Abstract
Our goal in the present work was to characterize the multiple steps involved in overcoming the anergy that exists in tumor hosts to tumor-associated antigen (TAA). Our studies showed that the subcutaneous injection of the Ad-sig-TAA/ecdCD40L vector resulted in secretion of the TAA/ecdCD40L protein for at least 10 days from infected cells. Binding of the TAA/ecdCD40L protein to dendritic cells (DCs) resulted in the induction of CCR-7 chemokine receptor expression and cytokine release. This was followed by migration of the DCs to regional lymph nodes. Tetramer staining, enzyme-linked immunospot (ELISPOT) assay, and cytotoxicity assay all showed that the Ad-sig-TAA/ecdCD40L vector increased the levels of splenic CD8+ T cells specific for the 2 TAAs (human MUC1 [hMUC1] and HPV E7) tested. Vaccination with the Ad-sighMUC1/ecdCD40L vector suppressed the growth of hMUC1 antigen-positive tumor cells in 100% of the test mice that were previously anergic to the hMUC1 antigen. These data suggest that Ad-sig-TAA-ecd/ecdCD40L vector injections may be of value in treating the many epithelial malignancies in which TAA-like hMUC1 is overexpressed. (Blood. 2004;104:2704-2713)
Introduction
We previously reported that subcutaneous injection of the Ad-sig-TAA/ecdCD40L vector can overcome the anergy in tumor hosts against tumor-associated antigen (TAA).1 Dendritic cells (DCs) are specialized cells of the immune system responsible for the initiation and regulation of cellular and humoral responses. The ability of DCs to regulate immunity is dependent on DC maturation. In the absence of costimulatory molecule expression on the DC surface, the presentation of TAA to naive T cells can lead to T-cell anergy caused by the induction of apoptosis in the T cells.2
Human DCs require multiple activation signals for the efficient generation of tumor antigen-specific T lymphocytes.3,4 These changes endow DCs with the ability to costimulate antigen-specific CD8+ and CD4+ T-cell responses and to foster CD8+ T-cell differentiation into cytotoxic lymphocytes (CTLs).5,6 The fact that antigen-loaded DCs can generate antitumor immune responses capable of eradicating established tumors in vivo has been documented in a number of animal tumor models. Strategies for loading DCs with TAA include the pulsing of tumor cell RNA into DCs, the mixing of tumor cell lysates with DCs, and the in vitro addition of recombinant peptides of proven binding capability to DCs.7-13 DC vaccination leads to tumor regression in selected patients with advanced cancer, but the weight of clinical trial data suggests that in vivo activation and tumor antigen loading of DCs might provide an advantage over in vitro activation strategies.
To develop an in vitro strategy of activation and tumor antigen-loading of DCs with which to overcome anergy to TAA, we built on the oral DNA vaccine/interleukin-2 (IL-2) targeting strategy of Xiang et al14 to create an adenoviral vector (Ad-sig-TAA/ecdCD40L) vaccine. The Ad-sig-TAA/ecdCD40L adenoviral vector encodes a secretable (sig) form of a TAA fused to the extracellular domain (ecd) of the CD40 ligand (CD40L). The ecd of CD40L contains all the sequences necessary to form a functional trimeric CD40L.15 Our previous studies with this vector show that subcutaneous injection of the Ad-sig-TAA/ecdCD40L vector induced immune resistance to the growth of TAA-positive cancer cells for more than 1 year.1
In the present work, we sought to characterize the multiple steps through which the Ad-sig-TAA/ecdCD40L vector induces an immune response to TAA in anergic animals. As shown in Figure 1A, this involves secretion of the TAA/ecdCD40L protein from the Ad-sig-TAA/ecdCD40L vector-infected cells near the subcutaneous injection site for more than 10 days. Binding of the TAA/ecdCD40L protein to the DCs resulted in activated cytokine release, increased levels of the CCR-7 chemokine, and increased membrane levels of the CD80 and CD86 receptors. This induced migration of DCs, which displayed TAA peptides on their surface major histocompatibility complex (MHC) class I molecules, and resulted in increases in the number of TAA-specific CD8+ T cells competent to recognize and kill cancer cells bearing the TAA.7,16
TAA/ecdCD40L protein produced by Ad-sig-TAA/ecdCD40-infected cells binds to DCs. (A) Proposed mechanism for induction of immune response by the Ad-sig-TAA/CD40L vector. Injecting Ad-sig-TAA/ecdCD40L induces in vivo activation and tumor-antigen loading of DCs, migration of the DCs to regional lymph nodes, and activation of CD8+ cytotoxic T cells, which are specific for cells carrying the tumor antigen. (B) In vitro expression of the E7/ecdCD40L transcription unit. Plasmid expression vectors encoding the nonsecretable E7/wtCD40 ligands (lane 1), the secretable ecd of the CD40 ligand (sig-ecdCD40L) alone (lane 2), and the secretable sig-E7/ecdCD40 ligand protein (lane 3) produced in a cell-free transcription/translation system are as predicted: lane 1, E7/wtCD40L is 39 kDa; lane 2, sig-ecdCD40L is 22 kDa; and lane 3, sig-E7/ecdCD40L is 32 kDa. Molecular weight markers are in the extreme right lane. (C) Western blot analysis of the expression of E7/ecdCD40L protein in 293 cells. Molecular weights of the TAA/ecdCD40L proteins produced from 293 cells infected by the Ad-sig-TAA/ecdCD40L vectors adenoviral vectors were as predicted: lane 1, lysates from cells infected with the Ad-sig-GFP/ecdCD40L vector; lane 2, lysates from cells infected with the Ad-sig-E7/ecdCD40L vector; lane 3, lysates from cells infected with the Ad-sig-ecdCD40L vector; and lane 4, lysates from the Ad-sig-ecdhMUC1/ecdCD40L vector. Molecular weight markers are in the extreme right lane. (D) Secretory form of TAA/ecdCD40L binds in vitro to DCs. Bone marrow-derived DCs were fractionated to 78% purity. (i-ii) FITC-labeled E7/ecdCD40L recombinant proteins released from Ad-sig-E7/ecdCD40L-infected 293 cells were incubated with bone marrow-derived DCs. Cells were portioned with light microscopy (left panels) to demonstrate the morphology of the DCs and then with fluorescence microscopy (right portion panels) to detect the binding of the fluoresceinated proteins. (i) DCs incubated with FITC-labeled proteins from the supernatant of cells infected with the Ad-sig-E7/ecdCD40L. (ii) DCs incubated with FITC-labeled proteins from the supernatants of cells infected with the Ad-sig-ecdCD40L vector. (iii-v) Proteins released from Ad-sig-ecdhMUC-1/ecdCD40L-infected 293 cells were fractionated on a Nickel column to purify the His-tagged ecdhMUC-1/ecdCD40L proteins. These proteins were fluorescein labeled, as outlined in “Materials and methods.” FITC-labeled ecdhMUC-1/ecdCD40L proteins and a PE-conjugated rat antimouse CD11C antibody were added to the purified DCs. (iii) Cells exposed to a laser excitatory for phycoerythrin. (iv) Cells exposed to a laser excitatory for FITC. (v) Overlay of the images from subpanels iii and iv. A Nikon Eclipse TE-2000-U microscope, which was equipped with a Perkin Elmer UltraView R55 spinning disk confocal attachment, was used at 20 × N.A. 0.5. Adobe Photoshop was the software used.
TAA/ecdCD40L protein produced by Ad-sig-TAA/ecdCD40-infected cells binds to DCs. (A) Proposed mechanism for induction of immune response by the Ad-sig-TAA/CD40L vector. Injecting Ad-sig-TAA/ecdCD40L induces in vivo activation and tumor-antigen loading of DCs, migration of the DCs to regional lymph nodes, and activation of CD8+ cytotoxic T cells, which are specific for cells carrying the tumor antigen. (B) In vitro expression of the E7/ecdCD40L transcription unit. Plasmid expression vectors encoding the nonsecretable E7/wtCD40 ligands (lane 1), the secretable ecd of the CD40 ligand (sig-ecdCD40L) alone (lane 2), and the secretable sig-E7/ecdCD40 ligand protein (lane 3) produced in a cell-free transcription/translation system are as predicted: lane 1, E7/wtCD40L is 39 kDa; lane 2, sig-ecdCD40L is 22 kDa; and lane 3, sig-E7/ecdCD40L is 32 kDa. Molecular weight markers are in the extreme right lane. (C) Western blot analysis of the expression of E7/ecdCD40L protein in 293 cells. Molecular weights of the TAA/ecdCD40L proteins produced from 293 cells infected by the Ad-sig-TAA/ecdCD40L vectors adenoviral vectors were as predicted: lane 1, lysates from cells infected with the Ad-sig-GFP/ecdCD40L vector; lane 2, lysates from cells infected with the Ad-sig-E7/ecdCD40L vector; lane 3, lysates from cells infected with the Ad-sig-ecdCD40L vector; and lane 4, lysates from the Ad-sig-ecdhMUC1/ecdCD40L vector. Molecular weight markers are in the extreme right lane. (D) Secretory form of TAA/ecdCD40L binds in vitro to DCs. Bone marrow-derived DCs were fractionated to 78% purity. (i-ii) FITC-labeled E7/ecdCD40L recombinant proteins released from Ad-sig-E7/ecdCD40L-infected 293 cells were incubated with bone marrow-derived DCs. Cells were portioned with light microscopy (left panels) to demonstrate the morphology of the DCs and then with fluorescence microscopy (right portion panels) to detect the binding of the fluoresceinated proteins. (i) DCs incubated with FITC-labeled proteins from the supernatant of cells infected with the Ad-sig-E7/ecdCD40L. (ii) DCs incubated with FITC-labeled proteins from the supernatants of cells infected with the Ad-sig-ecdCD40L vector. (iii-v) Proteins released from Ad-sig-ecdhMUC-1/ecdCD40L-infected 293 cells were fractionated on a Nickel column to purify the His-tagged ecdhMUC-1/ecdCD40L proteins. These proteins were fluorescein labeled, as outlined in “Materials and methods.” FITC-labeled ecdhMUC-1/ecdCD40L proteins and a PE-conjugated rat antimouse CD11C antibody were added to the purified DCs. (iii) Cells exposed to a laser excitatory for phycoerythrin. (iv) Cells exposed to a laser excitatory for FITC. (v) Overlay of the images from subpanels iii and iv. A Nikon Eclipse TE-2000-U microscope, which was equipped with a Perkin Elmer UltraView R55 spinning disk confocal attachment, was used at 20 × N.A. 0.5. Adobe Photoshop was the software used.
We studied 2 types of TAA in this vector vaccination strategy: the human papillomavirus (HPV) E7 foreign antigen, which has been shown to be a strong stimulus of the cellular immune response,17-20 and the ecd of the human Mucin-1 (hMUC1) self-antigen, which is expressed focally at low levels on normal epithelial cellular surfaces.21-24 The MUC1 antigen is expressed at high levels diffusely in neoplastic epithelial mucosal cells, thereby disrupting the regulation of anchorage-dependent growth, which leads to metastases.22,23 The MUC1 antigen is a self-protein overexpressed in carcinomas of the breast, ovary, lung, prostate, colon, and pancreas, among other carcinomas.21 Overexpression in epithelial cancers is thought to disrupt E-cadherin function, leading to anchorage-independent growth and metastases.22 Although non-MHC-restricted cytotoxic T-cell responses to MUC1 have been reported in patients with breast cancer,23 hMUC1 transgenic mice (MUC1.Tg) have been reported to be unresponsive to stimulation with hMUC1 antigen.24
Our results show that immunizing hMUC1 transgenic mice, which are anergic to the hMUC1 antigen,24 with the Ad-sig-hMUC1/ecdCD40L vector induces a CD8+ T cell-dependent systemic T-helper 1 (TH1) immune response that is antigen specific and HLA restricted and that overcomes the block in proliferation that exists in T cells in anergic hosts. Vaccination increases the frequency of hMUC1-specific T cells in the spleens of injected mice. This response requires the Ad-sig-ecdhMUC1/ecdCD40L adenoviral vector and cannot be produced by subcutaneous injection of the hMUC1/ecdCD40L protein alone. Using a similar vector system, but with the E7 antigen in place of the hMUC1 antigen, we showed that the Ad-sig-E7/ecdCD40L vector injection induced immune responses against E7-positive TC-1 tumor cells in 100% of the injected mice for up to 1 year. These results suggest that Ad-sig-TAA/ecdCD40L vector injections induce a memory cell response against TAA-positive tumor cells without the need for additional cytokine boosting treatments.
Materials and methods
Mice and cell lines
Six- to 8-week-old C57BL/6 mice were purchased from Harlan. MUC1 transgenic mice-C57/BL6/human MUC124 were obtained from Dr S. Gendler of Mayo Clinic Scottsdale and were bred on site.
Construction of recombinant adenoviruses
The E7/ecdCD40L fusion gene was constructed by ligating the amino terminal end of the ecd of CD40L to an octapeptide linker (NDAQAPKS), which was linked in turn to the carboxyl terminal end of a TAA, the amino terminal end of which was linked to a secretory signal sequence. The oligonucleotide for E7 was 5′-TGG GTT CCA GGT TCC ACT GGT GAC ATG CAT GGA G AT ACA CCT AC-3′ and 5′-CCG CTC GAG TGG TTT CTG AGA ACA GAT GGG GCA C -3′. This oligonucleotide was cloned to the pcDNA3TOPO vector. Coding sequences for the full-length mouse CD40 ligand were generated by using the following primers: 5′-GAGAC CTC GAG AAC GAC GCA CAA GCA CCA AAA AGC ATG ATA GAA ACA TAC AGC CAA C-3′ and 5′-CCG CGC CCC AAG CTT ATC AGA GTT TGA GTA AGC CAA AAG-3′. The CD40L template is the plasmid pDC406-mCD40L (American Type Culture Collection, Manassas, VA). Polymerase chain reaction (PCR) conditions are as per protocol from Tgo DNA polymerase kit (Roche Diagnostics, Mannheim, Germany): 94°C for 3 minutes, 25 cycles at 94°C for 30 seconds, 56°C for 45 seconds, 72°C for 45 seconds, and 1 cycle at 72°C for 7 minutes. The PCR fragment was inserted into the plasmid pcDNA3-E7 after restriction endonuclease digestion with XbaI (TCTAGA) and XhoI (CTCGAG). This vector was named pCDNA3CE7/wtCD40L. The E7/wt encoding DNA was cut from pCDNA3CE7/wtCD40L using HindIII-XbaI restriction endonuclease digestion that was then inserted into pShuttle-cytomegalovirus (CMV) downstream of the CMV promoter. This plasmid is designated pShuttle-E7/wtCD40L.
The ecdCD40L fragment for pShuttle-ecdCD40L was generated by PCR encoding the mouse immunoglobulin G (IgG) κ chain by 4 rounds of PCR amplification (first round, primers 1 and 5; second round, primers 2 and 5; third round, primers 3 and 5; fourth round, primers 4 and 5). Primers were as follows: (1) 5′-CTG CTCTGG GTT CCA GGT TCC ACT GGT GAC AAG GTC GAA GAG GAA GTA AAC C-3′; (2) 5′-TG CTC TGG GTT CCA GGT TCC ACT GGT GAC ATG CAT G-3′; (3) 5′-TC CTG CTA TGG GTA CTG CTG CTC TGG GTT CCA GGT TC3′; (4) 5′-ACG ATG GAG ACA GAC ACA C TC CTG CTA TGG GTA CTG CTG-3′; (5) 5′-CCG CGC CCC TCT AGA ATC AGA GTT TGA GTA AGC CAA AAG-3′.
The CD40L template is the plasmid pDC406-mCD40L (American Type Culture Collection). PCR conditions are per protocol from Tgo DNA polymerase kit (Roche Diagnostics). Conditions are the same as given earlier in this section. Fragments of ecdCD40L were cloned into the pcDNA3.1TOPO vector (Invitrogen, Carlsbad, CA), then cut from the pCDNA3-hMUC1/ecdCD40L vector using HindIII-XbaI restriction endonuclease digestion and inserted into pShuttle-CMV downstream of the CMV promoter and named pShuttle-ecdCD40L.
A transcription unit that included DNA encoding the signal sequence of the mouse IgG κ chain gene upstream of DNA encoding hMUC-1 was generated by PCR using plasmid pcDNA3-hMUC-1 (gift of O.J. Finn, University of Pittsburgh School of Medicine, PA) and the following primers. DNA encoding the mouse IgG κ chain METDTLLLWVLLLWVPGSTGD (single-letter amino acid code) was prepared by PCR amplification to generate the full 21-amino acid mouse IgG κ chain signal sequence: (1) 5′-CCACC ATG GAG ACA GAC ACA CTC CTG CTA TGG GTA CTG CTG-3′; (2) 5′-TC CTG CTA TGG GTA CTG CTG CTC TGG GTT CCA GGT TC-3′; (3) 5′-TG CTC TGG GTT CCA GGT TCC ACT GGT GAC GAT G -3′; (4) 5′-GGT TCC ACT GGT GAC GAT GTC ACC TCG GTC CCA GTC-3′; (5) 5′-GAG CTC GAG ATT GTG GAC TGG AGG GGC GGT G-3′. K/hMUC-1 with the upstream κ signal sequence was generated by 4 rounds of PCR amplification (first round, primers 4 and 5; second round, primers 3 and 5; third round, primers 2 and 5; fourth round, primers 1 and 5). PCR conditions are the same as given earlier in this section. The hMUC-1 encoding DNA was cloned into the pcDNA3.1TOPO vector (Invitrogen) forming pcDNA-hMUC-1. A pair of PCR primers was designed for ecdCD40L without the cytoplasmic and transmembrane domains: 5′-CCG CTC GAG AAC GAC GCA CAA GCA CCA AAA TCA AAG GTC GAA GAG GAA GTA-3′;5′-GCG GGC CCG CGG CCG CCG CTA GTC TAG AGA GTT TGA GTA AGC CAA AAG ATG AG-3′. The CD40L template is the plasmid pDC406-mCD40L (American Type Culture Collection). PCR conditions are as per protocol from the Tgo DNA polymerase kit (Roche Diagnostics), which are the same as earlier in this section. The PCR fragment was inserted into the plasmid pcDNA-hMUC-1 after restriction endonuclease digestion with XbaI (TCTAGA) and XhoI (CTCGAG). This vector was named pCDNA3-hMUC1/ecdCD40L. The hMUC1/ecdCD40L encoding DNA was cut from the pCDNA3-hMUC1/ecdCD40L vector using HindIII-XbaI restriction endonuclease digestion and was inserted into pShuttle-CMV downstream of the CMV promoter. The plasmid is designated pShuttle-hMUC1/ecdCD40L.
Coding sequences for the full-length mouse CD40L were generated by using the following primers: 5′-GAG ACC TCG AGA ACG ACG CAC AAG CAC CAA AAA GCA TGA TAG AAA CAT ACA GCC AAC-3′ and 5′-CCG CGC CCC AAG CTT ATC AGA GTT TGA GTA AGC CAA AAG-3′. The CD40L template is the plasmid pDC4mCD40L (American Type Culture Collection). PCR conditions as per protocol from the Tgo DNA polymerase kit (Roche Diagnostics) are the same as given earlier in this section. Using PCR methods, in some vectors, we added the mouse HSF1 trimer domain between MUC-1 and CD40L and a His tag at the end of the CD40L. Fragments of the TAA/CD40L fusion were inserted downstream of the CMV promoter in the pShuttleCMV expression vector using the XhoI and XbaI restriction sites. The ecd of the CD40L and the full-length-wtCD40L was amplified by PCR primers and cloned into the pShuttleCMV plasmid using the HindIII and XbaI restriction endonuclease sites. Recombinant adenoviral vectors were generated using the AdEasy vector system.25
All populations of vector particles used in the experiments described in this paper were shown to contain fewer than 5 replication-competent adenoviral particles (RCAs) per 1 × 1010 viral particles (VPs).
Western blotting and in vitro expression of the E7/ecdCD40L transcription unit
Western blotting and in vitro cell-free transcription/translation were used to analyze protein expression from the vector transcription units as described previously.30 The coupled in vitro transcription-translation system of reticulocyte lysate (RRL) (TNT kits; Promega, Madison, WI) was used to synthesize the protein products of the transgenes of the following vectors: Ad-sig-E7/ecdCD40L, Ad-E7/wtCD40L (where wt indicates the full-length or wild-type CD40L gene), Ad-sig-ecdCD40L, Ad-wtCD40L, and Ad-sigecdhMUC1/ecdCD40L. The protein cell lysate derived from 293 cells infected by each adenoviral vector described in the preceding sentence at a multiplicity of infection (MOI) of 40 was fractionated on a 10% reducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel and transferred to an Immobilon-P membrane (Millipore, Bedford, MA). After blocking with 5% nonfat milk for 2 hours at room temperature, the membrane was probed with an antibody against the specific mouse CD40L (mCD40LM; eBioscience, San Diego, CA) in TBS-T buffer (20 mM Tris-HCl [pH 7.6], 137 mM NaCl, and 0.5% Tween 20) in the presence of 2% bovine serum albumin (BSA) overnight. After 4 washes with TBS-T buffer, the blot was incubated with a goat antihamster alkaline phosphatase-conjugated antibody (Jackson ImmunoResearch, Bar Harbor, ME) for 1 hour. Immunoreactive bands were visualized on membranes by using the ProtoBlot II AP system (Promega).
Assay for binding of the TAA/CD40L protein to DCs
DCs were derived from incubation of bone marrow mononuclear cells in granulocyte macrophage-colony-stimulating factor (GM-CSF) and IL-4 for 7 days, followed by purification to a purity of 78% DCs. The TAA/CD40L proteins were generated by exposing 293 cells to either the Ad-sig-E7/ecdCD40L vector (Figure 1Di-ii) or the Ad-sig-ecdhMUC-1/ecdCD40L(His-tagged) vector (Figure 1Diii-v). In Figure 1Di-ii, no purification of the proteins was carried out, whereas in panel C, nickel column purification of the ecdhMUC-1/ecdCD40L proteins was carried out. The TAA/CD40L proteins were fluorescently labeled with the Fluoreporter fluorescein isothiocyanate (FITC)-protein labeling kit (Molecular Probes), added to the DCs at a final concentration of 10 μg/mL, and incubated for 30 minutes. Cells were then washed 3 times with cold medium, fixed with 1% paraformaldehyde, and observed under a fluorescence microscope.
Assay for activation of bone marrow-derived DCs
DCs were incubated with the supernatant from 293 cells infected by Ad-sig-TAA/ecdCD40L adenoviral vectors, and then plated in 24-well plates at 2 × 105 cells/mL. After incubation for 24 hours and 48 hours at 37°C, the supernatant fluid (1 mL) was harvested and centrifuged to remove debris. The level of murine IL-12 or interferon-γ (IFN-γ) released into the culture medium from vector-infected cells was assessed by enzyme-linked immunoadsorbent assay (ELISA), using mouse IL-12 p70 or IFN-γ (R&D Systems, Minneapolis, MN), respectively. Bone marrow cells were incubated for 5 days in GM-CSF and IL-4. DCs were purified with the SpinSep Mouse Dendritic Cell enrichment kit (Stem Cell Technologies, Vancouver, BC, Canada). Forward and side scatter analyses of the populations before and after fractionation are given in Figure 1B-C. We then stained the bone marrow-derived DCs before and after fractionation with phycoerythrin (PE)-labeled CD11c antibody, incubating nonenriched and enriched cells for 10 minutes on ice with 5% normal rat serum to block the nonspecific background before adding fluorochrome-conjugated antibodies. Then we stained DC fractions with PE-labeled CD11c antibody.
Detection of CCR-7 mRNA by RT-PCR
Total RNA extracted from DCs was analyzed for CCR-7 mRNA as described previously.26 Primers for detecting CCR7 and the GAPDH control were as follows: for CCR7 sense, 5′-TCC TCC TAA TTC TTC CCT TC-3′; for CCR7 antisense, 5′-AAA CTC ATA GCC AGC ATA GG-3′); for GAPDH sense, 5′-TTG TGA TGG GTG AAC CAC-3′; and for GAPDH antisense, 5′-CCA TGT AGG CCA TGA AGT CC-3′. Expected sizes of the amplified fragments were 400 bp for CCR7 and 525 bp for GAPDH. Amplified samples were resolved on ethidium bromide-stained agarose gels. Total cellular RNA was extracted using the Trizol reagent (Life Technologies, Burlington, ON, Canada). Reverse transcription-polymerase chain reaction (RT-PCR) was performed on 5 μg RNA for the reverse transcription reaction. Half of each cDNA product was used to amplify CCR-7 and GAPDH.
DC migration assays
Bone marrow-derived DCs were loaded with the carboxyfluorescein diacetate succinimidyl ester (CFDA SE) supravital dye for 15 minutes at 37°C (Molecular Probes, Eugene, OR). Rinsed DCs were mixed with each recombinant adenoviral vector at an MOI of 200 and were injected into the left flank of the test mouse. Three days later, axillary lymph nodes draining the region of the injection site for the DCs were removed, and frozen tissue sections were made and observed under the fluorescence microscope.
Immunohistochemical staining
Immunized mice were killed 3 and 10 days after injection of the Ad-sig-E7/ecdCD40 vector. Skin at each site of subcutaneous vector injection was subjected to biopsy, embedded in optimum cutting temperature (OCT) solution, and cut into 5-μm sections. Slides were incubated with rat anti-CD40L antibody (eBioscience) and exposed to biotinylated goat anti-rat IgG antibody (1:200 dilution) and avidin-biotin complex (Vector Laboratories, Burlingame, CA). Stained slides were then mounted and studied under a fluorescence microscope.
Tetramer and ELISPOT assays
PE-labeled H-2Db tetramers containing HPV16 E749-57 peptide (RA-HYNIVTF) were purchased from Beckman Coulter (Hialeah, FL) and were used for the fluorescence-activated cell sorter (FACS) analysis of peptide-specific CTL immunity. Tetramer-positive and CD8+ cells are shown as percentages of total spleen cells. The presence of E7- and hMUC1-specific effector T cells in the immunized mice was also assessed by carrying out enzyme-linked immunospot (ELISPOT) assays, as previously described.27
Cytotoxicity assay
E7-positive TC-1 target cells or LL2/LL1hMUC1-positive target cells (5 × 103) were incubated with splenic mononuclear cells (effector cells) at varying effector-target ratios (100:1, 20:1, and 5:1) for 4 hours at 37°C, in culture media containing 5% fetal bovine serum (FBS). Effector cells had been prestimulated with the TAA-positive cancer cells for 5 days in vitro before the in vitro cytotoxicity assay. Cell-mediated cytotoxicity was determined using a nonradioactive lactate dehydrogenase (LDH) release assay. Student unpaired t test was used to determine differences among the various groups in cytotoxicity assays. Statistical significance was defined by the P less than .05 level.
In vivo efficacy experiment in mouse model
Mice (5 or 10 per group) were vaccinated through subcutaneous injection with 1 × 108 plaque-forming units (pfus) of the Ad-sig-TAA/ecdCD40L, Ad-TAA, Ad-TAA/wtCD40L, Ad-sig-CD40L, Ad-wtCD40L, or Ad-sigecdhMUC1/ecdCD40L vectors. One week later, mice were boosted with the same adenoviral vector regimen as the first vaccination. One week after the last vaccination, mice were challenged by subcutaneous injection of 5 × 105 TAA-positive cancer cells. Tumor volumes were measured in centimeters by caliper, and the volumes were calculated as tumor volume = length × (width2)/2 (this assumes an elliptical shape).
Analysis of p44/p42 mitogen-activated protein kinase and SAPK/JNK phosphorylation
Western blot analysis of p44/p42 and SAPK/JNK was carried out with kits (no. 9100 for p44/p42 and no. 9250 for SAPK/JNK) from New England Biolabs (Beverly, MA). Responder splenocytes were isolated from vaccinated mice and enriched in CD8+ cells using a murine CD8 T-cell enrichment kit (catalog 13033; StemCell Technologies, Vancouver, BC, Canada). Bone marrow-derived DCs were infected with Ad-sig-ecdlMUC1/ecdCD40L for 2 hours, then washed with phosphate buffered saline and incubated for 48 hours.28 Responder cells were mixed in a 1:1 ratio with Ad-sig-ecdhMUC1/ecdCD40L infected antigen-presenting cells (APCs), and Western blot analysis was performed at the indicated time points.
Statistics
All parameters were analyzed using Student t test or analysis of variance (ANOVA), followed by the Scheffé procedure for multiple comparisons as post hoc analysis. All data shown are presented as mean ± SEM.
Results
TAA/ecdCD40L protein binds to DCs
Cell free-coupled transcription/translation and Western blot analysis of the E7/ecdCD40L, E7, ecdCD40L, E7/wtCD40L, and wtCD40L proteins were used to study the molecular weights of the proteins produced in cells infected by the Ad-sig-E7/ecdCD40L, Ad-sig-E7, Ad-sig-ecdCD40L, Ad-E7/wtCD40L, and Ad-wtCD40L vectors, respectively. As shown in Figure 1B-C, the molecular weights of these proteins are those predicted.
We then collected the TAA/ecdCD40L proteins from vector-infected 293 cells and labeled these proteins with fluorescein (see “Materials and methods”). These proteins were then incubated in vitro with bone marrow-derived DCs (fractionated to 78% purity) for 30 minutes at 4°C. The DCs were washed and portioned once using light microscopy and again using fluorescence microscopy. As shown in Figure 1Di-ii, the secretable form of E7/ecdCD40L can bind to the DCs.
A second experiment was carried out in which 293 cells were infected with the Ad-sig-ecdhMUC-1/ecdCD40L vector (His tag present), and the proteins were fluorescein labeled after purification of the MUC-1/ecdCD40L proteins on a Nickel column. The cells were exposed to a PE-conjugated anti-CD11C antibody and to the FITC-conjugated ecdhMUC-1/ecdCD40L proteins. The results (Figure 1Diii-v) show that the DCs bind the ecdhMUC-1/ecdCD40L proteins.
E7/ecdCD40L protein can be detected in vivo for up to 10 days in vivo after subcutaneous injection of the Ad-sig-E7/ecdCD40L vector
We then sectioned the skin at the site of intradermal injection of the Ad-sig-E7/ecdCD40L vector to determine when the secretable sig-E7/ecdCD40L protein was released from vector-infected cells. We double stained these sections with an FITC-labeled antibody to the CD40L (CD154), which stained green (Figure 2A), and DAPI, which stained the nuclear DNA blue (Figure 2A). As indicated in Figure 2A, double staining showed that the TAA/CD40L protein bound in vivo to cells near the vector-infected cells for up to 10 days after subcutaneous injection with the Ad-sig-E7/ecdCD40L vector, which carried the secretable TAA/ecdCD40L transcription unit. In contrast, a lower level of double-stained positive cells was observed in the epidermis 3 days after injection of the Ad-E7/wtCD40L, which contained a nonsecretable CD40L transcription unit (data not shown).
TAA/ecdCD40L protein from Ad-sig-TAA/ecdCD40L vector-infected cells binds to and activates DCs, which induce migration to regional lymphoid tissue. (A) Injection of the Ad-sig-E7/ecdCD40L vector generates the release of the E7/ecdCD40L protein around the vector injection site for up to 10 days. Skin section stained by anti-CD154 and DAPI 5 days (i) and 10 days (ii) after injection of the Ad-sig-E7/ecdCD40L vector. (B) Bone marrow-derived DCs release IL-12 and IFN-γ after exposure to the Ad-sig-E7/CD40L Vector. IL-12 (i) or IFN-γ (ii) released by vector-infected DCs into the supernatant medium was measured by ELISA in DCs stimulated for 24 hours (light gray bars) and 48 hours (dark gray bars) with the adenoviral vectors Ad-sig-E7/ecdCD40L, Ad-ecdCD40L, Ad-GFP, Ad-wtCD40L, and AD-E7/wtCD40L. (iii) Semiquantitative RT-PCR reaction was used to measure the levels of E7/CD40L RNA in 293 cells exposed to the Ad-sig-eE7/ecdCD40L vector or the Ad-E7/wtCD40L vector. 293 cells were infected with the vectors Ad-sig-ecdCD40L, Ad-E7/wtCD40L, and Ad-sig-E7/ecdCD40L at an MOI of 10. Then the RNA was isolated and PCR was carried out with primers specific for E7/CD40L mRNA. The cDNA generated was then fractionated on a molecular-weight gel. The electrophoretic species corresponding to the predicted molecular weight of the PCR product from the E7/CD40L template is indicated in the right-hand margin of the gel by the CD40L label. Electrophoretic mobility of a PCR cDNA product using the same RNA but primers specific for GAPDH (loading control) is indicated in the right-hand margin by glyceraldehyde phosphate dehydrogenase (GAPDH). (C) Up-regulation of CCR-7 mRNA in DCs exposed to the Ad-sig-E7/ecdCD40L vector. Lane 1: the Ad-sig-ecdCD40L vector. Lane 2: the Ad-wtCD40L vector. Lane 3: the Ad-sig-E7/ecdCD40L vector. Lane 4: the Ad-E7 vector. Lane 5: the Ad-E7-wtCD40L vector. Lane 6: uninfected cells (control). (D) In vivo study of migration of DCs to regional lymph nodes after loading of DCs with CFDA SE dye and infection with the Ad-sig-E7/ecdCD40L vector. Bone marrow-derived DCs were loaded in vitro with the CFDA SE supravital dye, exposed in vitro to the following vectors at an MOI of 200. (i) Ad-sig-E7/ecdCD40L. (ii) Ad-ecdCD40L. (iii) Ad-E7/wtCD40L. (iv) Ad-wtCD40L. DCs were then injected subcutaneously into the hind flanks of the test mice. Two days later, regional lymph nodes were dissected and frozen sections were studied under a fluorescence microscope. Color micrographs were obtained.
TAA/ecdCD40L protein from Ad-sig-TAA/ecdCD40L vector-infected cells binds to and activates DCs, which induce migration to regional lymphoid tissue. (A) Injection of the Ad-sig-E7/ecdCD40L vector generates the release of the E7/ecdCD40L protein around the vector injection site for up to 10 days. Skin section stained by anti-CD154 and DAPI 5 days (i) and 10 days (ii) after injection of the Ad-sig-E7/ecdCD40L vector. (B) Bone marrow-derived DCs release IL-12 and IFN-γ after exposure to the Ad-sig-E7/CD40L Vector. IL-12 (i) or IFN-γ (ii) released by vector-infected DCs into the supernatant medium was measured by ELISA in DCs stimulated for 24 hours (light gray bars) and 48 hours (dark gray bars) with the adenoviral vectors Ad-sig-E7/ecdCD40L, Ad-ecdCD40L, Ad-GFP, Ad-wtCD40L, and AD-E7/wtCD40L. (iii) Semiquantitative RT-PCR reaction was used to measure the levels of E7/CD40L RNA in 293 cells exposed to the Ad-sig-eE7/ecdCD40L vector or the Ad-E7/wtCD40L vector. 293 cells were infected with the vectors Ad-sig-ecdCD40L, Ad-E7/wtCD40L, and Ad-sig-E7/ecdCD40L at an MOI of 10. Then the RNA was isolated and PCR was carried out with primers specific for E7/CD40L mRNA. The cDNA generated was then fractionated on a molecular-weight gel. The electrophoretic species corresponding to the predicted molecular weight of the PCR product from the E7/CD40L template is indicated in the right-hand margin of the gel by the CD40L label. Electrophoretic mobility of a PCR cDNA product using the same RNA but primers specific for GAPDH (loading control) is indicated in the right-hand margin by glyceraldehyde phosphate dehydrogenase (GAPDH). (C) Up-regulation of CCR-7 mRNA in DCs exposed to the Ad-sig-E7/ecdCD40L vector. Lane 1: the Ad-sig-ecdCD40L vector. Lane 2: the Ad-wtCD40L vector. Lane 3: the Ad-sig-E7/ecdCD40L vector. Lane 4: the Ad-E7 vector. Lane 5: the Ad-E7-wtCD40L vector. Lane 6: uninfected cells (control). (D) In vivo study of migration of DCs to regional lymph nodes after loading of DCs with CFDA SE dye and infection with the Ad-sig-E7/ecdCD40L vector. Bone marrow-derived DCs were loaded in vitro with the CFDA SE supravital dye, exposed in vitro to the following vectors at an MOI of 200. (i) Ad-sig-E7/ecdCD40L. (ii) Ad-ecdCD40L. (iii) Ad-E7/wtCD40L. (iv) Ad-wtCD40L. DCs were then injected subcutaneously into the hind flanks of the test mice. Two days later, regional lymph nodes were dissected and frozen sections were studied under a fluorescence microscope. Color micrographs were obtained.
Activation of DCs by the Ad-sig-E7/ecdCD40L vector
As shown in Figure 2Bi, there was a statistically significant increase in the level of induction of IL-12 production after in vitro exposure of the DCs to the supernatant of Ad-sig-E7/ecdCD40L vector-infected 293 cells. This vector carried a transcription unit encoding a secretable TAA/CD40L protein as in Figure 1. The results were compared with vectors encoding a nonsecretable TAA/CD40L protein, such as the Ad-E7/wtCD40L vector (P < .0001). IL-12 (6 ± 3 pg/2 × 105 cells per milliliter per 24 hours or 66 ± 18 pg/2 × 105 cells per milliliter per 48 hours) was produced by DCs exposed to the Ad-sig-E7/ecdCD40L vector supernatant, whereas exposing DCs to the Ad-E7/wtCD40L vector supernatant resulted in no measurable IL-12 at 24 hours or 48 hours.
Similarly, there was a statistically significant increase in the IFN-γ released from DCs exposed to the supernatant from the Ad-sig-E7/ecdCD40L vector-infected cells: 24 ± 3 pg in the first 24 hours and 132 ± 6 pg during the next 24 hours, compared with 0 pg released from DCs exposed to supernatant from 293 cells infected with nonsecretable CD40L vectors or other control vectors (Figure 2Bii). These experimental data suggest that the TAA/ecdCD40L fusion protein secreted from the Ad-sig-TAA/ecdCD40L-infected cells bound to the CD40 receptor on DCs to generate the observed effect on cytokine release.
Differences between the cytokine release induced in bone marrow-derived DCs exposed to the supernatant from 293 cells infected with CD40L secretable or nonsecretable transcription units could be attributed to the E7/CD40L RNA levels generated by the Ad-sig-E7/ecdCD40L (encoding the secretable E7/CD40L protein) compared with the Ad-E7/wtCD40L (encoding the nonsecretable E7/CD40L protein). Another possibility is that one vector encodes a secretable or a nonsecretable protein. To test this question, RNA was extracted from 293 cells that had been infected by either the Ad-sig-E7/ecdCD40L vector or the Ad-E7/wtCD40L vector at an MOI of 10. The cDNA was synthesized by using the superscript first-strand system (Invitrogen, Carlsbad, CA). RT-PCR was performed using 5 μg total RNA extracted from the vector-infected cells and the reverse transcription reaction with a random primer. The cDNA product was split into 2 halves; one half was used as a template for a PCR reaction with primers specific for the E7/CD40L cDNA, and the other half was used to prime a PCR reaction with primers specific for GAPDH as a control. Results shown Figure 2Biii, indicate no difference in the E7/CD40L mRNA levels using the secretable or the nonsecretable vectors. Thus, it appears that cytokine release is greater from bone marrow-derived DCs exposed to the supernatant from 293 cells infected with the Ad-sig-E7/ecdCD40L rather than the Ad-E7/CD40L vector because of the secretable nature of the E7/CD40L protein from the Ad-sig-E7/ecdCD40L-infected cells.
In vitro and in vivo exposure of DCs to the Ad-sig-E7/ecdCD40L vector elevates CC chemokine receptor-7 (CCR-7) expression in mature DCs and induces the migration of DCs to regional lymph nodes
On antigen exposure, DCs become activated, express CCR-7, and migrate in response to differential gradients of the chemokine ligands CCL 19 and CCL 21.26 Therefore, we investigated the effect of exposing DCs to supernatants from Ad-sig-E7/ecdCD40L-infected 293 cells to determine whether the level of CCR-7 expression increased. As shown in Figure 2C, the level of CCR-7 mRNA in DCs increased significantly when DCs were cultured with supernatants from Ad-sig-E7/ecdCD40L or Ad-sig-E7/ecdCD40L vector-infected 293 cells.26
To formally test whether the subcutaneous injection of the Ad-sig-E7/ecdCD40L vector induces migration of the DCs to the regional lymph nodes in vivo,26 1 × 106 DCs were loaded with the CFDA SE dye and were exposed to adenoviral vectors at an MOI of 200. Then, the dye-loaded DCs were injected into the left flanks of the C57BL/6 mice. Three days after these injections, the mice were killed, and the regional axillary lymph nodes on the side of the injection were harvested and studied for the presence of the dye-loaded DCs. As shown by the green dots visible in Figure 2Di, CFDA SE-stained DCs are detectably present in the regional lymph nodes after injection of the vector carrying the secretable E7/ecdCD40L transcription unit, whereas no other vector (Figure 2Dii-iv) was associated with detectable fluorescence-labeled DCs in the regional lymph nodes. No CFDA SE-labeled cells were observed in the nondraining, contralateral lymph nodes. One of the sections was stained with PE-labeled CD11C antibody to confirm that the green-stained cells were DCs (data not shown).
Injection of Ad-sig-E7/ecdCD40L suppresses growth of E7-positive cancer cells in syngeneic mice
To assess the effect of subcutaneous injection of the Ad-sig-E7/ecdCD40L vector on the engraftment of the E7-positive TC-1 cell line in C57BL/6 mice, we injected 1 × 108 pfu of each vector subcutaneously into each animal. Mice were vaccinated again 1 week later with the same vector. One week after this boost, 5 × 105 E7-positive TC-1 cells were injected subcutaneously on the backs of the C57BL/6 mice at a site different from that of the vector injections. All mice injected with the Ad-sig-E7/ecdCD40L vector remained tumor free throughout the study (up to 18 days after injection), whereas mice injected with all other vectors listed in Figure 3A, including the Ad-E7/wtCD40L vector, which did not carry a secretable TAA/CD40L transcription unit, had measurable tumors within 13 days of tumor challenge (Figure 3A).
Mechanism of the Ad-sig-E7/ecdCD40L vector-induced suppression of the growth of E7-positive TC-1 tumor cells in C57BL/6 mice. (A) Resistance to the subcutaneous growth of 5 × 105 E7-positive TC-1 cancer cells in mice after 2 injections with 1 × 108 pfu of the Ad-sig-E7/ecdCD40L vector 7 days apart. (▪) Ad-sig-E7/ecdCD40L. (□) Ad-sig-ecdCD40L. (▴) Ad-E7/wtCD40L. ( ) Ad-wtCD40L. (B) Survival of mice vaccinated with Ad-sig-E7/ecdCD40L vectors. The following vectors were injected into C57BL/6 mice, after which the E7-positive TC-1 cancer cells were injected into the subcutaneous spaces of the mice: bold continuous line, mice treated with 2 subcutaneous injections 7 days apart of 1 × 108 pfu of the Ad-sig-E7/ecdCD40L vector; thin continuous line, mice treated with subcutaneous injections of the Ad-wtCD40L vector; broken thin line, control mice, which were not treated with vector injections. (C) Comparison of the effects of 2 subcutaneous injections of 1 × 108 pfu of the Ad-sig-E7/ecdCD40L vector on the in vivo growth of the E7-positive TC-1 cells (♦) and the E7-negative EL-4 cell line (▦). Sizes of the subcutaneous tumors were estimated by measuring with calipers in 2 separate orthogonal directions and then calculating the volume assuming an elliptical shape. (D) Use of tetramers to measure the level of E7-specific CD8+ T cells in the spleens of Ad-sig-E7/ecd/CD40L vector-immunized C57BL/6 mice. Spleen cells were harvested 10 days after the completion of 2 subcutaneous injections 7 days apart with 1 × 108 pfu of vectors Ad-sig-E7/ecdCD40L, Ad-E7/wtCD40L, Ad-wtCD40L, and Ad-sig-ecdCD40L. T cells were then analyzed for the percentage of E749-57 peptide-specific CD8+ T-cell lymphocytes by H-2Db tetramer staining. (E) ELISPOT assay shows increase in the level of IFN-α-secreting cells in the spleen cells of mice injected subcutaneously twice (7 days apart) with 1 × 108 pfu Ad-sig-E7/ecdCD40 vector. Mice were injected twice with the following vectors: Ad-sig-E7/ecdCD40L, Ad-sig-ecdCD40L, Ad-E7/wtCD40L, and Ad-wtCD40L. Splenic T cells taken from the mice 1 week later were analyzed by ELISPOT assay for the presence of IFN-γ. (F) Increase in the level of E7-specific CTLs in the spleens of Ad-sig-e7/ecdCD40L-injected mice. Mice were injected subcutaneously twice (7 days apart) with 1 × 108 pfu of vectors Ad-sig-E7/ecdCD40L, Ad-E7/wtCD40L, Ad-sig-ecdCD40L, Ad-wtCD40L, and control (no vector injection). T cells were harvested from the spleens of the test mice 1 week after the second adenoviral vector injection and were restimulated in vitro with TC-1. After 7 days, restimulated effector cells (spleen cells exposed to TC-1 cells in vitro) were mixed at varying ratios with TC-1 (E7-positive) and EL-4 (E7-negative) target cells. Then the LDH released from the target cells was measured. No LDH was detectable from any of the mixtures of EL-4 and the restimulated effector cells isolated from the vaccinated mice, whereas significant levels of LDH were released from the TC-1 target cells when they were mixed with the restimulated effector cells isolated from the mice vaccinated with the Ad-sig-E7/ecdCD40L vector.
) Ad-wtCD40L. (B) Survival of mice vaccinated with Ad-sig-E7/ecdCD40L vectors. The following vectors were injected into C57BL/6 mice, after which the E7-positive TC-1 cancer cells were injected into the subcutaneous spaces of the mice: bold continuous line, mice treated with 2 subcutaneous injections 7 days apart of 1 × 108 pfu of the Ad-sig-E7/ecdCD40L vector; thin continuous line, mice treated with subcutaneous injections of the Ad-wtCD40L vector; broken thin line, control mice, which were not treated with vector injections. (C) Comparison of the effects of 2 subcutaneous injections of 1 × 108 pfu of the Ad-sig-E7/ecdCD40L vector on the in vivo growth of the E7-positive TC-1 cells (♦) and the E7-negative EL-4 cell line (▦). Sizes of the subcutaneous tumors were estimated by measuring with calipers in 2 separate orthogonal directions and then calculating the volume assuming an elliptical shape. (D) Use of tetramers to measure the level of E7-specific CD8+ T cells in the spleens of Ad-sig-E7/ecd/CD40L vector-immunized C57BL/6 mice. Spleen cells were harvested 10 days after the completion of 2 subcutaneous injections 7 days apart with 1 × 108 pfu of vectors Ad-sig-E7/ecdCD40L, Ad-E7/wtCD40L, Ad-wtCD40L, and Ad-sig-ecdCD40L. T cells were then analyzed for the percentage of E749-57 peptide-specific CD8+ T-cell lymphocytes by H-2Db tetramer staining. (E) ELISPOT assay shows increase in the level of IFN-α-secreting cells in the spleen cells of mice injected subcutaneously twice (7 days apart) with 1 × 108 pfu Ad-sig-E7/ecdCD40 vector. Mice were injected twice with the following vectors: Ad-sig-E7/ecdCD40L, Ad-sig-ecdCD40L, Ad-E7/wtCD40L, and Ad-wtCD40L. Splenic T cells taken from the mice 1 week later were analyzed by ELISPOT assay for the presence of IFN-γ. (F) Increase in the level of E7-specific CTLs in the spleens of Ad-sig-e7/ecdCD40L-injected mice. Mice were injected subcutaneously twice (7 days apart) with 1 × 108 pfu of vectors Ad-sig-E7/ecdCD40L, Ad-E7/wtCD40L, Ad-sig-ecdCD40L, Ad-wtCD40L, and control (no vector injection). T cells were harvested from the spleens of the test mice 1 week after the second adenoviral vector injection and were restimulated in vitro with TC-1. After 7 days, restimulated effector cells (spleen cells exposed to TC-1 cells in vitro) were mixed at varying ratios with TC-1 (E7-positive) and EL-4 (E7-negative) target cells. Then the LDH released from the target cells was measured. No LDH was detectable from any of the mixtures of EL-4 and the restimulated effector cells isolated from the vaccinated mice, whereas significant levels of LDH were released from the TC-1 target cells when they were mixed with the restimulated effector cells isolated from the mice vaccinated with the Ad-sig-E7/ecdCD40L vector.
Mechanism of the Ad-sig-E7/ecdCD40L vector-induced suppression of the growth of E7-positive TC-1 tumor cells in C57BL/6 mice. (A) Resistance to the subcutaneous growth of 5 × 105 E7-positive TC-1 cancer cells in mice after 2 injections with 1 × 108 pfu of the Ad-sig-E7/ecdCD40L vector 7 days apart. (▪) Ad-sig-E7/ecdCD40L. (□) Ad-sig-ecdCD40L. (▴) Ad-E7/wtCD40L. ( ) Ad-wtCD40L. (B) Survival of mice vaccinated with Ad-sig-E7/ecdCD40L vectors. The following vectors were injected into C57BL/6 mice, after which the E7-positive TC-1 cancer cells were injected into the subcutaneous spaces of the mice: bold continuous line, mice treated with 2 subcutaneous injections 7 days apart of 1 × 108 pfu of the Ad-sig-E7/ecdCD40L vector; thin continuous line, mice treated with subcutaneous injections of the Ad-wtCD40L vector; broken thin line, control mice, which were not treated with vector injections. (C) Comparison of the effects of 2 subcutaneous injections of 1 × 108 pfu of the Ad-sig-E7/ecdCD40L vector on the in vivo growth of the E7-positive TC-1 cells (♦) and the E7-negative EL-4 cell line (▦). Sizes of the subcutaneous tumors were estimated by measuring with calipers in 2 separate orthogonal directions and then calculating the volume assuming an elliptical shape. (D) Use of tetramers to measure the level of E7-specific CD8+ T cells in the spleens of Ad-sig-E7/ecd/CD40L vector-immunized C57BL/6 mice. Spleen cells were harvested 10 days after the completion of 2 subcutaneous injections 7 days apart with 1 × 108 pfu of vectors Ad-sig-E7/ecdCD40L, Ad-E7/wtCD40L, Ad-wtCD40L, and Ad-sig-ecdCD40L. T cells were then analyzed for the percentage of E749-57 peptide-specific CD8+ T-cell lymphocytes by H-2Db tetramer staining. (E) ELISPOT assay shows increase in the level of IFN-α-secreting cells in the spleen cells of mice injected subcutaneously twice (7 days apart) with 1 × 108 pfu Ad-sig-E7/ecdCD40 vector. Mice were injected twice with the following vectors: Ad-sig-E7/ecdCD40L, Ad-sig-ecdCD40L, Ad-E7/wtCD40L, and Ad-wtCD40L. Splenic T cells taken from the mice 1 week later were analyzed by ELISPOT assay for the presence of IFN-γ. (F) Increase in the level of E7-specific CTLs in the spleens of Ad-sig-e7/ecdCD40L-injected mice. Mice were injected subcutaneously twice (7 days apart) with 1 × 108 pfu of vectors Ad-sig-E7/ecdCD40L, Ad-E7/wtCD40L, Ad-sig-ecdCD40L, Ad-wtCD40L, and control (no vector injection). T cells were harvested from the spleens of the test mice 1 week after the second adenoviral vector injection and were restimulated in vitro with TC-1. After 7 days, restimulated effector cells (spleen cells exposed to TC-1 cells in vitro) were mixed at varying ratios with TC-1 (E7-positive) and EL-4 (E7-negative) target cells. Then the LDH released from the target cells was measured. No LDH was detectable from any of the mixtures of EL-4 and the restimulated effector cells isolated from the vaccinated mice, whereas significant levels of LDH were released from the TC-1 target cells when they were mixed with the restimulated effector cells isolated from the mice vaccinated with the Ad-sig-E7/ecdCD40L vector.
) Ad-wtCD40L. (B) Survival of mice vaccinated with Ad-sig-E7/ecdCD40L vectors. The following vectors were injected into C57BL/6 mice, after which the E7-positive TC-1 cancer cells were injected into the subcutaneous spaces of the mice: bold continuous line, mice treated with 2 subcutaneous injections 7 days apart of 1 × 108 pfu of the Ad-sig-E7/ecdCD40L vector; thin continuous line, mice treated with subcutaneous injections of the Ad-wtCD40L vector; broken thin line, control mice, which were not treated with vector injections. (C) Comparison of the effects of 2 subcutaneous injections of 1 × 108 pfu of the Ad-sig-E7/ecdCD40L vector on the in vivo growth of the E7-positive TC-1 cells (♦) and the E7-negative EL-4 cell line (▦). Sizes of the subcutaneous tumors were estimated by measuring with calipers in 2 separate orthogonal directions and then calculating the volume assuming an elliptical shape. (D) Use of tetramers to measure the level of E7-specific CD8+ T cells in the spleens of Ad-sig-E7/ecd/CD40L vector-immunized C57BL/6 mice. Spleen cells were harvested 10 days after the completion of 2 subcutaneous injections 7 days apart with 1 × 108 pfu of vectors Ad-sig-E7/ecdCD40L, Ad-E7/wtCD40L, Ad-wtCD40L, and Ad-sig-ecdCD40L. T cells were then analyzed for the percentage of E749-57 peptide-specific CD8+ T-cell lymphocytes by H-2Db tetramer staining. (E) ELISPOT assay shows increase in the level of IFN-α-secreting cells in the spleen cells of mice injected subcutaneously twice (7 days apart) with 1 × 108 pfu Ad-sig-E7/ecdCD40 vector. Mice were injected twice with the following vectors: Ad-sig-E7/ecdCD40L, Ad-sig-ecdCD40L, Ad-E7/wtCD40L, and Ad-wtCD40L. Splenic T cells taken from the mice 1 week later were analyzed by ELISPOT assay for the presence of IFN-γ. (F) Increase in the level of E7-specific CTLs in the spleens of Ad-sig-e7/ecdCD40L-injected mice. Mice were injected subcutaneously twice (7 days apart) with 1 × 108 pfu of vectors Ad-sig-E7/ecdCD40L, Ad-E7/wtCD40L, Ad-sig-ecdCD40L, Ad-wtCD40L, and control (no vector injection). T cells were harvested from the spleens of the test mice 1 week after the second adenoviral vector injection and were restimulated in vitro with TC-1. After 7 days, restimulated effector cells (spleen cells exposed to TC-1 cells in vitro) were mixed at varying ratios with TC-1 (E7-positive) and EL-4 (E7-negative) target cells. Then the LDH released from the target cells was measured. No LDH was detectable from any of the mixtures of EL-4 and the restimulated effector cells isolated from the vaccinated mice, whereas significant levels of LDH were released from the TC-1 target cells when they were mixed with the restimulated effector cells isolated from the mice vaccinated with the Ad-sig-E7/ecdCD40L vector.
As shown in Figure 3B, the survival of the mice injected with the Ad-sig-E7/ecdCD40L vector (bold, unbroken line at the top of the graph) and then injected with the E7-positive TC-1 cells was superior to the survival of mice injected with the Ad-E7/wtCD40L vector (thin, unbroken line), which does not encode a secretable E7/CD40L protein, or injected with no vector (thin, broken line) and then injected with the TC-1 cells.
We then tested whether inducing resistance to engraftment of the E7-positive TC-1 cells was specific for the E7 antigen. As shown in Figure 3C, subcutaneous injection of the Ad-sig-E7/ecdCD40L vector did not protect mice against the engraftment of E7-negative EL-4 cells but did protect against engraftment of the E7-positive TC-1 cells.
Mechanism of suppression of E7-positive tumor cells by Ad-sig-E7/ecdCD40L vector injections
Spleens were harvested 10 days after vector vaccination, and the percentage of E749-57 peptide-specific CD8+ T cells was determined by H-2Db tetramer staining. As shown in Figure 3D, the level of E7 peptide-specific T cells in the spleen cells from Ad-sig-E7/ecdCD40L injected animals was increased 3 times compared with the level observed after injection with other vectors, including the Ad-E7/wtCD40L vector.
The frequency of IFN-γ- and IL-4-secreting T cells from the spleens of mice vaccinated with the various vectors was determined by ELISPOT assays.27 As shown in Figure 3E, mice injected with the Ad-sig-E7/ecdCD40L vector had a greater number of IFN-γ-secreting T cells (117 ± 10.6 spots/1 × 105 spleen cells) than mice injected with the vector carrying the nonsecretable E7/wtCD40L transcriptional unit (26.3 ± 2.4 spots/1 × 105 spleen cells) or any of the other control vectors tested (P ≤ .05). The number of splenic T cells producing a TH2 cytokine (IL-4) was only (22.3 ± 3.68 spots/1 × 105 spleen cells). These data indicate that the Ad-sig-E7/ecdCD40L vector vaccination stimulates a TH1 rather than a TH2 immune response.
Spleen cells from mice injected with the Ad-sig-E7/ecdCD40L vector were prestimulated in vitro for 7 days with TC-1-positive cells and then mixed in a 100:1 ratio with E7-positive TC-1 cells in a cytotoxicity assay described in “Materials and methods.” These studies showed that the splenic T cells from the Ad-sig-E7/ecdCD40L vector-sensitized animals lysed 90% of the TC-1 target cells (Figure 3F). In contrast, spleen cells from uninjected mice or from mice injected with the Ad-E7/wtCD40L vector lysed 0% or 20% of the target cells, respectively.
To test whether the induced cytolytic immune response was mediated through an HLA-restricted process, we added anti-MHC class I antibody or an isotype-matched control antibody to the mixture of effector spleen cells from Ad-sig-E7/ecdCD40L vector-injected mice and E7-positive TC-1 target cancer cells. Adding the anti-HLA antibody suppressed cytotoxicity to the TC-1 target cells to 10.32%, which is significantly lower than the cytotoxicity found with control antibody (76.91%).
Injection of the Ad-sig-ecdhMUC1/ecd/CD40L vector overcomes anergy to hMUC1-positive cells in mice transgenic for the hMUC1 gene
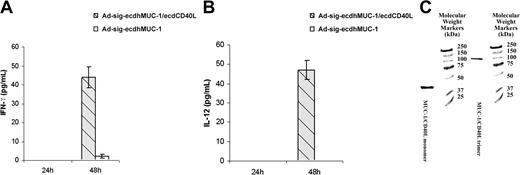
We first exposed bone marrow-derived DCs to the Ad-sigecdhMUC1/ecdCD40L vector or to the Ad-sig-ecdhMUC1 vector. As shown in Figure 4A-B, the ecdhMUC1/ecdCD40L fusion protein can significantly increase the levels of IFN-gamma and IL-12 cytokines secreted from DCs harvested from hMUC1.Tg transgenic mice 48 hours after exposure to the vector. These studies suggest that the ecdhMUC1/ecdCD40L fusion protein can bind to the CD40 receptors on DCs and induce DC activation.
The ecdhMUC1 protein released from Ad-sig-ecdhMUC1/ecdCD40L vector-infected cells forms functional trimers and activates DCs. (A) Induction of IFN-γ secretion from bone marrow-derived DCs induced by exposure to the Ad-sig-ecdhMUC1/ecdCD40L vector. Supernatant medium collected from DCs derived in vitro from hMUC1.Tg mice after exposure to the Ad-sig-ecdhMUC1/ecdCD40L vector or to the Ad-sig-ecdhMUC1 vector and then analyzed for the levels of IFN-γ. (B) Induction of IL-12 secretion from bone marrow-derived DCs induced by exposure to the Ad-sig-ecdhMUC1/ecdCD40L or the Ad-sig-ecdhMUC1 vectors. The same procedure outlined for panel A was carried out, except that the supernatant medium was analyzed for IL-12. (C) Nondenaturing gel analysis of molecular weights of the ecdhMUC1/ecdCD40L protein. A construct was created in which a His tag was placed at the carboxyl terminal end of the CD40L, and an HSF1 trimeric stabilization domain was added between the ecdhMUC1 and ecdCD40L domains. After release from vector-infected cells, the protein was purified using a His tag column, concentrated, and added to a nondenaturing gel. The protein in the lane labeled MUC1/CD40L trimer was added to the nondenaturing gel without treatment. The protein in the lane labeled MUC1/CD40L monomer was first treated with the denaturing conditions before loading on the gel. Molecular weight markers are given in the extreme right lane.
The ecdhMUC1 protein released from Ad-sig-ecdhMUC1/ecdCD40L vector-infected cells forms functional trimers and activates DCs. (A) Induction of IFN-γ secretion from bone marrow-derived DCs induced by exposure to the Ad-sig-ecdhMUC1/ecdCD40L vector. Supernatant medium collected from DCs derived in vitro from hMUC1.Tg mice after exposure to the Ad-sig-ecdhMUC1/ecdCD40L vector or to the Ad-sig-ecdhMUC1 vector and then analyzed for the levels of IFN-γ. (B) Induction of IL-12 secretion from bone marrow-derived DCs induced by exposure to the Ad-sig-ecdhMUC1/ecdCD40L or the Ad-sig-ecdhMUC1 vectors. The same procedure outlined for panel A was carried out, except that the supernatant medium was analyzed for IL-12. (C) Nondenaturing gel analysis of molecular weights of the ecdhMUC1/ecdCD40L protein. A construct was created in which a His tag was placed at the carboxyl terminal end of the CD40L, and an HSF1 trimeric stabilization domain was added between the ecdhMUC1 and ecdCD40L domains. After release from vector-infected cells, the protein was purified using a His tag column, concentrated, and added to a nondenaturing gel. The protein in the lane labeled MUC1/CD40L trimer was added to the nondenaturing gel without treatment. The protein in the lane labeled MUC1/CD40L monomer was first treated with the denaturing conditions before loading on the gel. Molecular weight markers are given in the extreme right lane.
Testing for functional trimers of ecdhMUC1/ecdCD40L proteins induced by the Ad-sig-ecdhMUC1/ecdCD40L vector injections that can activate DCs
To formally test whether trimeric ecdhMUC1/ecdCD40L proteins are released after the infection of cells with Ad-sig-ecdhMUC1/ecdCD40L vector, we purified (using a His Tag purification kit) the ecdhMUC1/ecdCD40L protein from the supernatant of 293 cells exposed to the Ad-sig-ecdhMUC1/ecdCD40L. In this vector, an HSF1 trimer stabilization domain had been placed between the ecdhMUC1 and the ecdCD40L fragments, and a His tag was placed at the carboxyl terminal domain of the ecdCD40L protein. As shown in Figure 4C, the molecular weight of the ecdhMUC1/ecdCD40L protein under nondenaturing conditions was close to 3 times that seen under denaturing conditions. This experiment showed that trimers could be formed by the ecdhMUC1/ecdCD40L fusion protein.
Subcutaneous injection of the Ad-sig-ecdhMUC1/ecdCD40L vector overcomes anergy for hMUC1 positive cells in mice, which are transgenic for hMUC1
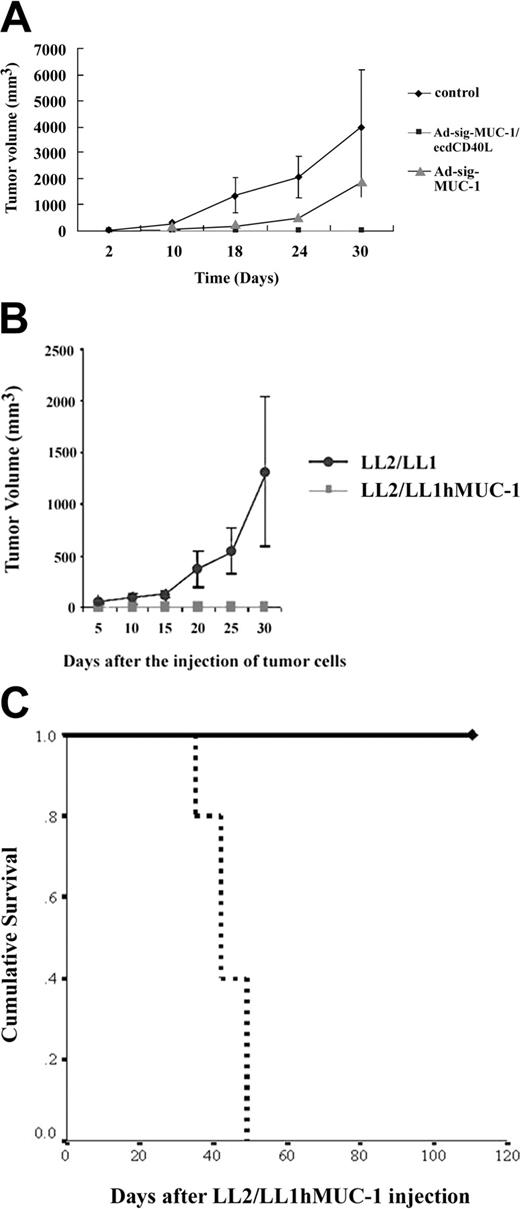
As shown in Figure 5A, mice injected subcutaneously with the Ad-sig-ecdhMUC1/ecdCD40L vector (solid squares) were resistant to engraftment by the hMUC1-positive LL2/LL1hMUC1 mouse cancer cells, whereas mice vaccinated with the Ad-sigecdhMUC-1 vector (solid triangles) or the untreated control animals not injected with vector (solid diamonds) were not resistant to the growth of the same cells. These data show that the full chimeric hMUC-1/ecdCD40L transcription unit is needed for complete suppression of the growth of the hMUC-1 cell line in the hMUC-1.Tg mice.
Effect of 2 subcutaneous injections (7 days apart) of 1 × 108 pfu of the Ad-sig-ecdhMUC1/ecdCD40L vector on the in vivo growth of the hMUC1-positive LL2/LL1hMUC1 cancer cell line in hMUC1.Tg mice. (A) Two subcutaneous injections (7 days apart) of 1 × 108 pfu Ad-sig-ecdhMUC1/ecdCD40L vector suppresses the growth of the human MUC1-positive LL2/LL1hMUC1 cancer cell line. The Ad-sig-ecdhMUC1/ecdCD40L vector or the Ad-sig-ecdhMUC-1 vector was injected twice at 7-day intervals or was not injected with any vector. One week after the second vector injection, the mice were injected with 5 × 105 LL2/LL1hMUC1 cancer cells, which were positive for hMUC1, and the growth of these cells was measured with calipers. (B) The Ad-sig-ecdhMUC1/ecdCD40L-induced suppression is specific for the hMUC1 antigen. hMUC1.Tg mice were injected twice subcutaneously (7 days apart) with 1 × 108 pfu Ad-sig-ecdhMUC1/ecdCD40L vector twice at 7-day intervals. One week after the second vector injection, the mice were injected with 5 × 105 LL2/LL1hMUC1 cells positive for the hMUC1 antigen or the same number of LL2/LL1 cells negative for the hMUC1 antigen. (C) Survival of LL2/LL1hMUC1 cell line-injected hMUC1.Tg mice that were twice (7 days apart) subcutaneously vaccinated or not vaccinated with 1 × 108 pfu Ad-sig-ecdhMUC1/ecdCD40L vector. Mice that received the injections outlined in panel A were monitored for survival after injection of the LL2/LL1hMUC1 cells. Continuous bold line indicates mice injected with the Ad-sig-ecdhMUC1/ecdCD40L vector. Broken bold line indicates mice not injected with a vector.
Effect of 2 subcutaneous injections (7 days apart) of 1 × 108 pfu of the Ad-sig-ecdhMUC1/ecdCD40L vector on the in vivo growth of the hMUC1-positive LL2/LL1hMUC1 cancer cell line in hMUC1.Tg mice. (A) Two subcutaneous injections (7 days apart) of 1 × 108 pfu Ad-sig-ecdhMUC1/ecdCD40L vector suppresses the growth of the human MUC1-positive LL2/LL1hMUC1 cancer cell line. The Ad-sig-ecdhMUC1/ecdCD40L vector or the Ad-sig-ecdhMUC-1 vector was injected twice at 7-day intervals or was not injected with any vector. One week after the second vector injection, the mice were injected with 5 × 105 LL2/LL1hMUC1 cancer cells, which were positive for hMUC1, and the growth of these cells was measured with calipers. (B) The Ad-sig-ecdhMUC1/ecdCD40L-induced suppression is specific for the hMUC1 antigen. hMUC1.Tg mice were injected twice subcutaneously (7 days apart) with 1 × 108 pfu Ad-sig-ecdhMUC1/ecdCD40L vector twice at 7-day intervals. One week after the second vector injection, the mice were injected with 5 × 105 LL2/LL1hMUC1 cells positive for the hMUC1 antigen or the same number of LL2/LL1 cells negative for the hMUC1 antigen. (C) Survival of LL2/LL1hMUC1 cell line-injected hMUC1.Tg mice that were twice (7 days apart) subcutaneously vaccinated or not vaccinated with 1 × 108 pfu Ad-sig-ecdhMUC1/ecdCD40L vector. Mice that received the injections outlined in panel A were monitored for survival after injection of the LL2/LL1hMUC1 cells. Continuous bold line indicates mice injected with the Ad-sig-ecdhMUC1/ecdCD40L vector. Broken bold line indicates mice not injected with a vector.
Mice injected with the Ad-sig-ecdhMUC1/ecdCD40L vector suppressed the growth of the hMUC1 antigen-positive LL2/LL1hMUC1 cell line, whereas this same vector did not suppress the growth of the parental cell line (LL2/LL1), which was not positive for the hMUC1 antigen (Figure 5B).This showed that the immune response was antigen specific.
Study of the cellular mechanisms through which Ad-sig-ecdhMUC1/ecdCD40L subcutaneous injections overcome anergy
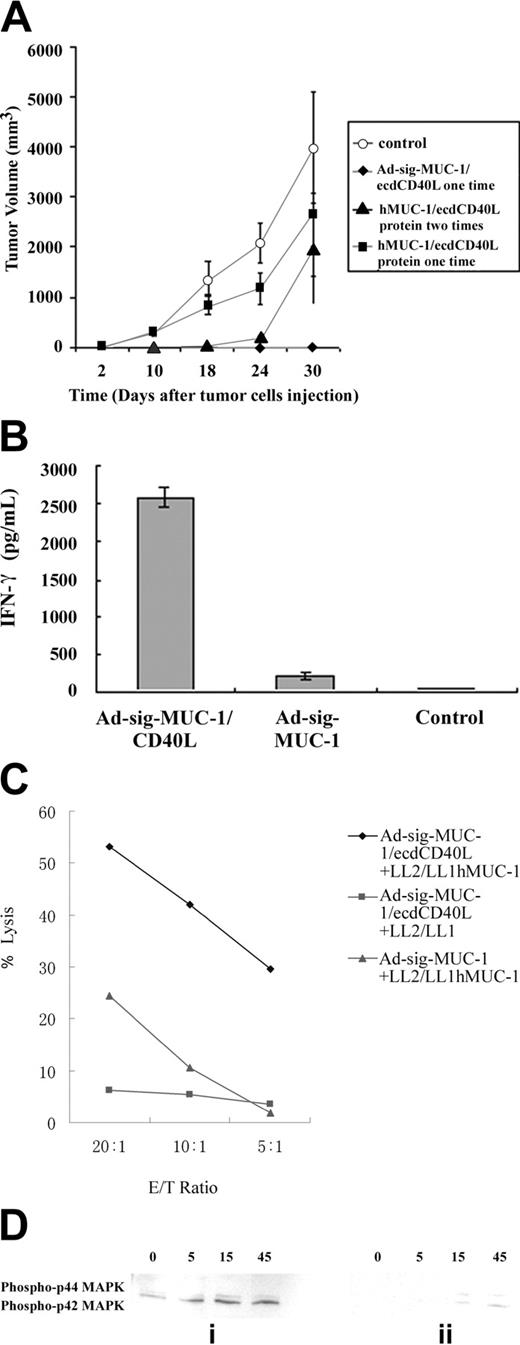
Will the injection of the ecdhMUC1/ecdCD40L protein overcome anergy in the hMUC1.Tg mouse without the vector danger signal? One question is whether the subcutaneous injection of the ecdhMUC1/ecdCD40L protein would induce the cellular immune response that was seen with the Ad-sig-ecdhMUC1/ecdCD40L vector injections. As shown by the data in Figure 6A, subcutaneous injection of the ecdhMUC1/ecdCD40L protein did not induce an immune response that could protect the hMUC1.Tg mice from the growth of the LL2/LL1hMUC1 cell line. It is possible that the use of the adenoviral vector injections provide the so-called danger signal2 necessary to induce the immune response in the hMUC1.Tg mice.
Mechanism of the suppressive effect of the Ad-sig-ecdhMUC1/ecdCD40L vector on induction of the immune suppression of the growth of the LL2/LL1hMUC1 cells in hMUC1.Tg mice. (A) Subcutaneous injection of the ecdhMUC1/ecdCD40L protein does not induce suppression of the growth of hMUC1-positive cells, which is equivalent to that seen with 2 subcutaneous injections of 1 × 108 pfu Ad-sig-ecdhMUC1/ecdCD40L vector. Five hundred thousand LL2/LL1hMUC1 cells were injected subcutaneously into the hMUC1.Tg mice. Two days after injection of the tumor cells, the ecdhMUC1/ecdCD40L protein was injected subcutaneously into hMUC1.Tg mice. (○) No protein injection. (♦) Ad-sig-ecdhMUC1/ecdCD40L vector. (▴) Two injections of the ecdhMUC1/ecdCD40L protein. (▪) One injection of the ecdhMUC1/ecdCD40L protein. (B) CD4+-depleted T cells from hMUC1 transgenic mice after 2 subcutaneous injections of 1 × 108 pfu Ad-sigecdhMUC1/ecdCD40L vector secrete increased levels of IFN-γ. CD8+ T cells were isolated from hMUC1.Tg mice that had been vaccinated twice with the Ad-sig-ecdhMUC1/ecdCD40L vector or with the Ad-sig-ecdhMUC-1 vector or that had been unvaccinated (labeled as control). Seven days after vaccination, CD8+ cells were harvested from the spleens of the test animals and were incubated for 24 hours. The supernatant medium was analyzed for IFN-γ levels. (C) Cytotoxicity of CTLs from hMUC1.Tg transgenic mice after 2 subcutaneous injections (7 days apart) of 1 × 108 pfu of Ad-sig-ecdhMUC1/ecdCD40L vector against LL2/LL1-MUC1 hMUC1-positive cancer cells or against LL2/LL1 cancer cells negative for the hMUC1 antigen. CD8+ T-cell lymphocytes were isolated from the spleens of hMUC1.Tg mice 1 week after vaccination with the Ad-sig-ecdhMUC1/ecdCD40L vector. Cells were restimulated in vitro with the LL2/LL1hMUC1 cell line for 5 days (♦) or the LL2/LL1 cell line (▪). CD8+ T-cell lymphocytes were also isolated from the spleens of hMUC1.Tg mice 1 week after vaccination with the Ad-sig-ecdhMUC1 vector, which was then stimulated in vitro with the LL2/LL1hMUC1 cell line (▴). Different effector/target ratios (20:1, 10:1, and 5:1) were used. The LDH released from each of these cell mixtures (ordinate) was then measured. (D) Phosphorylation of the ERK1/ERK2 proliferation pathway in CD8 T cells from hMUC1 transgenic mice after stimulation with bone marrow-derived DCs infected with the Ad-sig-ecdhMUC1/ecdCD40L vector. CD8 T cells were isolated by CD4 depletion from the spleen cells of hMUC1.Tg mice 1 week after the completion of 2 subcutaneous injections (1 week apart) with the Ad-sig-ecdhMUC1/ecdCD40L vector (i) or from mice that were not vaccinated (ii). DCs that had been infected with the Ad-sig-ecdhMUC1/ecdCD40L vector were then mixed in a 1:1 ratio with the restimulated CD8+ T cells. Proteins were isolated from these mixtures 0, 5, 15, and 45 minutes later and were separated using SDS-PAGE, transferred by Western blot analysis to a filter, and analyzed for phosphorylation of the p44 and p42 mitogen-activated kinase proteins using the New England BioLabs kit for phosphorylated proteins. The blot for the vaccinated mice is shown in panel i, and the blot for the unvaccinated mice is shown in panel ii.
Mechanism of the suppressive effect of the Ad-sig-ecdhMUC1/ecdCD40L vector on induction of the immune suppression of the growth of the LL2/LL1hMUC1 cells in hMUC1.Tg mice. (A) Subcutaneous injection of the ecdhMUC1/ecdCD40L protein does not induce suppression of the growth of hMUC1-positive cells, which is equivalent to that seen with 2 subcutaneous injections of 1 × 108 pfu Ad-sig-ecdhMUC1/ecdCD40L vector. Five hundred thousand LL2/LL1hMUC1 cells were injected subcutaneously into the hMUC1.Tg mice. Two days after injection of the tumor cells, the ecdhMUC1/ecdCD40L protein was injected subcutaneously into hMUC1.Tg mice. (○) No protein injection. (♦) Ad-sig-ecdhMUC1/ecdCD40L vector. (▴) Two injections of the ecdhMUC1/ecdCD40L protein. (▪) One injection of the ecdhMUC1/ecdCD40L protein. (B) CD4+-depleted T cells from hMUC1 transgenic mice after 2 subcutaneous injections of 1 × 108 pfu Ad-sigecdhMUC1/ecdCD40L vector secrete increased levels of IFN-γ. CD8+ T cells were isolated from hMUC1.Tg mice that had been vaccinated twice with the Ad-sig-ecdhMUC1/ecdCD40L vector or with the Ad-sig-ecdhMUC-1 vector or that had been unvaccinated (labeled as control). Seven days after vaccination, CD8+ cells were harvested from the spleens of the test animals and were incubated for 24 hours. The supernatant medium was analyzed for IFN-γ levels. (C) Cytotoxicity of CTLs from hMUC1.Tg transgenic mice after 2 subcutaneous injections (7 days apart) of 1 × 108 pfu of Ad-sig-ecdhMUC1/ecdCD40L vector against LL2/LL1-MUC1 hMUC1-positive cancer cells or against LL2/LL1 cancer cells negative for the hMUC1 antigen. CD8+ T-cell lymphocytes were isolated from the spleens of hMUC1.Tg mice 1 week after vaccination with the Ad-sig-ecdhMUC1/ecdCD40L vector. Cells were restimulated in vitro with the LL2/LL1hMUC1 cell line for 5 days (♦) or the LL2/LL1 cell line (▪). CD8+ T-cell lymphocytes were also isolated from the spleens of hMUC1.Tg mice 1 week after vaccination with the Ad-sig-ecdhMUC1 vector, which was then stimulated in vitro with the LL2/LL1hMUC1 cell line (▴). Different effector/target ratios (20:1, 10:1, and 5:1) were used. The LDH released from each of these cell mixtures (ordinate) was then measured. (D) Phosphorylation of the ERK1/ERK2 proliferation pathway in CD8 T cells from hMUC1 transgenic mice after stimulation with bone marrow-derived DCs infected with the Ad-sig-ecdhMUC1/ecdCD40L vector. CD8 T cells were isolated by CD4 depletion from the spleen cells of hMUC1.Tg mice 1 week after the completion of 2 subcutaneous injections (1 week apart) with the Ad-sig-ecdhMUC1/ecdCD40L vector (i) or from mice that were not vaccinated (ii). DCs that had been infected with the Ad-sig-ecdhMUC1/ecdCD40L vector were then mixed in a 1:1 ratio with the restimulated CD8+ T cells. Proteins were isolated from these mixtures 0, 5, 15, and 45 minutes later and were separated using SDS-PAGE, transferred by Western blot analysis to a filter, and analyzed for phosphorylation of the p44 and p42 mitogen-activated kinase proteins using the New England BioLabs kit for phosphorylated proteins. The blot for the vaccinated mice is shown in panel i, and the blot for the unvaccinated mice is shown in panel ii.
Cytokine release from vaccinated compared with nonvaccinated mice. To test whether the Ad-sig-ecdhMUC1/ecdCD40L induction of cellular immunity was mediated by CD8 T cells, the spleen T cells of the Ad-sig-ecdhMUC1/ecdCD40L vector vaccinated hMUC-1.Tg mice or the Ad-sig-ecdhMUC-1 vaccinated mice were depleted of CD4 T-cell lymphocytes with magnetic beads. As shown in Figure 6B, the CD8 T-cell lymphocytes isolated 7 days after injection from the spleens of hMUC1.Tg mice with the Ad-sig-ecdhMUC1/ecdCD40L vector released more than 2500 times the level of IFN-γ as did CD8 T cells taken from control vector-vaccinated MUC1.Tg mice and 50 times the levels of IFN-γ as did mice vaccinated with the Ad-sig-ecdhMUC-1 vector.
Cytotoxicity assay of splenic T cells from Ad-sig-ecdhMUC1/ecdCD40L vector injected mice against LL2/LL1hMUC1 or LL2/LL1 cancer cells. Splenic T cells were collected from hMUC1.Tg mice 7 days after injection with the Ad-sig-ecdhMUC1/ecdCD40L vector or the Ad-sig-ecdhMUC-1 vector and were then exposed to the hMUC1 antigen-positive LL2/LL1hMUC1 cancer cells for 7 days. Stimulated T cells were then mixed in varying ratios with either the hMUC1-positive LL2/LL1hMUC1 cells or the hMUC1-negative LL2/LL1 cancer cells. As shown in Figure 6C, T cells from Ad-sig-ecdhMUC1/ecdCD40L vaccinated mice can specifically kill cancer cells carrying the hMUC1 antigen but not the antigen-negative cells. Moreover, the level of hMUC-1 specific cytotoxic T cells in the Ad-sig-ecdhMUC-1/ecdCD40L mice was 6 times higher than in mice vaccinated with the Ad-sig-ecdhMUC-1 vector.
Ad-sig-ecdhMUC1/ecdCD40L vector injection overcomes resistance to expansion of hMUC1-specific T cells. Although anergic peripheral CD8+ T cells can be induced to lyse target cells in an antigen-specific manner, they have been found to exhibit a block in the activation of the ERK proliferation signal transduction pathway after antigenic stimulation.28 To determine whether CD8 cells from hMUC1.Tg mice expressed the active form of ERK1/2 on vector immunization, splenic CD8-positive T cells were obtained from noninjected hMUC1.Tg transgenic mice or mice injected 7 days earlier with the Ad-sig-ecdhMCU1/ecdCD40L vector and stimulated in vitro with the Ad-sig-ecdhMUC1/ecdCD40L vector-infected DCs.
CD8 T cells from unvaccinated hMUC1.Tg mice showed delayed kinetics and decreased total phosphorylation of ERK1 and ERK2 proteins (Figure 6Dii) compared with CD8 T cells from Ad-sig-ecdhMUC1/ecdCD40L-vaccinated hMUC1.Tg mice (Figure 6Di). These data suggest that Ad-sig-hMUC1/ecdCD40L vector injection induces an antigen-specific CD8 T-cell immune response to the MUC1 self-antigen through activation of the proliferation induction pathways in CD8 T cells.
Discussion
Our goal was to characterize the steps through which the vaccination of mice with the Ad-sig-TAA/ecdCD40L vector can induce an immune response to TAA-positive cells in anergic animals. Our experimental results suggest that subcutaneous injection of the Ad-sig-TAA/ecdCD40L vector leads to the continuous release of the TAA/ecdCD40L protein for at least a 10-day period. Binding of this protein to DCs induces increased levels of secondary signals of activation (CD80 and CD86) and the CCR-7 chemokine receptor on DCs, which lead to the migration of the TAA-loaded DCs to the regional lymph nodes. These events induce increases in the levels of the TAA-specific CD8+ cytotoxic T lymphocytes in the spleens of Ad-sig-TAA/ecdCD40L vector-injected mice.
This increase in the TAA-specific CD8+ lymphocytes in the Ad-sig-ecdhMUC1/ecdCD40L vector injected mice overcomes the anergy that exists to the hMUC1 antigen in hMUC1.Tg mice, which have expressed the hMUC1 antigen since birth. These experiments further show that inducing immunity is associated with the release of TH1 cytokines, is HLA restricted, and is accompanied by an increase in the total phosphorylation of ERK1 and ERK2 pathways in T cells from vector-injected hMUC1.Tg mice when the T cells are exposed to Ad-sig-ecdhMUC1/ecdCD40L vector-infected DCs.
In contrast to the subcutaneous injection of the Ad-sigecdhMUC1/ecdCD40L vector, the subcutaneous injection of the ecdhMUC1/ecdCD40L protein does not induce immune protection against the growth of the hMUC1-positive LL1/L2hMUC1 tumor cells (Figure 6A). This suggests that the danger signal2 associated with the adenoviral vector carrying the ecdhMUC1/ecdCD40L transcription unit is an important part of overcoming the anergy to the hMUC1 antigen that exists in the hMUC1.Tg mice.
The oral TAA/CD40L Salmonella typhimurium DNA vaccine of Xiang and coworkers14 had 3 potential limitations: the need for targeted IL-2 in addition to oral DNA bacterial vaccine; the use of a DNA vaccine that, because of its inefficiency of transfection, generated only low levels of expression for a short period of time; and the need to restrict the vaccination to the development of the antigen-loaded and activated DCs to the secondary lymphoid tissue of the gastrointestinal tract. Restriction to the T cells of the secondary lymphoid tissue of the gastrointestinal tract,29 in accordance with the method of Xiang et al,14 could be a limitation.
Because the adenoviral vector used in our work (1 and current results) can be administered to any part of the body, the homing of the T cells to the region of origin could be directed to the secondary lymphoid organs of any tissue by selection of the site of injection. In contrast to Xiang et al,14 we found no need to follow up the vaccination of mice with targeted IL-2 treatment to break tolerance or to induce resistance to the engraftment of cancer cell lines in 100% of the vaccinated mice in our studies. Finally, we showed that subcutaneous injection of the Ad-sig-TAA/ecdCD40L vector is able to overcome the anergy that develops to TAAs, which are present from birth.
We had many reasons for selecting an in vivo method of activating and TAA loading DCs. The first is that our goal was to study the steps involved in the in vivo activation and antigen loading of DCs, not to compare in vivo and ex vivo loading of DCs. In vivo activation was an attractive option to study for several reasons. First, the work of Xiang et al14 with the TAA/CD40L DNA vaccine involved in vivo vaccination, not ex vivo loading and activation. We wanted to determine whether we could improve on the in vivo activation and TAA loading seen when an adenoviral vaccine was used instead of a DNA vaccine. Second, in vivo activation by 1 or 2 subcutaneous injections of a vector could be vastly cheaper and simpler to administer than complex strategies involving ex vivo activation and TAA loading of DCs. Third, the in vitro activation approach was hampered by the limited number of DCs that could be produced, the inability to duplicate an in vivo environment in an in vitro culture system, and the short release as compared to the protracted in vivo TAA/CD40L protein release over a 10- to 14-day period when the ex vivo approach involved just a single injection. Finally, clinical trials involving ex vivo activation or tumor-antigen loading of DCs have proven to be less effective than in vivo methods of vaccination.13
A notable finding was that control experiments with vectors encoding TAA alone or CD40L alone were not as effective in activating DCs or inducing a cellular immune response against TAA-positive cancer cells in animal models. The question may be asked why the vaccination with vectors encoding the secretable fusion protein of the TAA/CD40L is more effective in inducing an immune response than vectors containing either TAA alone or CD40L alone. We have shown here that the chimeric TAA/CD40L fusion protein can form functional trimers, a requirement for binding the CD40L end of the fusion protein to the CD40 receptor on the DCs. Once the chimeric protein binds to the DCs, 2 things happen. DCs are activated to be effective at providing CD8 cells with the secondary signals necessary to activate CD8 TAA-specific T cells, and the chimeric TAA/CD40L protein is taken up into the DCs by endocytosis, thereby permitting the TAA to be processed in a way that results in its being available for presentation by MHC class I molecules. The fact that individual DCs are activated and TAA loaded is the advantage of the vectors encoding the TAA/CD40L fusion protein.
The immune response induced by the subcutaneous injection of the Ad-sig-TAA/ecdCD40L vector is antigen specific and is dependent on the activation of the DCs in and around the vector injection site and on the migration of the TAA-loaded and activated DCs to the regional lymph nodes. It is not possible to overcome anergy with subcutaneous injection of the TAA/ecdCD40L protein or the subcutaneous injection of an adenoviral vector that carries a transcription unit encoding a nonsecretable TAA/ecdCD40L protein. These experimental results suggest that this approach to the activation of the immune response against tumor cells merits further study in preclinical and clinical models.
Prepublished online as Blood First Edition Paper, July 6, 2004; DOI 10.1182/blood-2003-12-4319.
Supported by the Vaccine Development Fund of the Sidney Kimmel Cancer Center, the George and Barbara Bush Leukemia Research Fund, The Breast Cancer Research Program of the United States Army (DAMD 17 99 9457 and BC022063), and the Breast Cancer Research Foundation of New York City. We recognize the support of Sidney Kimmel to our cancer center, which made this work possible. We thank the following for their support: the Brian Schultz Cancer Research Fund, the Audrey Demas Melanoma Research Fund, and the Anthony Dewitt Frost Melanoma Research Fund.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

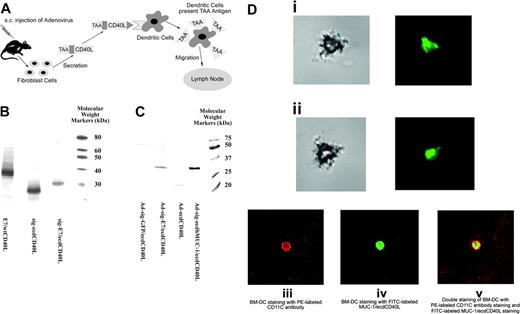
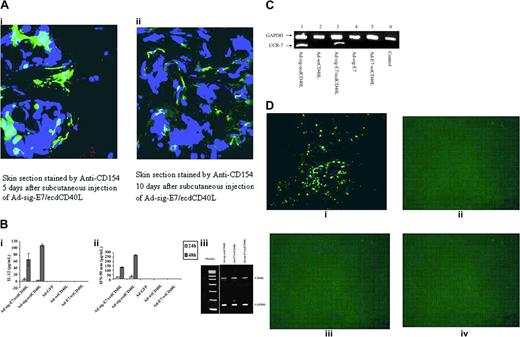
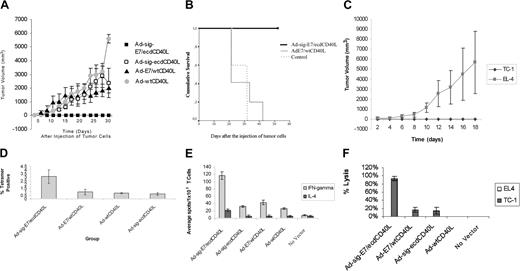
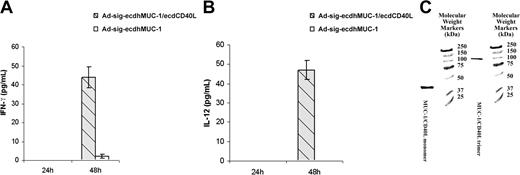
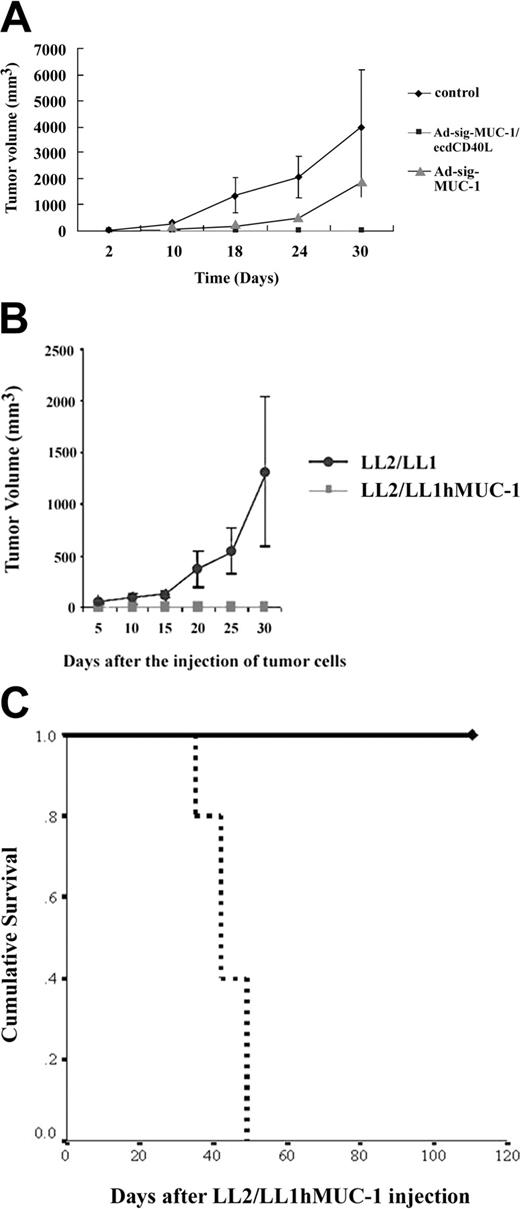
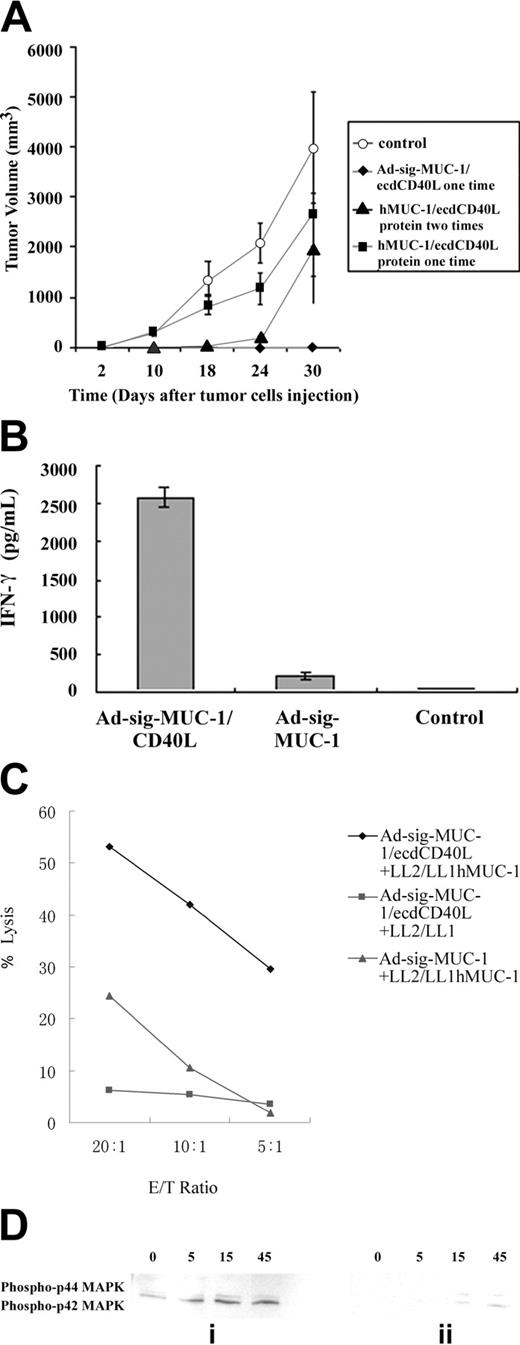

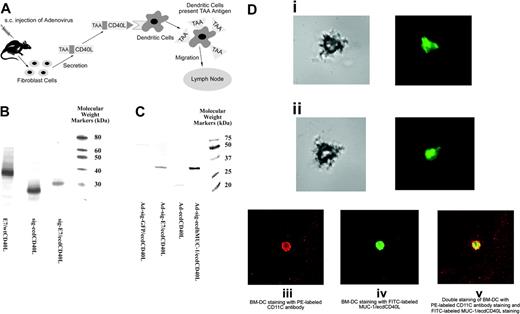
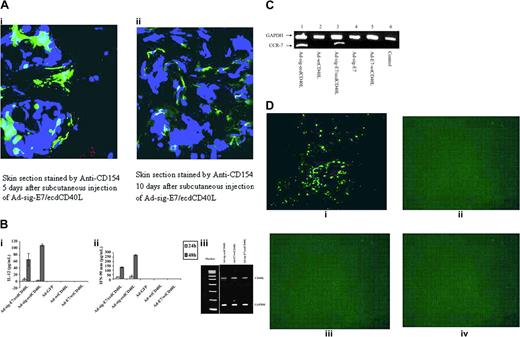
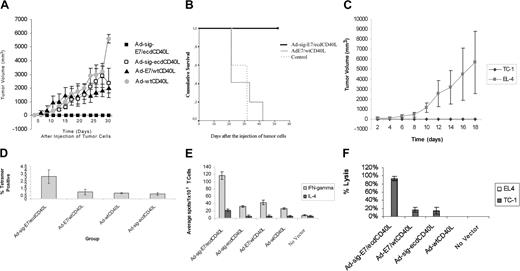
 ) Ad-wtCD40L. (B) Survival of mice vaccinated with Ad-sig-E7/ecdCD40L vectors. The following vectors were injected into C57BL/6 mice, after which the E7-positive TC-1 cancer cells were injected into the subcutaneous spaces of the mice: bold continuous line, mice treated with 2 subcutaneous injections 7 days apart of 1 × 108 pfu of the Ad-sig-E7/ecdCD40L vector; thin continuous line, mice treated with subcutaneous injections of the Ad-wtCD40L vector; broken thin line, control mice, which were not treated with vector injections. (C) Comparison of the effects of 2 subcutaneous injections of 1 × 108 pfu of the Ad-sig-E7/ecdCD40L vector on the in vivo growth of the E7-positive TC-1 cells (♦) and the E7-negative EL-4 cell line (▦). Sizes of the subcutaneous tumors were estimated by measuring with calipers in 2 separate orthogonal directions and then calculating the volume assuming an elliptical shape. (D) Use of tetramers to measure the level of E7-specific CD8+ T cells in the spleens of Ad-sig-E7/ecd/CD40L vector-immunized C57BL/6 mice. Spleen cells were harvested 10 days after the completion of 2 subcutaneous injections 7 days apart with 1 × 108 pfu of vectors Ad-sig-E7/ecdCD40L, Ad-E7/wtCD40L, Ad-wtCD40L, and Ad-sig-ecdCD40L. T cells were then analyzed for the percentage of E749-57 peptide-specific CD8+ T-cell lymphocytes by H-2Db tetramer staining. (E) ELISPOT assay shows increase in the level of IFN-α-secreting cells in the spleen cells of mice injected subcutaneously twice (7 days apart) with 1 × 108 pfu Ad-sig-E7/ecdCD40 vector. Mice were injected twice with the following vectors: Ad-sig-E7/ecdCD40L, Ad-sig-ecdCD40L, Ad-E7/wtCD40L, and Ad-wtCD40L. Splenic T cells taken from the mice 1 week later were analyzed by ELISPOT assay for the presence of IFN-γ. (F) Increase in the level of E7-specific CTLs in the spleens of Ad-sig-e7/ecdCD40L-injected mice. Mice were injected subcutaneously twice (7 days apart) with 1 × 108 pfu of vectors Ad-sig-E7/ecdCD40L, Ad-E7/wtCD40L, Ad-sig-ecdCD40L, Ad-wtCD40L, and control (no vector injection). T cells were harvested from the spleens of the test mice 1 week after the second adenoviral vector injection and were restimulated in vitro with TC-1. After 7 days, restimulated effector cells (spleen cells exposed to TC-1 cells in vitro) were mixed at varying ratios with TC-1 (E7-positive) and EL-4 (E7-negative) target cells. Then the LDH released from the target cells was measured. No LDH was detectable from any of the mixtures of EL-4 and the restimulated effector cells isolated from the vaccinated mice, whereas significant levels of LDH were released from the TC-1 target cells when they were mixed with the restimulated effector cells isolated from the mice vaccinated with the Ad-sig-E7/ecdCD40L vector.
) Ad-wtCD40L. (B) Survival of mice vaccinated with Ad-sig-E7/ecdCD40L vectors. The following vectors were injected into C57BL/6 mice, after which the E7-positive TC-1 cancer cells were injected into the subcutaneous spaces of the mice: bold continuous line, mice treated with 2 subcutaneous injections 7 days apart of 1 × 108 pfu of the Ad-sig-E7/ecdCD40L vector; thin continuous line, mice treated with subcutaneous injections of the Ad-wtCD40L vector; broken thin line, control mice, which were not treated with vector injections. (C) Comparison of the effects of 2 subcutaneous injections of 1 × 108 pfu of the Ad-sig-E7/ecdCD40L vector on the in vivo growth of the E7-positive TC-1 cells (♦) and the E7-negative EL-4 cell line (▦). Sizes of the subcutaneous tumors were estimated by measuring with calipers in 2 separate orthogonal directions and then calculating the volume assuming an elliptical shape. (D) Use of tetramers to measure the level of E7-specific CD8+ T cells in the spleens of Ad-sig-E7/ecd/CD40L vector-immunized C57BL/6 mice. Spleen cells were harvested 10 days after the completion of 2 subcutaneous injections 7 days apart with 1 × 108 pfu of vectors Ad-sig-E7/ecdCD40L, Ad-E7/wtCD40L, Ad-wtCD40L, and Ad-sig-ecdCD40L. T cells were then analyzed for the percentage of E749-57 peptide-specific CD8+ T-cell lymphocytes by H-2Db tetramer staining. (E) ELISPOT assay shows increase in the level of IFN-α-secreting cells in the spleen cells of mice injected subcutaneously twice (7 days apart) with 1 × 108 pfu Ad-sig-E7/ecdCD40 vector. Mice were injected twice with the following vectors: Ad-sig-E7/ecdCD40L, Ad-sig-ecdCD40L, Ad-E7/wtCD40L, and Ad-wtCD40L. Splenic T cells taken from the mice 1 week later were analyzed by ELISPOT assay for the presence of IFN-γ. (F) Increase in the level of E7-specific CTLs in the spleens of Ad-sig-e7/ecdCD40L-injected mice. Mice were injected subcutaneously twice (7 days apart) with 1 × 108 pfu of vectors Ad-sig-E7/ecdCD40L, Ad-E7/wtCD40L, Ad-sig-ecdCD40L, Ad-wtCD40L, and control (no vector injection). T cells were harvested from the spleens of the test mice 1 week after the second adenoviral vector injection and were restimulated in vitro with TC-1. After 7 days, restimulated effector cells (spleen cells exposed to TC-1 cells in vitro) were mixed at varying ratios with TC-1 (E7-positive) and EL-4 (E7-negative) target cells. Then the LDH released from the target cells was measured. No LDH was detectable from any of the mixtures of EL-4 and the restimulated effector cells isolated from the vaccinated mice, whereas significant levels of LDH were released from the TC-1 target cells when they were mixed with the restimulated effector cells isolated from the mice vaccinated with the Ad-sig-E7/ecdCD40L vector.