Abstract
A20 is a stress response gene in endothelial cells (ECs). A20 serves a dual cytoprotective function, protecting from tumor necrosis factor (TNF)–mediated apoptosis and inhibiting inflammation via blockade of the transcription factor nuclear factor–κB (NF-κB). In this study, we evaluated the molecular basis of the cytoprotective function of A20 in EC cultures and questioned whether its protective effect extends beyond TNF to other apoptotic and necrotic stimuli. Our data demonstrate that A20 targets the TNF apoptotic pathway by inhibiting proteolytic cleavage of apical caspases 8 and 2, executioner caspases 3 and 6, Bid cleavage, and release of cytochrome c, thus preserving mitochondrion integrity. A20 also protects from Fas/CD95 and significantly blunts natural killer cell–mediated EC apoptosis by inhibiting caspase 8 activation. In addition to protecting ECs from apoptotic stimuli, A20 safeguards ECs from complement-mediated necrosis. These data demonstrate, for the first time, that the cytoprotective effect of A20 in ECs is not limited to TNF-triggered apoptosis. Rather, A20 affords broad EC protective functions by effectively shutting down cell death pathways initiated by inflammatory and immune offenders.
Introduction
A20 is a zinc finger protein originally identified as a tumor necrosis factor (TNF)–responsive gene in endothelial cells (ECs).1 A20 is expressed in multiple cell types in response to a variety of stimuli that activate the transcription factor nuclear factor–κB (NF-κB), including interleukin 1 (IL-1), lipopolysaccharide (LPS), phorbol 12–myristate 13–acetate (PMA), H2O2, and CD40 ligand.2-7 We and others have demonstrated that A20, initially described as an antiapoptotic gene, is also a potent inhibitor of the transcription factor NF-κB.7-9 A20-null mice fail to terminate TNF-induced NF-κB activation, develop severe inflammation and cachexia, and die prematurely, indicating the importance of A20 in the hierarchy of anti-inflammatory defense processes.6,10
Elucidating the molecular basis and binding partner(s) that determine the inhibitory effect of A20 upon NF-κB activation is the focus of ongoing research.8 A20 has been shown to interact with components of the NF-κB signaling cascade upstream of inhibitor of NF-κB (IκB), including TNF receptor–associated factors (TRAFs)11,12 TRAF-1, TRAF-2, and TRAF-6; A20 binding inhibitors of NF-κB; and the signalosome IκB kinase–γ (IKKγ)/NF-κB essential modulator (NEMO) unit.13-15 Further work is required to test the relevance of these interactions in blocking NF-κB activation in response to stimuli other than TNF.16
In contrast to its so far universal inhibitory effect on NF-κB activation, the antiapoptotic activity of A20 remains controversial and appears to be specific to cell type and stimulus. Overexpression of A20 protects human breast carcinoma MCF-7 cells, murine fibrosarcoma WEHI 164, and murine embryonic NIH3T3, but not Hela and lung epithelial A459 cells from TNF-mediated apoptosis.9,17-19 In cultures derived from primary cells, we and other investigators have found that overexpression of A20 protects ECs, hepatocytes, and islets of Langerhans from cycloheximide (CHX)/TNF–, ceramide/TNF–, and IL-1–mediated apoptosis but not human aortic smooth muscle cells (SMCs), which were even sensitized to apoptosis when A20 was overexpressed.3,20-22
The protective effect of A20 extends, in some but not all cell types, to apoptotic stimuli other than TNF. A20 protects human umbilical vein endothelial cells (HUVECs) and BJAB B cells from serum starvation, H1299 epithelial cells from p53-mediated apoptosis, and the human microvascular EC line HMEC-1 from LPS.4,23 In contrast, it does not affect Fas/CD95 receptor lymphokine-activated killer (LAK)–, oxidative stress–, doxorubicine-, and serum starvation–induced apoptosis in MCF-7 and WEHI cells.7,24 Similarly, A20 protects Jurkat cells from TNF- but not Fas-mediated apoptosis.25 The precise mechanisms by which A20 protects from apoptosis are not known. Interaction between A20 and novel antiapoptotic proteins such as TAX-1 binding protein 151 (TXBP151) is suggested as one potential mechanism.26
Therefore, we undertook a thorough analysis of A20's antiapoptotic effect in normal, non–cell line–derived ECs. We studied the effect of A20 upon critical components within the molecular ordering of TNF-triggered apoptotic pathways and evaluated the effect of A20 upon other apoptotic and necrotic stimuli relevant to immune-based attack of the endothelium: namely, Fas, natural killer (NK) cells, and complement. Our data indicates that A20 protects ECs from TNF-mediated apoptosis by inhibiting proteolytic cleavage of initiator caspases 8 and 2, effector caspases 3 and 6, Bid processing, and release of cytochrome c, hence preserving mitochondrial integrity. Furthermore, we show for the first time that A20 protects from Fas, significantly blunts NK cell–mediated EC apoptosis, and safeguards ECs from complement-mediated necrosis.
Materials and methods
Cell culture
Porcine aortic endothelial cells (PAECs) and bovine AECs (BAECs) were isolated, grown without any added growth factors as described,2,3 and used between passages 4 and 8. Human coronary arterial endothelial cells (HCAECs) were purchased from Clonetics (San Diego, CA) and grown according to the manufacturer's instructions. NK 92 cell line (ATCC, Rockville, MD) was cultured in Myelocult H1500 (StemCell Technologies, Vancouver, BC, Canada) supplemented with 500 U/mL IL-2 (R&D Systems, Minneapolis, MN) as described.27
Recombinant adenoviral vectors
The recombinant adenoviral vector encoding human A20 (rAdA20) was generated in our laboratory as described.3 The control β-galactosidase adenovirus (rAdβ-gal) was a kind gift from Dr R. Gerard (University of Texas SW, Dallas, TX) and was propagated in our laboratory. The A20 expression plasmid used to generate the rAdA20 was a kind gift from Dr V. Dixit (Genentech, South San Francisco, CA). Both rAdA20 and rAdβ-gal were generated by recombination of PJM17 and the pAC shuttle vector in 293 cells as described.28 PJM17 is a circular plasmid comprising the full-length adenovirus 5 genome together with a 4.3 kb insert (pBRX). Cotransfection with the shuttle plasmid pAC, which contains the left end of Ad5 with a deletion in the early region 1 (E1) flanking a cytomegalovirus (CMV) promoter and a multiple cloning site, efficiently recombines with PJM17 and give rise to infectious viruses expressing the transgene cloned in pAC.29 PAECs were infected with rAdA20 and rAdβ-gal at a multiplicity of infection (MOI) of 25 to 500. Infection with an MOI of 100 resulted in 95% to 100% of cells expressing the transgene without causing toxicity as measured by lactate dehydrogenase (LDH) release.
Cell extracts and Western blotting
All cell extracts were recovered 6 hours following treatment with CHX and TNF and analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting with the use of standard techniques. For Bid, cell extracts were recovered according to the method described by Gross et al.30 Bid (24 kD) was detected by means of a rabbit anti-Bid polyclonal antibody from Pharmingen (San Diego, CA). For cytochrome c, cell pellets were suspended in an extraction buffer containing 220 mM mannitol, 68 mM sucrose, 50 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) pH 7.5, 50 mM KCL, 5 mM EGTA (ethylene glycol tetraacetic acid), 2 mM MgCl2, 1 mM dithiothreitol (DTT), 1 mM 4-(2-aminoethyl)benzenesulfonyl fluoride, 10 μg/mL pepstatin A, and leupeptin. Cytochrome c was detected with the mouse anti–cytochrome c monoclonal antibody (clone 7H8.2C12) from Pharmingen. For caspases, cell extracts were recovered as described.31 Procaspases 3, 8, and 9 were detected with mouse anti–human caspase 8 monoclonal antibody (mAb) (Oncogene Research Products, Cambridge, MA) and rabbit polyclonal anti–human caspases 3 and 9 (Pharmingen). A20 expression was detected by the polyclonal rabbit antiserum (A20-NT) used at a dilution of 1:800; this Ab recognizes an N-terminus peptide sequence of human A20 (IRERTPEDIFKPTN). Secondary antibodies used included goat anti–mouse immunoglobulin G (IgG) Fc and donkey anti–rabbit IgG (H [heavy chains] plus L [light chains]) conjugated to horseradish peroxidase (Pierce, Rockford, IL).
In vitro measurement of caspase activity
Cell extracts were recovered with the use of the BioVision extraction buffer (BioVision, Palo Alto, CA) and assessed for caspase 2, 3, 6, 8, and 9 activities by means of colorimetric probes (BioVision; also Chemicon International, Temecula, CA). Colorimetric caspase assay kits are based on detection of the chromophore p-nitroanilide (pNA) after cleavage from caspase-specific–labeled substrates. Colorimetric readings were performed in an LKB enzyme-linked immunosorbent assay (ELISA) plate reader at an optical density of 405 nm. A specific caspase 8 inhibitor IETD–Acetyl-IETD-C22H34N4O20 [Ac-IETD-CHO]) was used in some of the experiments (Chemicon).
Analysis of apoptosis
Apoptosis was quantified by fluorescence-activated cell sorter (FACS) analysis of DNA content as described.3 Cells with a normal DNA content (at least diploid [2N]) were scored as viable, whereas cells with a hyplodiploid DNA content (lower than 2N, termed Ao) were scored as apoptotic.
Cytotoxicity assay
Cytotoxicity was assessed by calcein fluorescence release assay. PAECs were plated in 96-well plates, labeled for 30 minutes at 37° C with 50 μM calcein–acetoxymethyl ester (Molecular Probes, Eugene, OR), and washed with 1 × phosphate-buffered saline (PBS). NK92 cells were then added at various effector (NK92)–to–target (PAECs) (E/T) ratios. At 6 hours following incubation, the plate was centrifuged and 75 μL culture supernatant was recovered for calcein measurement by means of the Wallac 1420 fluorescence multiwell plate reader (Perkin Elmer, Shelton, CT) at excitation and emission wavelengths of 485 nm and 530 nm, respectively. Percentage of cytotoxicity was calculated as a ratio as follows: OD [sample]-OD [SR]/OD [TR]-OD [SR], where OD is optical density, SR is spontaneous release, and TR is total release. SR corresponds to the background calcein released in supernatants of nontreated PAECs. TR was measured following disruption of PAECs with 1% Triton 100-X.
Measurement of mitochondrial transmembrane potential (Δψm)
The Δψm was quantified by means of a cationic fluorescent probe, rhodamine 123 (RH 123) (Molecular Probes), which accumulates in functional mitochondria having high Δψm. Active mitochondria in viable cells are stained bright green. Loss of gradient within nonviable cells results in loss of fluorescence. At 4 hours following treatment with CHX and TNF, cells were recovered, and 5 μL of 1 mg/mL rhodamine 123 was added to 106 cells per milliliter and incubated 5 minutes at 37° C. Cells were then analyzed by FACS analysis.
Complement-induced EC cytotoxicity assay
PAECs were grown in a 96-well plate and incubated with 10% and 20% human blood serum pool (hS), selected for normal complement levels and high titer of antiporcine ECs (kind gift of Dr Augustine Dalmasso, University of Minnesota, Minneapolis, MN). Complement-mediated cytotoxicity was evaluated 2.5 hours later by means of LDH release with the use of the CytoTox 9600 nonradioactive kit (Promega, Madison, WI) according to the manufacturer's instructions. PAECs treated with 10% heat-inactivated human serum (HIhS) served as the control for nonspecific cell death, and PAECs left in the growth medium served as controls for 100% viability.
Evaluation of apoptosis in a transient transfection assay
BAECs grown in 6-well plates were transfected with 0.2 μg reporter plasmid Rous sarcoma virus (RSV) β-gal; 0.7 μg A20 expression plasmid or the control pcDNA3; and 0.3 μg full-length human hemagglutinin (HA)–tagged Fas expression plasmid or 0.025 μg Fas-associated death domain (FADD) or the dominant negative FADD (FADD-DN) plasmids (Fas plasmid is a kind gift of Dr Richard Siegel, National Institutes of Health [NIH], Bethesda, MD; FADD and FADD-DN plasmids are kind gifts of Dr Vishva Dixit, Genentech, CA), with the use of lipofectamine (Gibco BRL, Grand Island, NY) or fugene (Roche, Indianapolis, IN). Additional pcDNA3 was added to adjust for a total of 1.6 μg DNA per well. At 24 hours after transfection, cells were either (1) fixed with 0.05% glutaraldehyde and stained with 5-bromo-4-chloro-3-indolyl β-D-galactopyranoside, and the number of living beta-galactosidase positive cells were evaluated in random 3 high-power fields (HPFs) per well,32 or (2) treated with 1 μg/mL mouse anti–human Fas IgM antibody (αFas (IPO-4; Kamiya Biomedical, Seattle, WA) for 6 hours prior to fixation, staining, and counting.
Porcine PBMC preparation and FACS analysis of Fas surface expression
Porcine blood was obtained from the same pig donors (miniature swine) that provided PAECs (kind gift of Dr David Sachs, Massachussetts General Hospital, Boston, MA). Peripheral blood mononuclear cells (PBMCs) were purified by means of a Ficoll gradient, and surface expression of Fas was evaluated by FACS analysis with the use of the same αFas antibody. Secondary antibodies included a goat anti–mouse IgM antibody and a fluorescein-labeled rabbit anti–goat IgG (Pierce). A control mouse IgM was also used in these studies (Accurate Chemical and Scientific, Westbury, NY).
Results
Expression of A20 in PAECs protects from CHX/TNF-mediated apoptosis
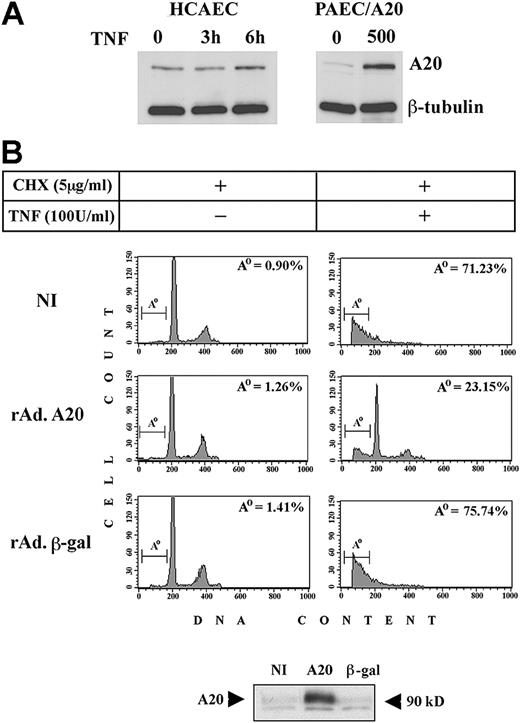
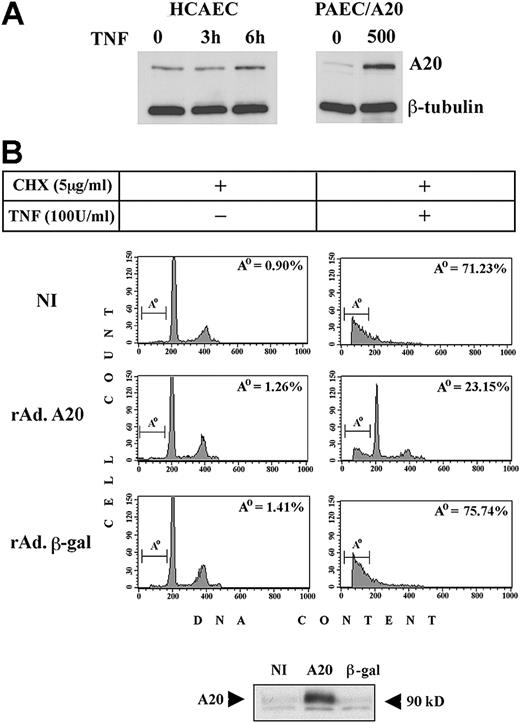
We first compared the levels of A20 protein in PAECs infected with rAdA20 at an MOI of 500 (the highest infection we used) and the physiologic up-regulation of A20 in human ECs (human coronary artery ECs [HCAECs]) treated with 100 U/mL TNF for 3 and 6 hours. Our results showed that A20 was detected in nontreated (NT) HCAECs and increased by 1.3- to 2.15-fold 6 hours following TNF treatment, as assessed by densitometry. In contrast, little or no A20 was detected in noninfected (NI) PAECs, and expression rose by 3.7- to 5.7-fold 48 hours after infection with rAdA20 (Figure 1A) (number of experiments [n] = 3). These data are in agreement with the 6-fold difference in mRNA levels detected by competitive quantitative reverse transcription–polymerase chain reaction (RT-PCR) (data not shown) and demonstrate that overexpression of A20 by means of the rAd we used is within an acceptable physiologic range. We then examined the protective effect of A20 against CHX/TNF-mediated apoptosis. Noninfected PAECs and PAECs infected with rAdA20 or the control rAdβ-gal at an MOI of 100 were pretreated with 5 μg/mL CHX for 1 hour followed by 100 U/mL TNF for 6 hours. Cells were recovered and analyzed for apoptosis by FACS analysis. The percentage (mean ± standard deviation [SD]) of cells undergoing apoptosis rose from 4.74% ± 2.66% to 68% ± 9.7%, and from 7.63% ± 5.83% to 68.9% ± 8.5%, respectively, in NI PAECs and PAECs infected with rAdβ-gal. In contrast, PAECs infected with rAdA20 were significantly protected from TNF-mediated apoptosis (P < .0001; n = 7). The percentage of apoptotic cells increased from 6% ± 2.9% to 27.8% ± 5% after treatment with CHX/TNF (Figure 1B). Expression of A20 in PAECs was verified by Western blot analysis (Figure 1B). Similar results were obtained when cells were infected with rAdA20 at an MOI of 25, but the percentage of infected cells was below 80%. Unless otherwise stated, all experiments were performed at an MOI of 100. By Western blot analysis of IκBα, we checked that expression of A20 in PAECs (MOI of 100) still inhibited its degradation following TNF stimulation and, hence, was still inhibitory of NF-κB activation (data not shown).
Effect of A20 overexpression on apoptotic fragmentation. Overexpression of A20 prevents apoptotic fragmentation of cellular DNA in CHX/TNF-treated PAECs. (A) Expression of A20 was evaluated by Western blot analysis in HCAECs before and 3 and 6 hours following treatment with TNF and compared with A20 expression in NI PAECs and rAdA20-infected PAECs at an MOI of 500. In this system, rAd-mediated expression of A20 reached 2.6- to 4.3-fold that of the physiologically induced levels. Data shown are representative of 3 experiments performed. (B) Noninfected PAECs and PAECs infected with rAdβ-gal or rAdA20 were treated with CHX (2 μg/mL) and TNF (100 U/mL), either alone or in combination, for 6 hours. Apoptosis was assessed by DNA content analysis by means of flow cytometry. The region below the G1/G0 peak, designated Ao, represents cells undergoing apoptosis with fractional DNA content (less than 2N) and is presented as a percentage of the total events collected. A20 significantly (P < .0001) protected PAECs from CHX/TNF-mediated apoptosis. Expression of the A20 transgene in each experiment was assessed by Western blot analysis of cell extracts from rAdA20-infected PAECs at an MOI of 100 (arrows). Data shown are representative of 7 independent experiments.
Effect of A20 overexpression on apoptotic fragmentation. Overexpression of A20 prevents apoptotic fragmentation of cellular DNA in CHX/TNF-treated PAECs. (A) Expression of A20 was evaluated by Western blot analysis in HCAECs before and 3 and 6 hours following treatment with TNF and compared with A20 expression in NI PAECs and rAdA20-infected PAECs at an MOI of 500. In this system, rAd-mediated expression of A20 reached 2.6- to 4.3-fold that of the physiologically induced levels. Data shown are representative of 3 experiments performed. (B) Noninfected PAECs and PAECs infected with rAdβ-gal or rAdA20 were treated with CHX (2 μg/mL) and TNF (100 U/mL), either alone or in combination, for 6 hours. Apoptosis was assessed by DNA content analysis by means of flow cytometry. The region below the G1/G0 peak, designated Ao, represents cells undergoing apoptosis with fractional DNA content (less than 2N) and is presented as a percentage of the total events collected. A20 significantly (P < .0001) protected PAECs from CHX/TNF-mediated apoptosis. Expression of the A20 transgene in each experiment was assessed by Western blot analysis of cell extracts from rAdA20-infected PAECs at an MOI of 100 (arrows). Data shown are representative of 7 independent experiments.
A20 expression in PAECs inhibits TNF-mediated activation of caspases
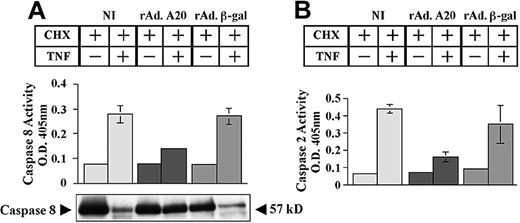
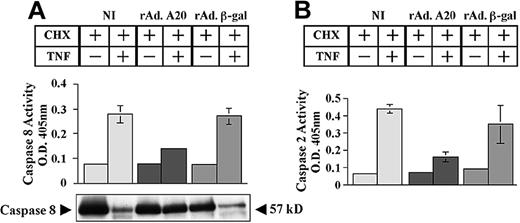
We questioned whether expression of A20 in PAECs affects caspase activation and mitochondrial integrity. NI PAECs and PAECs infected with rAdA20 and rAdβ-gal were treated with CHX/TNF, and caspase activity was evaluated 6 hours later by means of a colorimetric assay. Expression of A20 in PAECs inhibited the activation of initiator caspases 2 and 8. Caspase 8 activity (mean ± SD) increased from 0.07 ± 0.003 to 0.28 ± 0.035 and from 0.07 to 0.27 ± 0.03 in NI and rAdβ-gal–infected PAECs, respectively (Figure 2A). In contrast, rAdA20-infected PAECs showed minimal rise of caspase 8 activity (0.07 ± 0.001 to 0.14 ± 0.009, P < .0001, n = 4). A20 inhibited caspase 8 at the level of its processing. The 57 kD procaspase 8 protein was barely detected in NI and rAdβ-gal–infected PAECs following TNF treatment, whereas it remained intact in rAdA20-infected PAECs (Figure 2A).
Effect of A20 on TNF-induced activation of initiator caspases 8 and 2. A20 inhibits TNF-induced activation of the initiator caspases 8 and 2 by blocking their proteolytic cleavage. Noninfected PAECs and PAECs infected with rAd.β-gal or rAdA20 were treated with CHX (2 μg/mL) alone or with CHX and TNF (100 U/mL) for 6 hours. Caspase 8 (A) and caspase 2 (B) activities were then analyzed in the PAEC lysates by means of a colorimetric assay based on spectrophotometric detection of the chromophore p-nitroanilide (pNA) after cleavage from the caspase-specific–labeled substrates IETD-ρNA for caspase 8 (panel A) and VDVAD-pNA for caspase 2 (panel B). Expression of A20 significantly inhibited TNF-induced activation of caspases 8 and 2. Western blot analysis of procaspase 8 demonstrated that A20 inhibited TNF-induced proteolytic cleavage of caspase 8 (A). The molecular mass of the inactive proform (57 kDa) of the procaspase 8 is indicated (arrows). Data shown represent mean ± SD of triplicate values and are representative of 4 independent experiments. Light gray bars correspond to NI cells; dark gray bars to rAdA20-infected cells; and medium gray bars to rAdβ-gal–infected cells.
Effect of A20 on TNF-induced activation of initiator caspases 8 and 2. A20 inhibits TNF-induced activation of the initiator caspases 8 and 2 by blocking their proteolytic cleavage. Noninfected PAECs and PAECs infected with rAd.β-gal or rAdA20 were treated with CHX (2 μg/mL) alone or with CHX and TNF (100 U/mL) for 6 hours. Caspase 8 (A) and caspase 2 (B) activities were then analyzed in the PAEC lysates by means of a colorimetric assay based on spectrophotometric detection of the chromophore p-nitroanilide (pNA) after cleavage from the caspase-specific–labeled substrates IETD-ρNA for caspase 8 (panel A) and VDVAD-pNA for caspase 2 (panel B). Expression of A20 significantly inhibited TNF-induced activation of caspases 8 and 2. Western blot analysis of procaspase 8 demonstrated that A20 inhibited TNF-induced proteolytic cleavage of caspase 8 (A). The molecular mass of the inactive proform (57 kDa) of the procaspase 8 is indicated (arrows). Data shown represent mean ± SD of triplicate values and are representative of 4 independent experiments. Light gray bars correspond to NI cells; dark gray bars to rAdA20-infected cells; and medium gray bars to rAdβ-gal–infected cells.
Next, we examined the effect of A20 upon activation of the initiator caspase 2. Caspase 2 activity (mean ± SD) increased from 0.06 ± 0.001 to 0.4 ± 0.025 and from 0.09 ± 0.007 to 0.35 ± 0.11 in NI and rAdβ-gal–infected PAECs, respectively (Figure 2B). In contrast, rAdA20-infected PAECs showed minimal rise of caspase 2 activity (0.07 ± 0.002 to 0.16 ± 0.027, P = .001, n = 4).
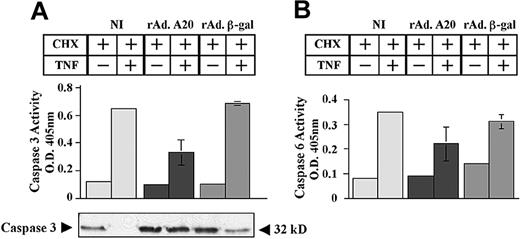
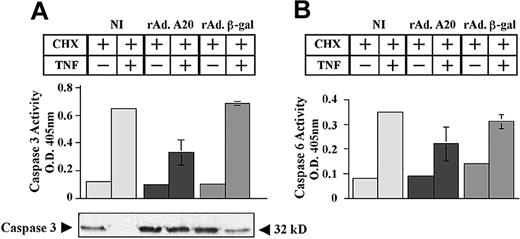
Consequently, expression of A20 halted the sequential activation of effector caspases 3 and 6 (Figure 3). Caspase 3 activity increased from 0.11 ± 0.003 to 0.65 ± 0.007 and from 0.1 ± 0.008 to 0.68 ± 0.014 in NI and rAdβ-gal–infected PAECs, respectively (Figure 3A). In contrast, rAdA20-infected PAECs showed minimal rise of caspase 3 activity (0.1 ± 0.01 to 0.3 ± 0.09, P < .0001, n = 4). Inhibition of caspase 3 activation by A20 occurred at the level of its proteolytic cleavage (Figure 3A).
Effect of A20 on TNF-induced activation of effector caspases 3 and 6. A20 inhibits TNF-induced activation of the effector caspases 3 and 6 by blocking their proteolytic cleavage. NI PAECs and PAECs infected with rAd.β-gal or rAdA20 were treated with CHX (2 μg/mL) alone or with CHX and TNF (100 U/mL) for 6 hours. Caspase 3 (A) and caspase 6 (B) activities were analyzed in the PAEC lysates by means of a colorimetric assay based on spectrophotometric detection of the chromophore ρNA after cleavage from the caspase-specific–labeled substrates DEVD-pNA for caspase 3 (A) and VEID-pNA for caspase 6 (B). Expression of A20 significantly inhibited TNF-induced activation of caspases 3 and 6. Western blot analysis of procaspase 3 demonstrated that A20 inhibited TNF-induced proteolytic cleavage of caspase 3 (A). The molecular mass of the inactive proform of the procaspase 3 (32 kD) is indicated (arrows). Data shown are mean ± SD of triplicate values and are representative of 4 independent experiments. Light gray bars correspond to NI cells; dark gray bars to rAdA20-infected cells; and medium gray bars to rAdβ-gal–infected cells.
Effect of A20 on TNF-induced activation of effector caspases 3 and 6. A20 inhibits TNF-induced activation of the effector caspases 3 and 6 by blocking their proteolytic cleavage. NI PAECs and PAECs infected with rAd.β-gal or rAdA20 were treated with CHX (2 μg/mL) alone or with CHX and TNF (100 U/mL) for 6 hours. Caspase 3 (A) and caspase 6 (B) activities were analyzed in the PAEC lysates by means of a colorimetric assay based on spectrophotometric detection of the chromophore ρNA after cleavage from the caspase-specific–labeled substrates DEVD-pNA for caspase 3 (A) and VEID-pNA for caspase 6 (B). Expression of A20 significantly inhibited TNF-induced activation of caspases 3 and 6. Western blot analysis of procaspase 3 demonstrated that A20 inhibited TNF-induced proteolytic cleavage of caspase 3 (A). The molecular mass of the inactive proform of the procaspase 3 (32 kD) is indicated (arrows). Data shown are mean ± SD of triplicate values and are representative of 4 independent experiments. Light gray bars correspond to NI cells; dark gray bars to rAdA20-infected cells; and medium gray bars to rAdβ-gal–infected cells.
Similarly, caspase 6 activity increased from 0.08 ± 0.005 to 0.35 ± 0.003 and from 0.15 ± 0.005 to 0.3 ± 0.03 in NI and rAdβ-gal–infected PAECs, respectively (Figure 3B). In contrast, rAdA20-infected PAECs showed minimal rise of caspase 6 activity (0.09 ± 0.003 to 0.2 ± 0.075, P = .0008, n = 4).
A20 expression in PAECs preserves mitochondrial integrity following TNF treatment
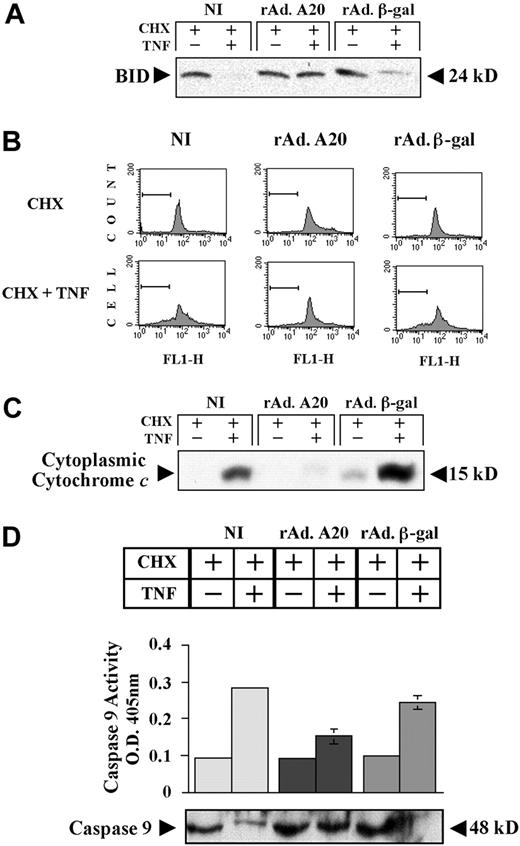
We questioned whether blockade of initiator caspases by A20 in ECs is sufficient to interrupt the amplification cascade leading to cleavage of the Bcl family member Bid by caspase 8 and subsequent disruption of the mitochondrial transmembrane potential, allowing release of cytochrome c into the cytoplasm.33 NI PAECs and PAECs infected with rAdβ-gal and rAdA20 were treated with CHX/TNF, and cells extracts were recovered 6 hours later and evaluated for Bid expression. The 24-kD full-length Bid protein detected in basal conditions was substantially decreased following CHX/TNF treatment in NI and rAdβ-gal–infected PAECs indicating its proteolytic cleavage, whereas it remained unchanged in A20-expressing PAECs (Figure 4A). We next examined the effect of A20 on the Δψm by FACS analysis using the dye RH 123. NI PAECs and PAECs infected with rAdβ-gal showed a loss of Δψm as indicated by a decrease in RH 123 fluorescence 4 hours after treatment with CHX/TNF (Figure 4B). In contrast, rAdA20-infected PAECs maintained their Δψm and showed minimal change in RH 123 fluorescence. Consequently, rAdA20-infected PAECs had no detectable cytochrome c in the cytoplasm as compared with NI and rAdβ-gal–infected cells, where a significant amount of cytochrome c was released in the cytoplasm following CHX/TNF (Figure 4C). Release of cytochrome c in NI and rAdβ-gal–infected cells was associated with the activation of caspase 9 as shown by colorimetric assay (caspase 9 activity [mean ± SD] increased from 0.1 ± 0.01 to 0.3 ± 0.014 and from 0.1 ± 0.003 to 0.25 ± 0.02, respectively) and the loss of the 48 kD procaspase 9 band by Western blot following CHX/TNF (Figure 4D). In contrast, rAdA20-infected PAECs demonstrated no activation of caspase 9 (caspase 9 activity increased from 0.095 ± 0.005 to 0.15 ± 0.022, P < .0001, n = 4), and the 48 kD procaspase 9 band was not altered by CHX/TNF treatment (Figure 4C). These data establish the antiapoptotic effect of A20 in ECs at the level of death receptor–associated caspases or upstream of them.
Effect of A20 preservation on mitochondrial integrity in PAECs after TNF treatment. A20 expression in PAECs preserves mitochondrial integrity following TNF treatment by inhibiting proteolytic cleavage of Bid, maintaining the Δψm, and preventing cytochrome c release and activation of caspase 9. (A) Western blot analysis of BID cleavage in NI PAECs and PAECs infected with rAdA20 or rAdβ-gal 6 hours following treatment with CHX/TNF. A20 expression in PAECs inhibited TNF-induced cleavage of BID (24 kD). Data shown are representative of 3 independent experiments. (B) NI PAECs and PAECs infected with rAdA20 or rAdβ-gal were treated with CHX/TNF for 4 hours. PAECs were then recovered and labeled with the RH 123 dye, and the Δψm was analyzed by FACS analysis in fluorescence channel 1 (FL-1) for green fluorescence. Cells with normal Δψm exhibit strong rhodamine fluorescence. Cells with low fluorescence reflect loss of the Δψm (gated area). Expression of A20 in PAECs significantly decreased the loss of the Δψm. Data shown are representative of 3 independent experiments. (C) NI PAECs and PAECs infected with rAdA20 or rAdβ-gal were treated with CHX or CHX/TNF for 6 hours. Cytoplasmic cell extracts were recovered and evaluated by Western blot analysis for cytochrome c expression. Expression of A20 inhibited TNF-mediated release of cytochrome c in the cytoplasm. Data shown are representative of 3 independent experiments. (D) NI PAECs and PAECs infected with rAd.β-gal or rAdA20 were treated with CHX alone or with CHX/TNF for 6 hours. Caspase 9 activity was analyzed in PAEC lysates by means of a colorimetric assay based on spectrophotometric detection of the chromophore pNA after cleavage from the caspase-specific–labeled substrate LEHD-pNA. Expression of A20 significantly inhibited TNF-induced activation of caspase 9. Western blot analysis of procaspase 9 demonstrated that A20 inhibited TNF-induced proteolytic cleavage of caspase 9. The molecular mass of the inactive proform of the procaspase 9 (48 kD) is indicated (arrows). Data are expressed as mean ± SD of triplicate values. Results shown are representative of 4 independent experiments.
Effect of A20 preservation on mitochondrial integrity in PAECs after TNF treatment. A20 expression in PAECs preserves mitochondrial integrity following TNF treatment by inhibiting proteolytic cleavage of Bid, maintaining the Δψm, and preventing cytochrome c release and activation of caspase 9. (A) Western blot analysis of BID cleavage in NI PAECs and PAECs infected with rAdA20 or rAdβ-gal 6 hours following treatment with CHX/TNF. A20 expression in PAECs inhibited TNF-induced cleavage of BID (24 kD). Data shown are representative of 3 independent experiments. (B) NI PAECs and PAECs infected with rAdA20 or rAdβ-gal were treated with CHX/TNF for 4 hours. PAECs were then recovered and labeled with the RH 123 dye, and the Δψm was analyzed by FACS analysis in fluorescence channel 1 (FL-1) for green fluorescence. Cells with normal Δψm exhibit strong rhodamine fluorescence. Cells with low fluorescence reflect loss of the Δψm (gated area). Expression of A20 in PAECs significantly decreased the loss of the Δψm. Data shown are representative of 3 independent experiments. (C) NI PAECs and PAECs infected with rAdA20 or rAdβ-gal were treated with CHX or CHX/TNF for 6 hours. Cytoplasmic cell extracts were recovered and evaluated by Western blot analysis for cytochrome c expression. Expression of A20 inhibited TNF-mediated release of cytochrome c in the cytoplasm. Data shown are representative of 3 independent experiments. (D) NI PAECs and PAECs infected with rAd.β-gal or rAdA20 were treated with CHX alone or with CHX/TNF for 6 hours. Caspase 9 activity was analyzed in PAEC lysates by means of a colorimetric assay based on spectrophotometric detection of the chromophore pNA after cleavage from the caspase-specific–labeled substrate LEHD-pNA. Expression of A20 significantly inhibited TNF-induced activation of caspase 9. Western blot analysis of procaspase 9 demonstrated that A20 inhibited TNF-induced proteolytic cleavage of caspase 9. The molecular mass of the inactive proform of the procaspase 9 (48 kD) is indicated (arrows). Data are expressed as mean ± SD of triplicate values. Results shown are representative of 4 independent experiments.
A20 protects ECs from Fas-mediated apoptosis
We next evaluated whether expression of A20 in ECs affects the Fas death receptor pathway. BAECs were cotransfected with the RSV β-gal reporter, the Fas expression plasmid, and the A20 expression plasmid or the control pcDNA3. Cell viability was evaluated 24 hours after transfection and 6 hours following treatment with αFas antibody by counting the number of blue cells per HPF. Three HPFs were counted per well in triplicate wells. The number of blue cells per HPF in Fas-transfected cells was 20 ± 7 (mean ± SD) 24 hours after transfection, whereas it reached 36 ± 15 in BAECs transfected with control pCDNA3 plasmid. Coexpression of A20 completely rescued Fas-expressing ECs from death (P < .0001, n = 3). The number of blue cells in Fas/A20-transfected BAECs was 59 ± 24 per HPF (Figure 5A). Treatment of Fas-transfected ECs with αFas led to a significant decrease in the number of blue cells reaching 4 ± 3 per HPF. A significant decrease in blue cells counts (9 ± 5 per HPF) was also noted in BAECs transfected with pcDNA3 alone, reflecting constitutive Fas expression in BAEC cultures. Coexpression of A20 totally abrogated αFas/Fas-mediated cell death. The number of blue cells remained at 46 ± 23 per HPF following αFas treatment (P < .0001, n = 3). Ligation of Fas leads to caspase 8 recruitment and subsequent activation of the caspase cascade via the adapter molecule FADD, whose overexpression is as effective as Fas ligation in inducing apoptotic cell death. We checked the effect of A20 expression upon FADD-mediated apoptosis and caspase 8 activation. BAECs were cotransfected with the RSV β-gal reporter, the FADD expression plasmid, and the A20 expression plasmid or the control pcDNA3. Cell viability was evaluated 24 hours after transfection by counting the number of blue cells per well. The number of blue cells per well in FADD-transfected cells was 150 ± 4 (mean ± SD) 24 hours after transfection, whereas it reached 650 ± 168 in BAECs transfected with the dominant negative FADD (FADD-DN). Coexpression of A20 with FADD completely rescued ECs from death (P = .003, n = 4). The number of blue cells in FADD/A20-transfected BAECs was 590 ± 114 per well (Figure 5B).
A20 protection from Fas- and FADD-induced cell death and caspase activation. A20 expression protects ECs from Fas- and FADD-induced cell death and caspase activation. BAECs were transfected with a control, a pcDNA3 plasmid or an A20 expression plasmid, and a β-gal expression plasmid. (A) Cotransfection with Fas alone (nontreated [NT]) resulted in cell death, which was enhanced by Fas cross-linking (αFas). Cell death was quantified by blue cell count per HPF. A20 significantly protected from Fas-mediated cell death. Data are expressed as mean ± SD of 3 HPFs per triplicate well. Results shown are representative of 3 independent experiments. (B) Similar results were obtained when cells were cotransfected with FADD. Cotransfection of BAECs with a dominant negative FADD was used as control. Caspase 8 (C) and caspase 3 (D) activities were determined in lysates of BAECs cotransfected with pcDNA3 or A20 plasmids and FADD expression plasmid by means of colorimetric assays based on the caspase-specific cleavage of labeled substrates (IETD-pNA for caspase 8 and DEVD-pNA for caspase 3). Expression of A20 significantly inhibited FADD-mediated activation of caspases 8 and 3. Data are expressed as mean ± SD of triplicate values. Results shown are representative of 4 independent experiments.
A20 protection from Fas- and FADD-induced cell death and caspase activation. A20 expression protects ECs from Fas- and FADD-induced cell death and caspase activation. BAECs were transfected with a control, a pcDNA3 plasmid or an A20 expression plasmid, and a β-gal expression plasmid. (A) Cotransfection with Fas alone (nontreated [NT]) resulted in cell death, which was enhanced by Fas cross-linking (αFas). Cell death was quantified by blue cell count per HPF. A20 significantly protected from Fas-mediated cell death. Data are expressed as mean ± SD of 3 HPFs per triplicate well. Results shown are representative of 3 independent experiments. (B) Similar results were obtained when cells were cotransfected with FADD. Cotransfection of BAECs with a dominant negative FADD was used as control. Caspase 8 (C) and caspase 3 (D) activities were determined in lysates of BAECs cotransfected with pcDNA3 or A20 plasmids and FADD expression plasmid by means of colorimetric assays based on the caspase-specific cleavage of labeled substrates (IETD-pNA for caspase 8 and DEVD-pNA for caspase 3). Expression of A20 significantly inhibited FADD-mediated activation of caspases 8 and 3. Data are expressed as mean ± SD of triplicate values. Results shown are representative of 4 independent experiments.
Protection of ECs from FADD-mediated apoptosis occurred at the level of caspase 8 activation. At 24 hours following transfection, caspase 8 activity (mean ± SD) was 0.1 ± 0.008 in FADD-transfected BAECs, twice as much the background activity detected in the control FADD-DN–transfected BAECs (0.05 ± 0.003) (Figure 5C). Cotransfection with A20 completely inhibited FADD-mediated activation of caspase 8 (0.05 ± 0.004, P < .0001, n = 4) (Figure 5C).
FADD-mediated activation of the downstream executioner caspase 3 was also blocked by expression of A20. Caspase 3 activity (mean ± SD) was at 0.3 ± 0.05 in FADD-transfected BAECs, twice the background activity detected in the FADD-DN–transfected BAECs (0.15 ± 0.03) (Figure 5D). Cotransfection with A20 inhibited FADD-mediated activation of caspase 3 (0.1 ± 0.006, P = .0003, n = 4) (Figure 5D). This is the first demonstration that expression of A20 is protective against Fas-mediated apoptosis.
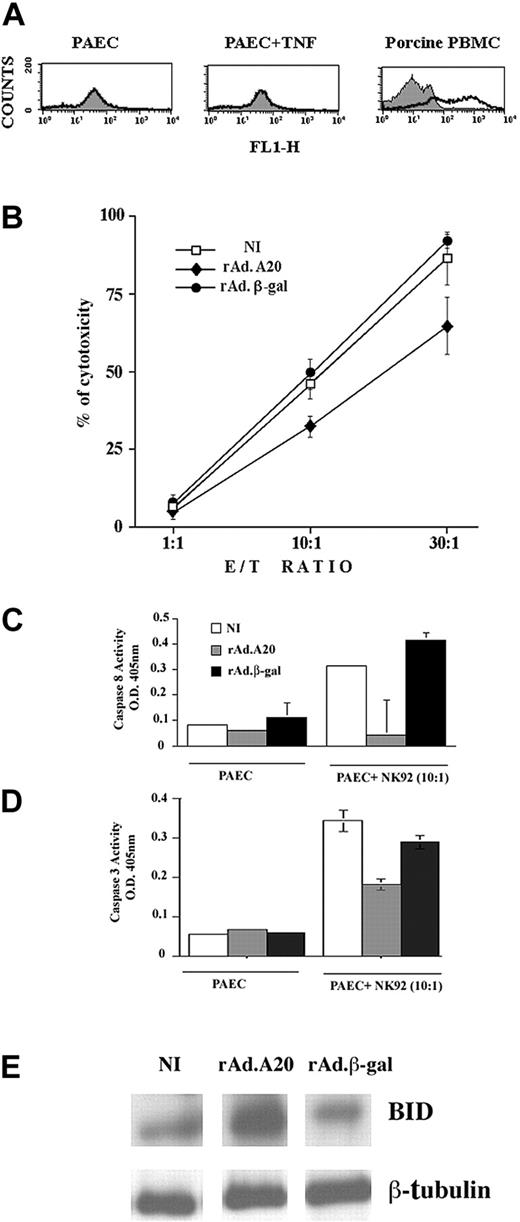
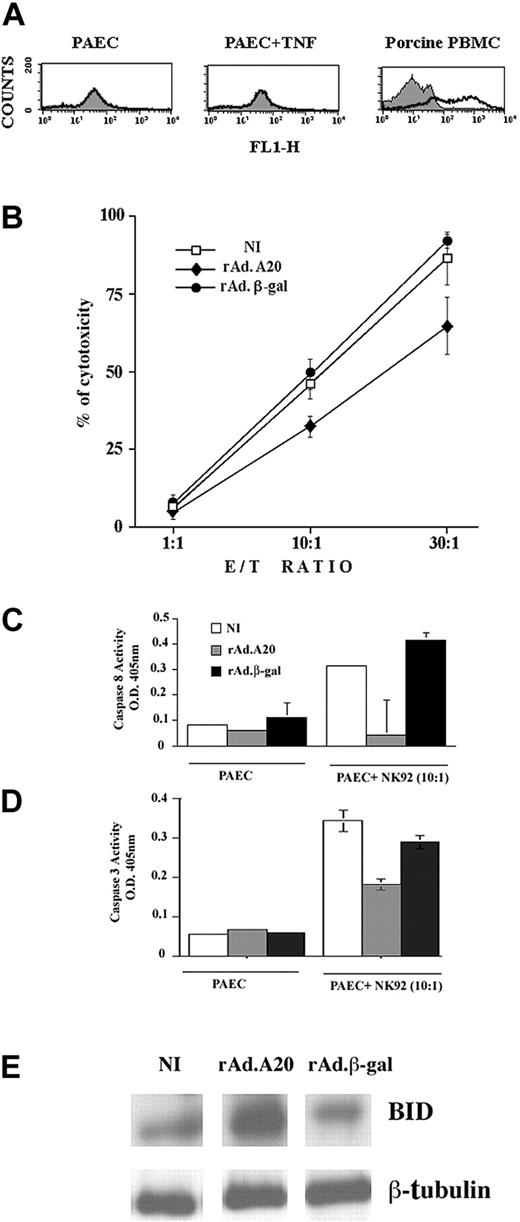
A20 protects ECs from NK-mediated cell death
Having demonstrated that A20 protected ECs from apoptosis triggered by death receptors, we questioned whether A20 would also interfere with cytotoxic pathways triggered by perforin/granzymes. Human NK cells lyse xenogeneic porcine ECs via the perforin/granzyme B pathway.34 We validated this system by showing that our PAEC cultures were devoid of Fas surface expression. FACS analysis of Fas expression using mouse IgM anti–human Fas showed no expression on resting PAECs and no induction at 6 and 24 hours following addition of 100 U/mL TNF. In contrast, Fas expression was readily detected with the use of the same αFas antibody on porcine PBMCs (Figure 6A). Calcein-labeled NI, rAdβ-gal–infected, and rAdA20-infected PAECs were then cocultured with the human NK92 cell line at E/T ratios of 1:1, 10:1, and 30:1. Cytotoxicity was analyzed 16 hours later by measuring calcein release. The percentage of cytotoxicity (mean ± SD) in NI PAECs increased from 6% ± 2% to 46% ± 8% and 87% ± 15% at E/T ratios of 1:1, 10:1, and 30:1, respectively (Figure 6B). Similar levels of cytotoxicity were observed in rAdβ-gal–infected PAECs, increasing from 7% ± 5% to 49% ± 8% and 92% ± 4%. Expression of A20 led to a significant 30% decrease in NK-mediated cytotoxicity (Figure 6B). The percentage of cytotoxicity in A20-expressing PAECs increased from 4% ± 4% to 32% ± 6% and 65% ± 16% at E/T ratios of 1:1, 10:1, and 30:1, respectively (n = 6, P < .0001 for rAdA20 versus NI and rAdβ-gal–infected PAECs). Addition of the pancaspase inhibitor DEVD-fmk to PAEC-NK cocultures led to 30% protection of ECs against NK-mediated toxicity, a percentage similar to that seen with A20 (data not shown), which suggests that the protective effect of A20 against NK-mediated toxicity (presumably granzyme/perforin in our system) related to blockade of caspase activity. Given the known involvement of caspases 8 and 3 in granule-mediated cytotoxicity, we measured caspase 8 and 3 activity in PAEC/NK cocultures using a colorimetric assay. Our results indicate that caspase 8 activity is increased 16 hours following addition of NK cells to NI and rAdβ-gal–infected PAECs (OD = 0.31 ± 0.004 and 0.41 ± 0.03, respectively). Expression of A20 in PAECs significantly decreased NK-mediated caspase 8 activity (OD = 0.04 ± 0.14) as compared with NI (P = .04) and rAdβ-gal–infected PAECs (P = .03, n = 4) (Figure 6C). Caspase 3 activity was similarly increased 16 hours following addition of NK cells to NI and rAdβ-gal–infected PAECs (OD = 0.34 ± 0.07 and 0.29 ± 0.04, respectively). Expression of A20 in PAECs significantly decreased NK-mediated caspase 3 activity (OD = 0.18 ± 0.04) as compared with NI (P = .0002) and rAdβ-gal–infected PAECs (P = .0005, n = 7) (Figure 6D). Concomitant with that, BID was cleaved 16 hours following addition of NK cells to NI and rAdβ-gal–infected PAECs as demonstrated by a substantial decrease in the 26 kD full-length BID signal. A20 substantially protected from BID cleavage (n = 3) (Figure 6E).
Mechanism of A20 protection from NK cell–mediated cell death. A20 expression protects ECs from NK cell–mediated cell death by blocking caspase 8 and 3 activation and inhibiting BID cleavage. (A) FACS analysis of Fas surface expression in PAECs and porcine PBMCs shows the absence of Fas expression in PAECs whether or not they were treated with TNF, while Fas is readily expressed on the surface of PBMCs collected from the same pig strain. (B) NI PAECs and PAECs infected with rAd.β-gal or rAdA20 were labeled with calcein and cocultured with NK 92 cells at an E/T ratio of 1:1, 10:1, and 30:1 for 4 hours. NK-induced cytotoxicity was measured by calcein release. Expression of A20 significantly protected PAECs from NK cell–mediated cytotoxicity. Data shown are representative of 6 experiments performed in triplicate. Caspase 8 (C) and caspase 3 (D) activities were analyzed in the PAEC/NK cocultures by means of a colorimetric assay based on spectrophotometric detection of the chromophore pNA after cleavage from the caspase-specific–labeled substrates. Expression of A20 significantly inhibited NK-induced activation of caspases 8 and 3. Data are expressed as mean ± SD of triplicate values. Results shown are representative of 4 independent experiments. (E) Western blot analysis of BID cleavage in NI PAECs and PAECs infected with rAdA20 or rAdβ-gal 6 hours following coculture with NK cells. A20 expression in PAECs inhibited NK-induced cleavage of BID (24 kD). Data shown are representative of 3 independent experiments. Equal loading was confirmed by β-tubulin expression.
Mechanism of A20 protection from NK cell–mediated cell death. A20 expression protects ECs from NK cell–mediated cell death by blocking caspase 8 and 3 activation and inhibiting BID cleavage. (A) FACS analysis of Fas surface expression in PAECs and porcine PBMCs shows the absence of Fas expression in PAECs whether or not they were treated with TNF, while Fas is readily expressed on the surface of PBMCs collected from the same pig strain. (B) NI PAECs and PAECs infected with rAd.β-gal or rAdA20 were labeled with calcein and cocultured with NK 92 cells at an E/T ratio of 1:1, 10:1, and 30:1 for 4 hours. NK-induced cytotoxicity was measured by calcein release. Expression of A20 significantly protected PAECs from NK cell–mediated cytotoxicity. Data shown are representative of 6 experiments performed in triplicate. Caspase 8 (C) and caspase 3 (D) activities were analyzed in the PAEC/NK cocultures by means of a colorimetric assay based on spectrophotometric detection of the chromophore pNA after cleavage from the caspase-specific–labeled substrates. Expression of A20 significantly inhibited NK-induced activation of caspases 8 and 3. Data are expressed as mean ± SD of triplicate values. Results shown are representative of 4 independent experiments. (E) Western blot analysis of BID cleavage in NI PAECs and PAECs infected with rAdA20 or rAdβ-gal 6 hours following coculture with NK cells. A20 expression in PAECs inhibited NK-induced cleavage of BID (24 kD). Data shown are representative of 3 independent experiments. Equal loading was confirmed by β-tubulin expression.
A20 protects ECs from complement-mediated but not heat-induced necrotic cell death
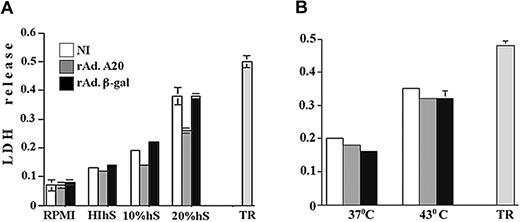
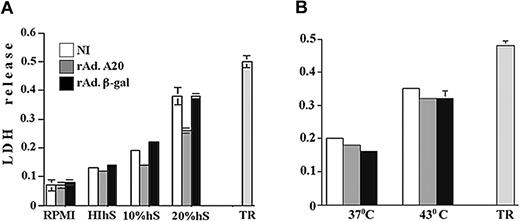
To determine whether A20 exclusively protects from caspase-dependent apoptotic pathways or also interferes with caspase-independent cytotoxic pathways, we examined whether expression of A20 in ECs affects necrotic cell death. We chose to evaluate the effect of A20 expression upon complement-mediated necrotic cell death. NI PAECs and PAECs infected with rAdβ-gal and rAdA20 were incubated with 10% complement-efficient human blood group AB+ serum, HIhS, or RPMI. Complement cytotoxicity was evaluated 6 hours later by the measurements of LDH release. The LDH release was similar among the 3 groups treated with RPMI (OD reaching 0.07 to 0.08) or HIhS (OD = 0.12 to 0.14). Treatment with 10% and 20% complement-efficient human serum led to a substantial increase in the LDH release, with OD (mean ± SD) reaching 0.19 ± 0.01.and 0.38 ± 0.09 in NI PAECs and 0.22 ± 0.01 and 0.38 ± 0.04 in rAdβ-gal–infected PAECs, respectively (Figure 7A). Expression of A20 inhibited LDH release; the OD for 10% hS was similar to that measured in cells treated with HIhS (OD = 0.13 ± 0.002, P < .0001, n = 4) and was significantly lower when PAECs were treated with 20% hS, reaching 0.26 ± 0.03 (P = .004 [NI] and P < .0001[β-gal]], n = 3 experiments done in replicates of 7) (Figure 7A). TR was measured following addition of 0.1% Triton 100-X to PAECs and showed an OD of 0.5 ± 0.05 (n = 3).
Effect of A20 expression on complementand heat-mediated necrotic cell death. A20 expression protects ECs from complement- but not heat-mediated necrotic cell death. NI PAECs and PAECs infected with rAdA20 or rAdβ-gal were treated with 10% and 20% human serum (hS) as a source of active complement (panel A) or subjected to heat injury by incubating them at 43° C (panel B), and lactose dehydrogenase (LDH) release was evaluated 2.5 hours and 16 hours later by means of CytoTox 9600 nonradioactive kit. Optical density was measured at 490 nm. PAECs treated with 10% heat-inactivated human serum (HIhS) and left at 37° C served as the control for nonspecific cell death, and PAECs left with growth medium alone served as controls for 100% viability. Data for panel A are expressed as mean ± SD of 7 replicates and are representative of 3 independent experiments, and data for panel B are the mean ± SD of 10 replicates and are representative of 7 independent experiments. Total LDH release (TR) was measured in PAECs treated with 0.1% Triton 100X.
Effect of A20 expression on complementand heat-mediated necrotic cell death. A20 expression protects ECs from complement- but not heat-mediated necrotic cell death. NI PAECs and PAECs infected with rAdA20 or rAdβ-gal were treated with 10% and 20% human serum (hS) as a source of active complement (panel A) or subjected to heat injury by incubating them at 43° C (panel B), and lactose dehydrogenase (LDH) release was evaluated 2.5 hours and 16 hours later by means of CytoTox 9600 nonradioactive kit. Optical density was measured at 490 nm. PAECs treated with 10% heat-inactivated human serum (HIhS) and left at 37° C served as the control for nonspecific cell death, and PAECs left with growth medium alone served as controls for 100% viability. Data for panel A are expressed as mean ± SD of 7 replicates and are representative of 3 independent experiments, and data for panel B are the mean ± SD of 10 replicates and are representative of 7 independent experiments. Total LDH release (TR) was measured in PAECs treated with 0.1% Triton 100X.
This is the first demonstration that expression of A20 protects ECs from complement-mediated cytotoxicity. To check whether A20 protects from other forms of necrotic cell death, NI PAECs and PAECs infected with rAdA20 and rAdβ-gal were cultured in hyperthermic conditions at 43° C, and necrosis was measured 16 hours later by LDH release. The LDH release was similar in all groups, reaching an OD of 0.22 ± 0.008, 0.16 ± 0.013, and 0.18 ± 0.01 for NI, rAdβ-gal–, and rAdA20-infected cells, respectively (n = 7; done in 10 replicates) (Figure 7B). SR reached 0.01 in all groups, and TR reached 0.48 ± 0.04.
Discussion
We have previously demonstrated that A20 is an induced cytoprotective gene in ECs. The cytoprotective function of A20 in ECs has a dual aspect, including protection from TNF-induced apoptosis and inhibition of inflammatory responses via blockade of NF-κB activation.3 The mechanisms underlying the antiapoptotic function of A20 remain largely unknown. In this study, we wished to analyze the molecular basis of the protective function of A20 against TNF-mediated apoptosis in ECs and evaluate its effectiveness against other relevant apoptotic and necrotic pathways triggered during immune and inflammatory responses.
The precise molecular ordering of apoptotic pathway(s) initiated upon signaling through the TNF receptor I (TNF-RI) is a subject of controversy. Classically, TNF-RI activation leads to the recruitment of the adapter molecules TNF receptor–associated death domain (TRADD), receptor-interacting protein (RIP), and FADD to the membrane.35,36 FADD recruits the initiator procaspase 8, leading to its autoactivation by proteolytic cleavage.37,38 Active caspase 8 in turn activates the executioner caspase 3, either directly by proteolytic processing or indirectly via cleavage of Bid. Cleaved Bid localizes to the mitochondrial membrane, resulting in loss of the transmembrane potential and triggering the release of apoptogenic cytochrome c from the inner mitochondrial membrane.39-42 Cytochrome c amplifies the apoptotic process by complexing to apoptotic protease–activating factor 1 (APAF-1) and procaspase 9, leading to activation of caspase 9 and subsequently caspase 3.43 Active caspase 3 proteolyses key proteins involved in cell survival and homeostasis, leading to cellular disassembly and death. Active caspase 3 may also trigger a feedback amplification loop, leading to proteolysis of caspases 2, 6, 8, and 9.44,45 FADD-knockout mice are resistant to TNF-mediated apoptosis, indicating that FADD has an obligatory role in this pathway.46 TNF-RI can also engage another adapter protein called RIP-associated interleukin-1 converting enzyme/ced-3 homolog (ICH)-1 homologous protein with a death domain (RAIDD). RAIDD binds through death domains to RIP and through a caspase recruitment domain (CARD) motif to a similar sequence in procaspase 2, resulting in its activation and hence setting apoptosis in motion.47,48 Controversy still exists as to whether or not caspase 2 activation is required for TNF-mediated apoptosis.49
Our current knowledge of these pathways stems from studies performed mostly in transformed cells lines. Given the likelihood of cell-type specificity to these pathways, we evaluated how treatment with CHX/TNF affects caspase activation and mitochondrial integrity in EC cultures derived from primary cells. Our data demonstrate that treatment of ECs with CHX/TNF activates caspases 8, 2, 3, and 6; cleaves BID; and disrupts mitochondrial transmembrane potential, releasing cytochrome c and activating caspase 9. Overexpression of A20 protected from TNF-mediated apoptosis by blocking the activation of the apical caspases 8 and 2, preventing subsequent activation of downstream effectors (caspases 3 and 6 and BID) and maintaining the Δψm and retaining cytochrome c in the inner mitochondrial membrane.
Several reports suggested that the antiapoptotic function of A20 is limited to protection from TNF-RI death pathways. Specifically, these reports did not show any protective effect of A20 against Fas/Fas ligand (Fas/FasL)– and FADD-mediated apoptosis.7,24,25,50 Because these studies were performed mainly in cell lines and in cell types other than ECs (T cells and breast cell carcinoma cell lines), we questioned their validity in ECs derived from primary cultures. Studies from our own laboratory showing that A20 has no antiapoptotic function in SMCs and renal tubular epithelial cells made us cautious in drawing general conclusions when working with a given cell type (Virendra Patel et al, manuscript submitted; and Uta Kunter et al, manuscript submitted). Therefore, we sought to determine whether expression of A20 in ECs affects Fas-mediated apoptosis. Within seconds of Fas receptor engagement, a death-inducing signaling complex (DISC) associates with activated Fas. First, FADD binds via its own death domain to the death domain in Fas and via its death domain recruits procaspase 8. Clustered procaspase 8 is activated by autoproteolytic cleavage and then released in the cytoplasm, where it activates the executioner caspase 3 either directly or indirectly via an amplification loop involving the mitochondria.38,41,42,51,52 Most ECs express low levels of Fas and are readily resistant to apoptosis triggered by Fas cross-linking. Fas-mediated apoptosis could be augmented in ECs by pretreatment with interferon-γ (IFN-γ) and pretreatment with CHX.53 Because our human and porcine EC cultures were particularly resistant to apoptosis triggered by Fas cross-linking, we induced Fas-mediated apoptosis by overexpressing human Fas in BAECs. Overexpression of Fas led to cell death that was drastically increased by Fas cross-linking. A20 totally protected BAECs from apoptosis induced by overexpression of Fas as well as following Fas cross-linking. Similar results were obtained when FADD was overexpressed in BAECs. A20-mediated protection from Fas occurred at the level of the key caspase 8. The inability of A20 to protect MCF-7 or Jurkat cells from Fas-mediated apoptosis may be attributable to their being cell lines and/or different cell types.
Our data showing that A20 inhibits both TNF- and Fas-mediated apoptosis suggest that A20 interferes at a common step in their signaling pathways. Apoptotic molecules recruited by TNF-RI and Fas are highly homologous. The 2 death receptors differ only in their use of proximal signaling molecules. TNF-RI requires the adapters TRADD and RIP to transduce signals to FADD whereas Fas interacts directly with FADD and activates caspase 8. Our data showing that A20 inhibits both of these death pathways in ECs support an effect of A20 at the level of FADD/caspase 8. Although we cannot completely rule it out, any effect of A20 on TRADD and RIP recruitment to the TNF-RI (as shown in Jurkat cells) would not explain A20's blockade of FADD-mediated apoptosis.25
Beyond apoptosis triggered by death receptors, we demonstrate that A20 significantly protects ECs from NK-mediated cytotoxicity. NK cells trigger multiple cytotoxic pathways.54 Their lytic granules contain perforin, which assembles in aggregates and inserts into membrane of target cells in a calcium-dependent manner, providing a channel leading to osmotic lysis of cells.54,55 Perforin also enables other granule components such as granzymes to enter cells and induce apoptosis.56 Additionally, interactions between FasL expressed by human NK and the Fas receptor on target cells may also induce apoptosis. Our PAEC cultures do not express Fas, ruling out Fas/FasL involvement in this system of NK-mediated cytotoxicity.34 This result is in agreement with data demonstrating that human NK cells lyse porcine “xenogeneic” targets via granule exocytosis–triggered death pathways, predominantly perforin/granzyme B,34 but contradicts the report of Zheng at al57 showing that PAECs can be killed by FasL. These contradictory results probably relate to different pig strains (in this study, we used miniature swine). Granzyme B is a serine proteinase that can process caspases 8 and 3 in vitro, suggesting that it can access the apoptotic program in a way similar to Fas.58 Granzyme B could also obviate the need for caspase 8 activity by directly cleaving Bid and triggering mitochondrial-dependent apoptosis.59 We demonstrate that A20 inhibits NK-mediated activation of caspases 8 and 3 and Bid cleavage in ECs. A20 had limited protection against NK-mediated cytotoxicity (approximately 30%) as compared with stronger protection from TNF- or Fas-mediated apoptosis (approximately 70% to 90%). This may relate to the fact that cell lysis by granule exocytosis engages caspase-dependent and caspase-independent death pathways.60,61
To our knowledge, these are the first data showing a protective, although limited, effect of A20 against cell-mediated cytotoxicity. Other studies have shown that A20 was unable to protect MCF-7 cells from LAK-mediated toxicity.7 Several possibilities may explain this discrepancy, including the use of cell lines versus normal cells derived from primary cultures; different cell type (breast cells versus ECs); killer cells (NK vs LAK), different and higher levels of A20 expression achieved with the use of adenovirus versus expression plasmids.
In addition to protecting from apoptosis, A20 was also able to protect ECs from necrotic cell death. We chose to evaluate the effect of A20 upon a necrotic stimulus relevant to immunologic insults: namely, complement. The vascular endothelium is the target of antibodies and complement in vascular diseases and certain forms of graft rejection.62 The membrane attack complex, which is the final product of activated complement, induces a loss in membrane integrity and a classic rapid necrotic-type cell death.63 In a previous report, Heyninck et al64 demonstrated that A20 partially protected the fibroblast cell line L929 from TNF-mediated necrosis by delaying the production of mitochondrial reactive oxygen species, implying that A20 specifically interfered with TNF signaling to block necrotic and apoptotic cell death. Our data demonstrating that A20 totally abrogated complement-mediated necrosis argues that the antinecrotic effect of A20 is not limited to TNF. To our knowledge, this is the first demonstration that A20 protects ECs from complement-mediated necrosis. The molecular basis of the protective effect of A20 against complement-mediated necrosis warrants further study. The protective effect of A20 against necrotic cell death did not extend to heat-induced necrosis.
In summary, we present strong evidence that A20 is a pancyto-protective gene in ECs. A20 arms the endothelium against death receptor–mediated apoptosis via inhibition of initiator caspases and safeguards mitochondrial membrane integrity. Additionally, A20 protects ECs against NK-mediated cytotoxicity and complement-induced necrotic cell death. The fact that A20 is rapidly induced in ECs in response to inflammatory and immune insults supports its critical role in maintaining the physical and functional integrity of the endothelial layer. A20 may represent an ideal gene therapy candidate to protect the vessel wall against inflammatory and immune-mediated injuries such as sepsis, vasculitis, atherosclerosis, and graft rejection.
Prepublished online as Blood First Edition Paper, July 13, 2004; DOI 10.1182/blood-2003-02-0635.
Supported by National Institutes of Health (NIH) grant HL57791 (C.F.); a grant from the Roche Organ Transplantation Research Foundation (C.F.); and fellowship grants from the Institut National d'Etudes et de Recherche Medicales (INSERM) France and the Association pour la Recherche sur le Cancer France (ARC) (S.D.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Vishva Dixit for providing the FADD, FADD-DN, and A20 plasmids; Dr Craig Gerard for providing rAdβ-gal; and Dr Richard Siegel for providing the Fas expression plasmid. We also thank Drs Roya Khosravi-Farr and Nordine Benhaga for helpful insights into the Fas experiments and Dr Fritz H. Bach for thoughtful discussion and support.


![Figure 5. A20 protection from Fas- and FADD-induced cell death and caspase activation. A20 expression protects ECs from Fas- and FADD-induced cell death and caspase activation. BAECs were transfected with a control, a pcDNA3 plasmid or an A20 expression plasmid, and a β-gal expression plasmid. (A) Cotransfection with Fas alone (nontreated [NT]) resulted in cell death, which was enhanced by Fas cross-linking (αFas). Cell death was quantified by blue cell count per HPF. A20 significantly protected from Fas-mediated cell death. Data are expressed as mean ± SD of 3 HPFs per triplicate well. Results shown are representative of 3 independent experiments. (B) Similar results were obtained when cells were cotransfected with FADD. Cotransfection of BAECs with a dominant negative FADD was used as control. Caspase 8 (C) and caspase 3 (D) activities were determined in lysates of BAECs cotransfected with pcDNA3 or A20 plasmids and FADD expression plasmid by means of colorimetric assays based on the caspase-specific cleavage of labeled substrates (IETD-pNA for caspase 8 and DEVD-pNA for caspase 3). Expression of A20 significantly inhibited FADD-mediated activation of caspases 8 and 3. Data are expressed as mean ± SD of triplicate values. Results shown are representative of 4 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/8/10.1182_blood-2003-02-0635/6/m_zh80200467890005.jpeg?Expires=1767842415&Signature=wbfvl-vV7vKXMrZrvnDmohjtY96kWHp6qd~DYg55HZycuCyzEl2xdqwrl2OBZ~6uzbtmq-S~uQJmeWH4xXO5QCo2kPTnrtHA-XsR1smp8xyynRlbHSAqSWV4rsCm0WK8HCLZJ~vXHjkXMVBWcRm8Ih5~96uRVruBfyJtsbmmcnEVP9Wdc6OeX2D1wCXlEcZHfsN8zq7HKcLbw-O-Od4p1Mu4yUBlixGLfdmSqId6XegPkRYuP95Fhb2-dkRPoTKz9Tk4h7FJkjT~nf-5PAJnCzHxvedoPO9IkRmaWCL1G2DtnnX2WXCsZGo-zlvQNYQX4fWq0THgj6mhPCWlkh0HuQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 5. A20 protection from Fas- and FADD-induced cell death and caspase activation. A20 expression protects ECs from Fas- and FADD-induced cell death and caspase activation. BAECs were transfected with a control, a pcDNA3 plasmid or an A20 expression plasmid, and a β-gal expression plasmid. (A) Cotransfection with Fas alone (nontreated [NT]) resulted in cell death, which was enhanced by Fas cross-linking (αFas). Cell death was quantified by blue cell count per HPF. A20 significantly protected from Fas-mediated cell death. Data are expressed as mean ± SD of 3 HPFs per triplicate well. Results shown are representative of 3 independent experiments. (B) Similar results were obtained when cells were cotransfected with FADD. Cotransfection of BAECs with a dominant negative FADD was used as control. Caspase 8 (C) and caspase 3 (D) activities were determined in lysates of BAECs cotransfected with pcDNA3 or A20 plasmids and FADD expression plasmid by means of colorimetric assays based on the caspase-specific cleavage of labeled substrates (IETD-pNA for caspase 8 and DEVD-pNA for caspase 3). Expression of A20 significantly inhibited FADD-mediated activation of caspases 8 and 3. Data are expressed as mean ± SD of triplicate values. Results shown are representative of 4 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/8/10.1182_blood-2003-02-0635/6/m_zh80200467890005.jpeg?Expires=1767842416&Signature=uO3x~T96wVj~83y~riXbO9F-MI-RO07vsXNmzCgDnzNFgtXGFUTQAx4NxFxsxj1jTo8lb2RYasOaKGyzXb2NhokQWCbdTc36GEDghK80n9NGJKvNsCB3RUSj~myg1YZHh4DaUEem4500tGbO~DamPaid8W51rdgzhOMwbWQVteDSfh1YeOtOJlIJ1VoBeFX0X5KDE-c2~XlOBdUapQv~486fFz3WM0ySobt6SX0~jvvDr8Nf7r-KwgKbSyS7g6~6Dl6MCr96rwh2A~9GDnt~eS9ngOdGqOYRueFwwChMYwrHfaOnxd9u84uk6fbEIWd6RNrzWxlaGP1prWFKThFyxw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)