IL-4 shortly after stimulation of these cells through the TCR.22,41 We found that, despite their decreased NKT cell numbers, on stimulation with α-GalCer, spleen cells from AD-fed WT mice produced levels of IFN-γ, IL-4, and IL-10 comparable to those from chow-fed WT mice. Of note, the cytokine production pattern of spleen cell cultures of AD-fed WT mice shifted toward a TH1 profile, especially 2 hours after α-GalCer stimulation (Figure 2). This pattern of cytokine production would be expected to promote atherosclerosis.10-14 However, the mechanism underlying this altered cytokine production pattern, with the concomitant decrease of NKT cells, remains elusive. One possibility is that the decrease of NKT cells in AD-fed WT mice is caused by a depletion of this population by activation-induced cell death (AICD). This may be mediated by the CD1d-restricted presentation of lipid antigens such as OxLDL, which accumulate during hyperlipidemia. An alternative explanation would be that chronic stimulation of the NKT cell population results in the continuous down-modulation of NK1.1 and TCR marker expression, resulting in the apparent loss of these cells.43 Furthermore, the diminished population of NKT cells could be attributed to the migration of these cells from liver or spleen to other peripheral tissues, such as the atherosclerotic lesion. In this context, we were able to detect mRNA corresponding to the invariant Vα14Jα18 TCR, which is characteristic of NKT cells and prerequisite for α-GalCer stimulation, within atherosclerotic lesions of AD-fed but not chow-fed WT mice by nested RT-PCR (Figure 6). The mechanisms that lead to NKT cell loss during AD feeding will be further addressed in future studies.
To examine the role of NKT cells in a more advanced atherosclerosis model, we reconstituted lethally irradiated Ldlr-/- mice with BM cells from CD1d-/- or WT mice. It has been reported that lack of LDL receptors aggravates the development of atherosclerosis in AD-fed mice. Indeed, using [WT→Ldlr-/-] chimeras, Boisvert et al44 reported that AD-fed chimeras showed severe atherosclerotic lesions where donor-derived leukocytes were present. In the present study, we observed that significantly large atherosclerotic lesions developed in [WT→Ldlr-/-] chimeras compared with [CD1d-/-→Ldlr-/-] chimeras (Figure 3). These findings demonstrate that NKT cell deficiency indeed ameliorates atherosclerosis in AD-fed animals. Immunohistochemistry studies in this BMT model demonstrated that the number of CD3+ cells within the lesion was significantly larger in [WT→Ldlr-/-] than that in [CD1d-/-→Ldlr-/-] mice, suggesting that these CD3+ cells contain NKT cells. Furthermore, we demonstrated NKT cell (Vα14Jα18) messages in lesions of other animal models by RT-PCR (Figure 6). However, immunohistochemical identification of NKT cells in the lesion has thus far been unsuccessful and will be pursued in future studies.
Complementary to the above results, we showed that activation of NKT cells by α-GalCer or OCH in apoE-/- mice, before significant lesions had been formed (early-phase study), resulted in increased areas of atherosclerotic lesions (Figure 4). We did not expect the results with OCH because it was reported that OCH favors a TH2 shift of NKT cells.33 It has been suggested that a TH2 bias suppresses atherogenesis.10,11 Consistent with previous reports,33 we found that a single injection of α-GalCer induced prominent production of IFN-γ and IL-4, whereas OCH induced little IFN-γ but similar levels of IL-4 (Figure 4E). However, after multiple administrations, IFN-γ induction in response to α-GalCer became reduced to levels similar to those for OCH. In contrast, repeated injection of these glycoplipids did not alter IL-4 induction. Although a number of studies support the idea that the TH1 cytokine IFN-γ is proatherogenic, the precise role of the TH2 cytokine IL-4 in atherogenesis remains elusive.45 Our finding that both α-GalCer and OCH exacerbate atherosclerosis during the early stage of the disease process, but to a different degree (Figure 4A, D), may be attributed to differences in the amounts and kinetics of IFN-γ and IL-4 production.
We found that peritoneal exudate macrophages expressed augmented levels of CD1d after culture with OxLDL (either 10 μg/mL or 50 μg/mL) or LDL (50 μg/mL) (Figure 7A-B). Although intact LDL is not captured by macrophages, it is plausible that LDL is degraded by peroxidases released from macrophages during the incubation period and is involved in the enhancement of CD1d expression. Furthermore, these macrophages with high CD1d expression stimulated NKT cells to produce low but significant levels of IFN-γ in vitro (Figure 7C). Thus, the enhancement of CD1d expression on OxLDL-pulsed macrophages appeared to result in their augmented capacity to induce IFN-γ production by HMNCs. This finding may be of importance because physiologically degraded lipids are abundantly present in the atherosclerotic lesions and may provide a source of physiologic ligands for NKT cells.
In the late-phase study to evaluate the effects of α-GalCer on atherosclerosis in apoE-/- mice, α-GalCer administration failed to enlarge lesions but instead decreased collagen content (Figure 5C-D) and increased total cell numbers (Figure 5E) within the atherosclerotic lesions. It has been reported that IFN-γ decreases collagen synthesis13 and plays a role in plaque stability. Accordingly, it is possible that IFN-γ, which is produced on α-GalCer stimulation, decreases collagen synthesis. Thus, NKT cell activation at the late phase may alter the lesion structure from a stable to an unstable state. Ostos et al29 demonstrated that LPS-treated apoE-/- mice have larger atherosclerotic lesions than PBS-treated control apoE-/- mice. In atherosclerotic lesions of these LPS-treated mice, increased numbers of IL-4-producing NK1.1+ cells were detected by immunohistochemistry. In our present study, the invariant Vα14Jα18 TCR was detected in aortic specimens with atherosclerotic lesions of either AD-fed WT or apoE-/- mice (Figure 6). These findings again favor the idea that NKT cells play a proatherogenic role in situ. However, it is also possible that NKT cells are activated in other tissues, such as the liver or spleen, and systemically affect the atherogenic process. Thus, the precise location where NKT cells are activated and demonstrate their effector functions during progression of atherosclerosis remains to be elucidated. Although TH1 and TH2 cytokines are probably important, other factors, such as chemokines and the capacity of NKT cells to exhibit cytotoxicity, should be considered in further investigations.23,34 In summary, we have demonstrated that NKT cells accelerate atherogenesis in mouse models for this disease. In addition, we show that NKT cell activation in the early phase of the disease process exacerbates atherogenesis and that NKT cell activation in the late phase of the disease promotes plaque instability. Because NKT cells and CD1d molecules are highly conserved among different species,46 our present results may be applicable to elucidation of the pathophysiology of atherosclerosis in humans, and they offer a novel approach for controlling the atherogenic process by intervening with certain NKT cell functions.
Prepublished online as Blood First Edition Paper, April 27, 2004; DOI 10.1182/blood-2003-10-3485.
Supported in part by Grant-in-Aid for Scientific Research S and B, Grant-in-Aid for Exploratory Research from the Ministry of Education, Culture, Science, Sports and Technology, Japan (K.O., S.F., K.I.); by grants from the Noastec Foundation (K.I., S.F., A.K., K.O.), the Mochida Memorial Foundation for Medical and Pharmaceutical Research (K.I.), the Akiyama Foundation (K.I., Y.N., T.M., C.I.), Daiwa Securities Health Foundation (K.I., C.I., K.N., S.F.), the Suhara Memorial Foundation (K.I., Y.N., N.D., C.I., S.F.), and the Program for the Promotion of Fundamental Studies in Health Sciences of the Pharmaceutical and Medical Devices Agency (T.Y.); and by National Institutes of Health grants AI50953, NS44044, HL68744 (L.V.K.), and AI42284 (S.J.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the staffs at Kirin Brewery Company and Takeda Chemical Industries for providing α-GalCer and recombinant human IL-2, respectively. We also thank Keiko Kato and Mizuho Kasai for technical assistance.

![Figure 3. Atherosclerotic lesions in Ldlr-/- mice reconstituted with BM cells from CD1d-/- or WT mice. (A) Representative CD1d expression pattern on thymocytes from [WT→Ldlr-/-] and [CD1d-/-→Ldlr-/-] mice. Red lines and filled histograms indicate CD1d staining and isotype control, respectively. (B) Thy1.1 and Thy1.2 expression on thymocytes from [WT→Ldlr-/-] and [CD1d-/-→Ldlr-/-] chimeras. Representative results of Thy1.1 and Thy1.2 stainings are shown in the right panels with their respective isotype controls (left panels). (C) Representative histologic sections of [WT→Ldlr-/-] and [CD1d-/-→Ldlr-/-] mice stained with oil red O (original magnification, × 40). Arrowheads represent oil red O-positive lesions. (D) Lesion area in [WT→Ldlr-/-] and [CD1d-/-→Ldlr-/-] mice. Each symbol represents the lesion area of an individual mouse. Horizontal bars and numbers represent the mean of all mice within each group. **P < .01 (E) Representative immunohistochemical section of [WT→Ldlr-/-] and [CD1d-/-→Ldlr-/-] mice. Sections were stained with anti-MOMA-2, anti-CD3, and anti-IFN-γ mAb (original magnification, × 200). Arrowheads represent respective mAb-positive cells. (F) Numbers of CD3+ cells per cross-section of lesion area. **P < .01.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/7/10.1182_blood-2003-10-3485/6/m_zh80190467250003.jpeg?Expires=1769780739&Signature=SzH3RhicGABYgL~lMm9i1Cci7nCyc6mXHSlrFqIZZ85Ja7gyXkWgV8TqmLt3UdN9oER96u5v9s6u7VkS~M8c41mebQ6NwV2UJ3fWW5-P-21nYN5MM1i9u4c001We2XLSHojwou248NFrLugD0O8q8Mc2W6xrg4F~rABcic02y0NvULsJYfKacS3jcWa~xQl7eYSfqZ7s3s0VU7Z1ZzkngcSNW9uOfkg~WD-eHrZmxAjjvRDn33jHgokzvFDMVtuiokYDEqY7zqTvTCPLg3qfCpPuWXcKX2IsV3LCgtE42cwZPOUKl9Jn~8NOf3N7cdtl7buWj85qkO6huArAXc8ARw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 3. Atherosclerotic lesions in Ldlr-/- mice reconstituted with BM cells from CD1d-/- or WT mice. (A) Representative CD1d expression pattern on thymocytes from [WT→Ldlr-/-] and [CD1d-/-→Ldlr-/-] mice. Red lines and filled histograms indicate CD1d staining and isotype control, respectively. (B) Thy1.1 and Thy1.2 expression on thymocytes from [WT→Ldlr-/-] and [CD1d-/-→Ldlr-/-] chimeras. Representative results of Thy1.1 and Thy1.2 stainings are shown in the right panels with their respective isotype controls (left panels). (C) Representative histologic sections of [WT→Ldlr-/-] and [CD1d-/-→Ldlr-/-] mice stained with oil red O (original magnification, × 40). Arrowheads represent oil red O-positive lesions. (D) Lesion area in [WT→Ldlr-/-] and [CD1d-/-→Ldlr-/-] mice. Each symbol represents the lesion area of an individual mouse. Horizontal bars and numbers represent the mean of all mice within each group. **P < .01 (E) Representative immunohistochemical section of [WT→Ldlr-/-] and [CD1d-/-→Ldlr-/-] mice. Sections were stained with anti-MOMA-2, anti-CD3, and anti-IFN-γ mAb (original magnification, × 200). Arrowheads represent respective mAb-positive cells. (F) Numbers of CD3+ cells per cross-section of lesion area. **P < .01.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/7/10.1182_blood-2003-10-3485/6/m_zh80190467250003.jpeg?Expires=1769872063&Signature=qHi1hRxizg9P0S5RIxKXtofMxP~37Rn~XJlxXhM17ZXndEonbXKuBAF~eP-mxeei3YYi7b1fcjuSzUth8k73wbxNpOeRQuDbVo8-HSutQ4klEvcLFIjiXHZXJ6emxNrnkmWWK9-lv4Ti9toMoZfdrJ~HpIHEwcqDtlCUrwLzFTEriDO4GXNsNtXPszzqKCpbzULeEICSFNt7h1-wuk53w4lH6HOuZkY2rzWcS5ELA-0JXounMMmp~oaMtNaF7uERawt3j19BIBUiOI364vClfSCVm~jhLMce8~HMzV~mwVfn1X0XPk6I1K5eXOshU7L1NUoTe8ulNNHYtocfCiok2w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)