Comment on Karamatic Crew et al, page 2217
Blood groups have always thrown up surprises that far transcend their function in blood. Karamatic Crew and colleagues present a remarkable new example.
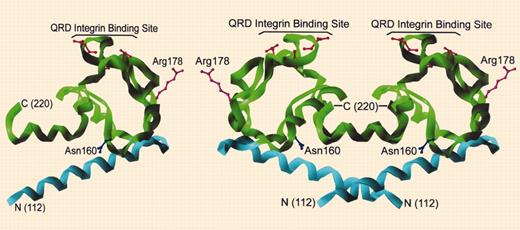
Tetraspanins are a distinct and fascinating family of proteins, so named because they span the membrane 4 times. Tetraspanins are palmitoylated and form intramolecular and intermolecular interactions crucial for biosynthesis and assembly of a network of multi-molecular membrane microdomains called the “tetraspanin web.” Numerous reports link tetraspanins with cell motility and signaling events, as well as with suppression or promotion of tumor cell invasion to metastasis. In 2004, knowing all we do about the red blood cell (RBC) membrane, it is astonishing to learn that it harbors one of these extraordinary molecules, namely CD151 (which is known to complex with the laminin-binding integrins α3β1, α6β1, α6β4, and α7β1).FIG1
Structure of CD151 large extracellular region. See the complete figure in the article beginning on page 2217.
Structure of CD151 large extracellular region. See the complete figure in the article beginning on page 2217.
It was the location of the genes encoding CD151 and the blood group system MER2 on chromosome 11p15 and the sensitivity to extracellular protease and thiol treatment that led Karamatic Crew and her colleagues to contemplate the possibility that MER2 (RAPH) might be a marker on CD151. This proved indeed to be the case, and the availability of samples from patients with alloanti-RAPH in their serum, suffering from nephritic syndrome with end-stage renal failure, pretibial bullous skin lesions, neurosensory deafness, bilateral lacrimal duct stenosis, and nail dystrophy, provided critical clues toward an understanding of the function of CD151. Two probands had a single nucleotide insertion (G383) in exon 5 of their CD151, which precluded expression of the protein, and a third had a variant form of CD151 (G533A in exon 6). Thus, the connection between CD151 and MER2 allowed the authors to infer from what they knew about the blood group that CD151 is present in the erythrocyte membrane and that null and variant forms of the protein exist. In the CD151-null individuals, the pathology in multiple tissues can be explained by the absence of CD151. Interactions between CD151 and laminin-binding integrins appear then to be critical for the correct assembly of kidney basement membranes to play a role in the stability of basement membranes in skin, inner ear, and other tissues. Yet again, then, the discovery of a blood group system null phenotype has led us to new insights into an aspect of human biology and disease. ▪