Abstract
Sézary syndrome (SzS) is an advanced form of cutaneous T-cell lymphoma characterized by peripheral blood involvement, impaired cell-mediated immunity, and T-helper 1 (TH1) cytokine production. To understand the mechanism of these defects, we studied the expression and function of CD40L in peripheral blood mononuclear cells (PBMCs) of patients with SzS. We found that PBMCs of patients with SzS have a defect in interleukin-12 (IL-12) and tumor necrosis factor-α (TNF-α) production upon anti-CD3 stimulation and that tumor CD4+ T lymphocytes have a specific defect in CD40L induction after anti-CD3 ligation in vitro. This defect may explain the poor IL-12 production, because IL-12 production by anti-CD3-stimulated PBMCs was dependent on CD40L in healthy donors. The observed defect in tumor cell CD40L expression appears to be due to inappropriate T-cell signaling upon CD3 ligation, because expression of other T-cell activation antigens such as CD25, and to a lesser extent CD69, are also impaired on tumor cells. Importantly however, the inability of SzS PBMCs to appropriately produce IL-12 and TNF-α could be restored by recombinant hexameric CD40L. Taken together, our results demonstrate that impaired IL-12 and TNF-α production in SzS is associated with defective CD4+ T lymphocyte CD40L induction and indicate that CD40L may have therapeutic potential in SzS. (Blood. 2005;105:219-225)
Introduction
Sézary syndrome (SzS) is a leukemic form of cutaneous T-cell lymphoma (CTCL) characterized by the clonal proliferation of skin-invasive CD4+ T lymphocytes that have the phenotype of mature helper T cells.1 Patients with SzS manifest erythroderma, generalized lymphadenopathy, and prominent immunologic defects, including depressed cell-mediated immunity, and as a consequence have a poor prognosis with a median survival of 32 months.2
Studies on the nature of the malignant T cells in SzS have provided evidence that these cells are at least partially responsible for the generation of the immune defects observed in this disease by way of the production of immunosuppressive T-helper 2 (TH2) cytokines and by the depressed production of TH1 cytokines.1,3-6 The molecular cause of this TH2 shift remains to be identified, but its identification is likely to provide new therapeutic possibilities, because biologic response modifiers such as interferon-α (IFN-α), IFN-γ, and interleukin-12 (IL-12), which stimulate TH1 responses, have significant therapeutic effects in SzS.7,8
Interactions between CD40 on antigen-presenting cells (APCs) and CD40 ligand (CD40L) on T cells enhance the development of TH1 responses through inducing production of IL-12 and by enhancing expression of costimulatory molecules of the B7 family on APCs.9-14 This is substantiated by the study of CD40L knock-out mice in which deficient CD40L expression on activated T cells leads to impaired IL-12 production and reduced TH1 T-cell responses.15,16 It is also supported by the demonstration of defective T-cell effector function and TH1 cytokine production in patients with X-linked hyper-IgM syndrome, a disease resulting from mutations in the gene encoding CD40L.17 Because of the known link between CD40-CD40L signaling and the development of TH1 responses, we performed an analysis of CD40 and CD40L function on mononuclear cells derived from patients with SzS.
Patients, materials, and methods
Patients, peripheral blood mononuclear cells (PBMCs), and dendritic cell preparation
All 5 patients participating in this study were diagnosed with Sézary syndrome (SzS),18 the leukemic form of CTCL, on the basis of clinical, histopathologic, and immunohistologic criteria (Table 1).19 Patients' circulating malignant T cells were analyzed on 1-μm sections of formalin-fixed peripheral blood buffy coats by the detection of mononuclear cells with cerebriform nuclear morphology.19 Additionally, for this report, patients with SzS were divided into 3 groups based on tumor load. Those with 5% to 20% circulating Sézary cells were defined as having low tumor burden; those with more than 20% to 50% circulating Sézary cells were defined as having medium tumor burden; and those with more than 50% circulating Sézary cells were defined as having high tumor burden. White blood cell counts of patients with SzS selected for these studies ranged from 3.5 × 106 to 5 × 106 cells per milliliter. All patients with SzS underwent identical treatment consisting of the use of extracorporeal photopheresis approximately every 4 weeks.20 None of the patients studied had been treated with chemotherapy, radiation, or other immunosuppressive drugs. As controls, blood samples from age-matched healthy donors were used. Donations of blood by patients or healthy volunteers in this study conformed to institutional review board (IRB)-approved protocol, and informed consents were obtained.
PBMCs were prepared as described previously.21 Briefly, venous blood was collected into heparinized syringes using uniform standards for both patients with SzS and healthy volunteers. The blood was then diluted 2-fold with Dulbecco phosphate-buffered saline (DPBS; BioWhittaker, Walkersville, MD), pH 7.2, layered over Ficoll-Hypaque (Amersham, Uppsala, Sweden), and centrifuged at 500g for 30 minutes at room temperature. The interface containing the mononuclear cell fraction was collected, and cells were washed twice with PBS. Cells were used immediately after purification. Cell cultures were set up at a final concentration of 1 × 106 cells per milliliter in RPMI 1640 media (GIBCO, Grand Island, NY) supplemented with 10% heat-inactivated fetal calf serum (FCS), 200 mM/L l-glutamine, and 100 U/mL penicillin-streptomycin.
Dendritic cells (DCs) were derived from human cord blood CD34+ cells amplified in a primary culture with FLT3-ligand, thrombopoietin, and stem cell factor as previously described.22 Briefly, DC precursors recovered from primary culture were washed, counted, and seeded at 2 × 105/mL in 24-well plates containing 1 mL DC medium (RPMI 1640 medium supplemented with 10% heat-inactivated FCS, 10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 1% nonessential amino acids, 1 mM sodium pyruvate, 2 mM l-glutamine, 100 U/mL penicillin, 100 mg/mL streptomycin). Cells were induced for 3 days with granulocyte-macrophage colony-stimulating factor (GM-CSF) (20 ng/mL) and IL-4 (20 ng/mL) (Strathmann Biotec, Hamburg, Germany) in the presence of 50 μM 2-mercaptoethanol (Sigma, St Louis, MO). After 3 days, cells were refed with 0.5 mL fresh DC medium, cytokines were renewed, and cells were further cultured for 3 days. Maturation factors such as tumor necrosis factor-α (TNF-α) (100 ng/mL), lipopolysaccharide (LPS) (20 ng/mL), or ACRP30-CD40L (megaCD40L, 100 ng/mL) as well as control molecules such as adipocyte complement-related protein of 30K (ACRP30) (amino acids [aa] 16 to 108, 100 ng/mL) or boiled megaCD40L were added for the last 48 hours. Flow cytometric analysis of DC surface CD1a, CD83, and HLA-DR expression was performed after 6 days of DC induction.23
Antibodies and reagents
Fluorescein-isothiocyanate (FITC)-conjugated monoclonal antibodies (mAbs) specific for human CD40L (clone TRAP1, mIgG1), CD40, CD80, and CD86 were purchased from BD Biosciences Pharmingen (San Jose, CA). Phycoerythrin (PE)-labeled mAbs specific for CD3 and CD64 and peridinin chlorophyll protein (PerCP)-labeled mAbs specific for CD4 were purchased from BD Biosciences Pharmingen. Anti-CD69 (FITC), anti-CD25 (PE), anti-CD7 (PerCP), anti-CD14 (allophycocyanin) were also purchased from BD Biosciences Pharmingen. Unlabeled mAbs specific for CD3 (clone HIT3a, mIgG2a) used for T-cell activation and unlabeled antagonistic mAbs specific for CD40L (TRAP1) used for CD40L blockade were purchased from BD Biosciences Pharmingen. Neutralizing Abs to IL-4, IL-10, and tumor growth factor-β1 (TGF-β1) were purchased from R&D Systems (Minneapolis, MN). Phorbol myristate acetate (PMA) (50 ng/mL), ionomycin (250 nM), and Staphylococcus aureus Cowan strain I (SAC) were purchased from (Calbiochem, La Jolla, CA) and IFN-γ (1000 U/mL) from InterMune (Brisbane, CA).
CD3 activation and ELISA quantification of secreted cytokines
Anti-CD3 was dissolved in carbonate buffer (pH 9.6) at a concentration of 1 μg/mL and aliquoted into 24-well tissue culture plates at 250 μL per well. After a 2-hour incubation at 37°C, the plates were washed twice in sterile PBS. Cell suspensions were then added. When necessary, human IFN-γ was used at 1000 U/mL, SAC (Calbiochem) at 1:10 000 (wt/vol), and LPS from Escherichia coli (Sigma) at 1 μg/mL. For fluorescence-activated cell sorter (FACS) analysis, SzS or healthy-donor PBMCs were incubated at 37°C for 6 hours, after which cells were collected and analyzed. For quantification of cytokine secretion into the media, PBMCs were similarly incubated but for 48 hours, supernatants collected, centrifuged twice to pellet contaminant cells, and frozen at -20°C until analysis. IFN-γ, IL-12, and TNF-α concentrations were determined by specific enzyme-linked immunosorbent assay (ELISA) (R&D Systems) according to the manufacturer's instructions.
Generation of soluble hexameric CD40L
Cloning, expression, and purification of recombinant proteins were performed essentially as described previously.24 Expression constructs for ACRP30 and ACRP30-CD40L (megaCD40L) were generated according to standard molecular biology protocols and cloned in the PCR-3 vector (Invitrogen, Leek, The Netherlands). The constructs encode the signal peptide of hemagglutinin (MAIIYLILLFTAVRG), the Flag sequence (DYKDDDDK), a linker (GPGQVQLQ), the collagen domain of huACRP30 (amino acids [aa] 16 to 108), and the C-terminal portion of human CD40L (huCD40L; aa 116 to 261). Recombinant proteins were also expressed from stable HEK-293 clones and were purified by affinity on M2-agarose.24
Immunoscope analysis of the Vβ repertoire
Total RNAs from CD4+CD7+ cells were extracted with Qiagen RNeasy Kit upon manufacturer's instructions (Qiagen, Basel, Switzerland) and converted to cDNA by standard methods using reverse transcriptase and an oligo(dT) primer (Invitrogen). The cDNAs were amplified in nonsaturating polymerase chain reaction (PCR) conditions (30 cycles) with a panel of previously validated 5′ sense primers specific for 22 Vβ subfamilies (Proligo, Paris, France) and one 3′ antisense primer specific for the corresponding C gene segment.25 Amplification products were run in a 2% agarose gel stained with ethidium bromide and visualized under a UV lamp.
Statistical analysis
To determine the statistical significance, levels of cytokines were compared between healthy donors and patients with SzS using the unpaired Student t test. Level of significance assumed in these comparisons was a P value of less than .05.
Results
Impaired IL-12 and TNF-α production by APCs from patients with SzS
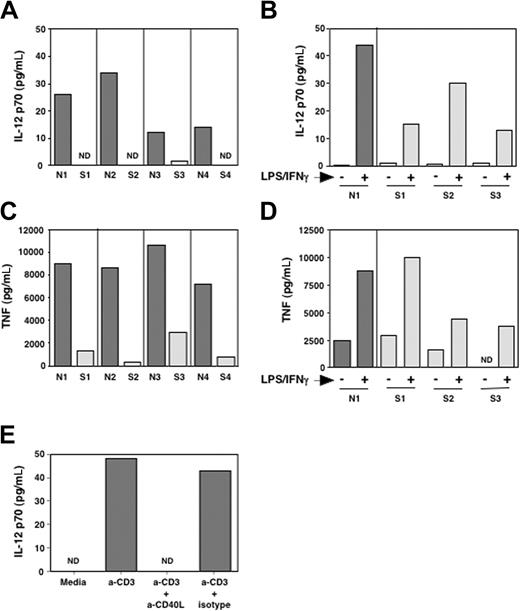
In our study of the immune function in patients with SzS, we determined the capacity of PBMCs from patients with SzS to produce IL-12 and TNF-α when stimulated with plate-bound anti-CD3. To this end we cultured PBMCs from patients with SzS and healthy donors with anti-CD3 and measured IL-12 and TNF-α secretion into the culture media by ELISA after 48 hours. As shown in Figure 1A, PBMCs of patients with SzS produced significantly reduced levels of both IL-12p70 and TNF-α as compared with healthy donors. In patients with SzS, PBMC IL-12 and TNF-α levels were more than 6-fold reduced, as they produced a mean of 0.5 ± 1 pg/mL IL-12p70 and 1350 ± 1100 pg/mL TNF-α as compared with a mean of 21 ± 10 pg/mL IL-12p70 and 8925 ± 1456 pg/mL TNF-α in healthy donors (P = .025 and .0002 for IL-12 and TNF-α, respectively). This reduced production could not solely be attributed to an intrinsic inability to synthesize these cytokines, because in patients with SzS, LPS/IFN-γ (Figure 1B,D) and SAC/IFN-γ (not shown) were able to induce significant IL-12 and TNF-α production.
Impaired IL-12p70 and TNF-α production in patients with SzS. Total PBMCs from healthy donors and patients with SzS were activated with anti-CD3 (A,C) or a mixture of LPS (1 μg/mL) and IFN-γ (500 IU/mL) (B,D). After 24 hours, supernatants were harvested and assayed for IL-12p70 (A,B) and TNF-α (C,D) production. (E) Total PBMCs from a healthy donor were activated as in panel A in the presence of a blocking mAb against CD40L (10 μg/mL) or an isotype-control mAb. The production of IL-12p70 after 24 hours is shown.
Impaired IL-12p70 and TNF-α production in patients with SzS. Total PBMCs from healthy donors and patients with SzS were activated with anti-CD3 (A,C) or a mixture of LPS (1 μg/mL) and IFN-γ (500 IU/mL) (B,D). After 24 hours, supernatants were harvested and assayed for IL-12p70 (A,B) and TNF-α (C,D) production. (E) Total PBMCs from a healthy donor were activated as in panel A in the presence of a blocking mAb against CD40L (10 μg/mL) or an isotype-control mAb. The production of IL-12p70 after 24 hours is shown.
In our assay for anti-CD3 stimulation of total PBMCs, production of IL-12 is thought to be due to induction of APC stimulatory molecules on T cells upon anti-CD3 stimulation. TNF-α production in this assay could occur by this indirect effect but also by anti-CD3-stimulated T cells themselves. Because CD40 signaling in APCs is known to induce IL-12, CD40L is likely to be one of the costimulatory molecules involved in our assay. To determine if blockade of CD40L signaling could reproduce the same defect in IL-12 production in an identical experimental setting, we performed the same experiment with PBMCs from healthy donors (n = 2) in the presence of an antagonistic antibody to CD40L. As shown in Figure 1E, inhibiting CD40L-CD40 interaction abrogated the capacity of PBMCs from healthy donors to produce IL-12 upon CD3 stimulation. Deficient CD40L signaling by CD3-stimulated T cells of patients with SzS could thus possibly explain the inability of PBMCs from patients with SzS to produce cytokines such as IL-12 that are essential for the generation of TH1 responses.
Defective induction of CD40L expression on SzS tumor cells
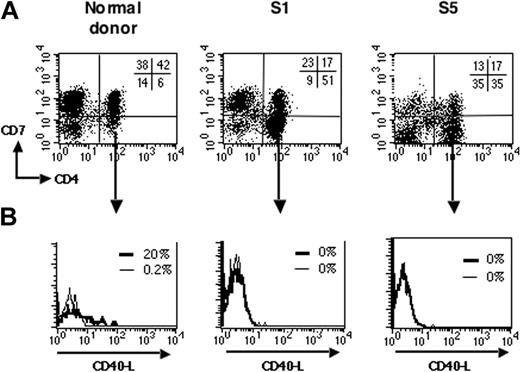
Previous studies have shown that T-cell activation leads to rapid surface expression of CD40L, thus enabling T cells following CD40L-CD40 interaction to stimulate APCs to produce cytokines.26-29 We next analyzed induction of CD40L upon CD3 ligation of PBMCs from patients with SzS. To discriminate between the tumor and nontumor CD4+ T-cell populations, we took advantage of the loss of the CD7 marker reported on CD4+ tumor cells from some patients with SzS.30 In healthy donors, most of the CD4+ T cells expressed CD7 (Schanberg et al31 and Figure 2A, left panel). As previously reported, several of the patients with SzS studied here harbored a CD4+ T-cell tumor population with a marked loss of CD7 expression, as evidenced by an abnormally increased CD4+CD7- cell population in total PBMCs (Figure 2A, middle and right panels; Table 1). We analyzed induction of CD40L expression upon anti-CD3 stimulation on tumor T cells. Figure 2B shows that stimulated tumor CD4+CD7- T cells did not express CD40L on their surface (Figure 2A, middle and right panels) compared with stimulated CD4+CD7- T cells from healthy donors (left panel). This absence of CD40L induction upon anti-CD3 stimulation was observed in all the patients with a CD4+CD7- tumor (n = 3, Table 1) and 1 patient with a CD4lowCD7+ tumor phenotype (Table 1). One patient with a CD4+CD7+ tumor and a high burden (S3) also had a complete absence in CD40L induction on tumor cells (Table 1). On the contrary, the CD4+CD7- T cells from healthy donors (n = 12) expressed CD40L upon anti-CD3 stimulation (data not shown; mean of 10% ± 7% CD40L+ cells). Altogether, this demonstrates that in patients with SzS there is a marked deficit in the ability for CD40L induction on tumor T cells.
Impaired CD40L induction on SzS tumor cells. (A) PBMCs from healthy donors and patients with SzS were double-stained for CD4 and CD7 expression. Representative dot plots are shown for a healthy donor (left panel), patients S1 (middle panel), and S5 (right panel), which both have a CD4+CD7- tumor. The percentage of labeled cells for each quadrant is indicated in the upper right quadrant. (B) PBMCs were activated with anti-CD3 as in Figure 1. After 6 hours, cells were immunostained for CD40L and analyzed by flow cytometry. Fluorescence histograms are shown for unstimulated (thin lines) and anti-CD3-stimulated (thick lines) gated CD4+CD7-cells.
Impaired CD40L induction on SzS tumor cells. (A) PBMCs from healthy donors and patients with SzS were double-stained for CD4 and CD7 expression. Representative dot plots are shown for a healthy donor (left panel), patients S1 (middle panel), and S5 (right panel), which both have a CD4+CD7- tumor. The percentage of labeled cells for each quadrant is indicated in the upper right quadrant. (B) PBMCs were activated with anti-CD3 as in Figure 1. After 6 hours, cells were immunostained for CD40L and analyzed by flow cytometry. Fluorescence histograms are shown for unstimulated (thin lines) and anti-CD3-stimulated (thick lines) gated CD4+CD7-cells.
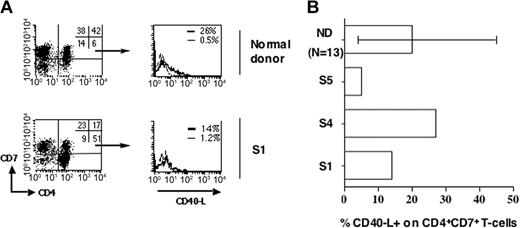
We next studied whether normal CD4+ T cells (nontumor cells) from patients with SzS are also defective for CD40L induction. We performed our analysis on CD4+CD7+ T cells. Patients S2 and S3, whose tumors are CD4+CD7+, were excluded. To confirm that we were analyzing a nontumor population, polyclonality was assessed by the immunoscope technique. Table 2 shows that the Vβ repertoire of the CD4+CD7+ population of 2 selected patients with SzS is as polyclonal as the normal counterpart from a healthy donor, indicating its nontumor characteristic. Figure 3A shows that a fraction (14%) of normal CD4+CD7+ T cells from patient S1 expressed CD40L upon anti-CD3 stimulation. When 3 patients with SzS with a detectable population of nontumoral CD4+CD7+ cells were analyzed, we observed that the mean percentage of CD40L+ cells was not significantly different on the normal CD4+CD7+ T cells of patients with SzS (14% ± 13%) as compared with those of healthy donors, which ranged from 5% to 46% (n = 13) (Figure 3B). This suggests that the remaining normal CD4+ T cells in patients with SzS are capable of expressing CD40L upon stimulation, in contrast to their tumor counterpart.
Conserved CD40L induction on normal polyclonal CD4+CD7+ cells of patients with SzS. (A) PBMCs were activated with anti-CD3. After 6 hours, cells were immunostained for CD40L, CD4, and CD7 and analyzed by flow cytometry. Dot plots show CD4 and CD7 expression for a healthy donor and patient S1 with a CD4+CD7-tumor (left panels). The percentage of labeled cells for each quadrant is indicated in the upper right quadrant. Histograms representing CD40Lexpression on unstimulated (thin lines) and anti-CD3-stimulated (thick lines) gated CD4+CD7+ T cells are shown (right panel). (B) CD4+CD7+ nontumor T cells were analyzed as in panel A for CD40L expression upon anti-CD3 stimulation. Percentage of CD40L+ cells obtained in the CD4+CD7+ T-cell fraction of healthy donors (n = 13) and patients with SzS (n = 3) is shown. Minimum and maximum values obtained with healthy donors are indicated.
Conserved CD40L induction on normal polyclonal CD4+CD7+ cells of patients with SzS. (A) PBMCs were activated with anti-CD3. After 6 hours, cells were immunostained for CD40L, CD4, and CD7 and analyzed by flow cytometry. Dot plots show CD4 and CD7 expression for a healthy donor and patient S1 with a CD4+CD7-tumor (left panels). The percentage of labeled cells for each quadrant is indicated in the upper right quadrant. Histograms representing CD40Lexpression on unstimulated (thin lines) and anti-CD3-stimulated (thick lines) gated CD4+CD7+ T cells are shown (right panel). (B) CD4+CD7+ nontumor T cells were analyzed as in panel A for CD40L expression upon anti-CD3 stimulation. Percentage of CD40L+ cells obtained in the CD4+CD7+ T-cell fraction of healthy donors (n = 13) and patients with SzS (n = 3) is shown. Minimum and maximum values obtained with healthy donors are indicated.
The tumor T-cell population of patients with SzS is refractory to TCR/CD3 signaling
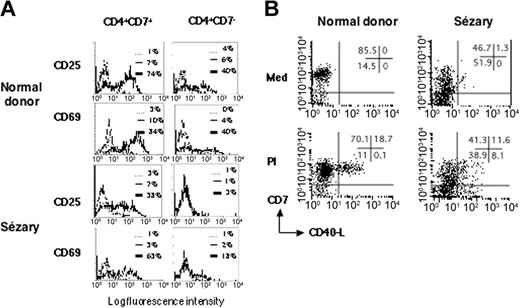
To determine if the CD40L defect in SzS tumor cells was limited to this activation antigen or whether it resulted from a more general activation defect, we looked for induction of other T-cell activation antigens on SzS tumor cells. To this end, PBMCs from patients with SzS or healthy donors were cultured in the presence of immobilized CD3 mAbs and then analyzed for expression of the T-cell activation markers CD25 and CD69. As shown in a representative experiment (Figure 4A), an absence of CD25 expression was observed in the tumor T-cell population (CD4+CD7-) of patients with SzS (n = 3). This defect was not seen in the normal T-cell population (CD4+CD7+) of such patients with SzS or in the CD4+CD7+ or CD4+CD7- populations of the healthy donors studied (n = 3). For CD69, a marked defect was also observed in the CD4+CD7- tumor T-cell population, but some cells did express detectable CD69 at their surface, showing that the T-cell receptor (TCR)/CD3 signaling was severely impaired but not completely abolished in the SzS tumor cells.
SzS patient tumor cells are refractory to TCR/CD3 signaling. (A) PBMCs from a representative healthy donor and patient S5 were activated with anti-CD3. In patients with SzS who had a CD4+CD7-tumor, nontumor (CD4+CD7+), and tumor (CD4+CD7-), T cells were analyzed for induction of CD25 and CD69 membrane expression upon anti-CD3 stimulation. The counterparts in healthy donors were also analyzed. Fluorescence histograms are shown for unstimulated cells (thin lines) and anti-CD3-stimulated cells (thick lines). Dashed lines represent isotype-control staining of unstimulated cells. Similar results were obtained in 2 other patients with SzS. (B) PBMCs from a healthy donor and patient S5 were cultured in medium alone or activated with PMA/ionomycin (PI) for 6 hours. CD40L induction was monitored by flow cytometry. Dot plots for CD7 and CD40L staining are shown. The tumor T cells in the patients with SzS are contained in the CD7- fraction. The percentage of labeled cells for each quadrant is indicated in the upper right quadrant. Similar results were obtained in 3 other patients with SzS.
SzS patient tumor cells are refractory to TCR/CD3 signaling. (A) PBMCs from a representative healthy donor and patient S5 were activated with anti-CD3. In patients with SzS who had a CD4+CD7-tumor, nontumor (CD4+CD7+), and tumor (CD4+CD7-), T cells were analyzed for induction of CD25 and CD69 membrane expression upon anti-CD3 stimulation. The counterparts in healthy donors were also analyzed. Fluorescence histograms are shown for unstimulated cells (thin lines) and anti-CD3-stimulated cells (thick lines). Dashed lines represent isotype-control staining of unstimulated cells. Similar results were obtained in 2 other patients with SzS. (B) PBMCs from a healthy donor and patient S5 were cultured in medium alone or activated with PMA/ionomycin (PI) for 6 hours. CD40L induction was monitored by flow cytometry. Dot plots for CD7 and CD40L staining are shown. The tumor T cells in the patients with SzS are contained in the CD7- fraction. The percentage of labeled cells for each quadrant is indicated in the upper right quadrant. Similar results were obtained in 3 other patients with SzS.
It has been shown that agents that increase intracellular calcium potently induce T-cell CD40L expression.32 Total PBMCs from patients with SzS (n = 4) were stimulated with PMA and ionomycin (PI) and then analyzed for expression of CD40L by flow cytometry. Because PMA is known to down-regulate CD4 from the surface of T cells,33 CD4 gating could not be applied for PMA-stimulated cells. We therefore studied tumor cells present in the CD7- fraction of total PBMCs. Figure 4B shows that the CD7- fraction of SzS PBMCs contained cells expressing CD40L upon PI stimulation. Notably, no CD40-L+ cells could be detected in the CD7- fraction of healthy donors, due here to the dilution by other PBMCs that are CD7- such as B cells and monocytes. In patients with SzS, the significant expansion of tumor CD7- cells allows the detection of CD40L in this population. Taken together, these results indicate that defective CD40L expression on tumor T cells of patients with SzS is most likely due to inappropriate membrane-proximal TCR/CD3 signaling.
We also analyzed CD40L and CD69 expression after CD3 stimulation of T cells from SzS patient number 5 at different times over the course of disease. The absence of CD40L expression on CD4+CD7- tumor cells was stable over the 13-month period studied as well as reduced induction of CD69 (not shown). This indicates that the deficiency in TCR/CD3 signaling and CD40L expression in tumor T cells is stable over time.
Deficient IL-12 production by APCs of patients with SzS can be restored by recombinant CD40L
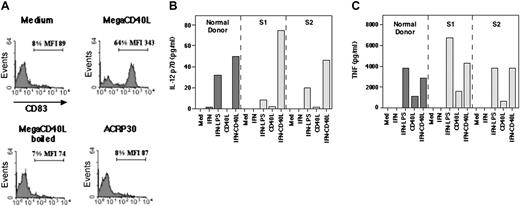
To demonstrate if the reduced ability of PBMCs from patients with SzS to produce IL-12 following incubation with anti-CD3 is due to defective production of CD40L, we have generated a recombinant soluble form of human CD40L and studied its effect on patient samples of PBMCs in vitro. Certain TNF ligands, including CD40L, induce optimal signaling upon binding to their receptor only when they are in membrane-bound or oligomerized form.34 To generate a fully human soluble form of CD40L that retains activity similar to the membrane-bound form, the extracellular portion of human CD40L was fused to a serum protein ACRP30, which results in the synthesis of hexameric CD40L, herein referred to as megaCD40L.34 MegaCD40L was produced in HEK-293 cells, purified, and shown to potently and specifically induce the expression of CD83 (Figure 5A) and HLA-DR (not shown) in DCs derived from CD34+ stem cells in vitro. No effect on the expression of these molecules was observed in the presence of ACRP30 alone or boiled megaCD40L, the latter showing that the observed effect is specific for CD40L and not due to contaminant endotoxins.
Recombinant megaCD40L induces IL-12p70 and TNF production in patients with SzS. (A) Dendritic cells derived from CD34+ stem cells of healthy donors were stimulated with the indicated reagents. MegaCD40L (ACRP30-CD40L) and ACRP30 were used at 100 ng/mL. After 18 hours, CD83 surface expression was monitored by immunostaining and flow cytometry analysis. Fluorescence histograms are shown. (B-C) Total PBMCs from a healthy donor and 2 patients with SzS were stimulated with the indicated reagents. IFN-γ was used at 500 IU/mL, LPS at 1 μg/mL, and megaCD40L at 100 ng/mL. After 24 hours, supernatants were harvested and assayed for IL-12p70 (B) and TNF-α (C) production.
Recombinant megaCD40L induces IL-12p70 and TNF production in patients with SzS. (A) Dendritic cells derived from CD34+ stem cells of healthy donors were stimulated with the indicated reagents. MegaCD40L (ACRP30-CD40L) and ACRP30 were used at 100 ng/mL. After 18 hours, CD83 surface expression was monitored by immunostaining and flow cytometry analysis. Fluorescence histograms are shown. (B-C) Total PBMCs from a healthy donor and 2 patients with SzS were stimulated with the indicated reagents. IFN-γ was used at 500 IU/mL, LPS at 1 μg/mL, and megaCD40L at 100 ng/mL. After 24 hours, supernatants were harvested and assayed for IL-12p70 (B) and TNF-α (C) production.
Using this form of CD40L, we determined the capacity of PBMCs from patients with SzS to produce IL-12 and TNF-α in response to CD40 signaling in vitro. To this end, PBMCs were cultured for 48 hours with vehicle, IFN-γ alone, or IFN-γ and megaCD40L and cytokine secretion measured in the culture supernatants by ELISA. As shown in Figure 5B-C, megaCD40L stimulation of IFN-γ-treated SzS PBMCs resulted in significant production of IL-12 and TNF-α. The mean levels of IL-12 (60 ± 19 pg/mL) and TNF-α (4000 ± 210 pg/mL) observed after IFN-γ and megaCD40L exposure of SzS patient PBMCs were similar to the levels of IL-12 (49 pg/mL) and TNF-α (3850 pg/mL) observed in healthy-donor PBMCs. This shows that the deficiency in IL-12 and TNF-α production observed in patients with SzS can be restored in vitro by exposure of PBMCs to an active form of soluble human CD40L (megaCD40L).
Discussion
Our findings demonstrate that T cells from patients with SzS have an important defect in expressing CD40L after CD3 ligation. This deficiency is restricted to the clonal tumor T-cell population. Furthermore, our data suggest that the reduced ability of PBMCs from patients with SzS to produce IL-12 and TNF-α after exposure to CD3 is due to the inappropriate induction of CD40L expression on tumor CD4+ T cells and the reduced number of normal CD4+ T cells because: (1) a similar defect in cytokine production is reproduced when PBMCs from healthy donors are stimulated by anti-CD3 in the presence of CD40L-blocking antibodies; and (2) production of IL-12 and TNF-α can be virtually completely restored in this setting in the presence of active soluble hexameric CD40L. It is thus likely that the abnormalities in TH1 cytokine and IL-12 production that characterize SzS are in part attributable to deficient CD40L signaling.
Several mechanisms could potentially account for the diminished CD40L expression in patients with SzS. Because certain TH2-derived cytokines such as IL-10 inhibit antigen-dependent IL-12 secretion by DCs, we considered the possibility that they may also affect CD40L expression by CD4+ T cells in SzS.35 However, culture of SzS patient T cells with either anti-IL-10, anti-IL-4, or anti-TGF-β1 neutralizing antibodies failed to restore the ability of CD3 ligation to up-regulate T-cell CD40L expression (data not shown). The role of prostaglandin E2 was not examined in our study. Whether increased production of this factor occurs in advanced CTCL and whether it plays a role in the deficient expression of CD40L deserves additional investigation. A second potential mechanism for the defective expression of CD40L following CD3 ligation is inadequate T-cell activation by CD3 cross-linking in SzS. In support of this, in our experimental system CD3 cross-linking did not induce comparable T-cell activation in SzS and healthy-donor PBMCs, as illustrated by the reduced levels of CD25 and, to a lesser extent, CD69 expression on the tumor T-cell population of patients with SzS. Furthermore, it has previously been reported that triggering of TCR/CD3 in vitro elicits only a weak mitogenic response in the malignant cells from patients with CTCL due to decreased activities of selected protein tyrosine kinases (Zap70, Syk, membrane-associated Csk) that are required for coupling cell surface receptors to intracellular signaling pathways.36,37 Taken together with the fact that CD40L expression could be induced on tumor T cells from patients with SzS upon PMA and ionomycin exposure, the observed defect is most likely due to inappropriate membrane-proximal CD3 signaling.
Cell surface interaction between CD40L on CD4+ T cells and CD40 on APCs (dendritic cells or monocyte/macrophages) is now well recognized as a requirement for optimal T-cell and dendritic cell differentiation. Notably, activated T cells expressing CD40L induce up-regulation of T-cell costimulatory molecules such as CD80/86 and IL-12 secretion upon interaction with CD40 on APCs. IL-12 secretion by APCs leads to the differentiation of naive T cells into TH1 cells. The importance of this interaction in vivo has been demonstrated in several models including a TH1 T cell-dependent murine colitis model (TNBS [2,4,6-trinitrobenzene sulfonic acid]-colitis), in which CD40L blockade by anti-CD40L prevented inflammation and colitis.11 Furthermore, CD40L-CD40 interaction has also been shown to be critical for the maturation of antigen-specific CD8+ T cells into effector cytotoxic T cells.38-40 Recently, evidence has also been provided showing that in vivo activation of CD40 (using agonistic anti-CD40 Abs) can replace T-cell help that is required for priming of effector T cells and can augment antitumor vaccine efficacy by reverting peripheral tolerance.41,42 The role of CD40L-CD40 interaction in TH1 development and cell-mediated immunity thus provides a basis for the finding that patients with SzS present diverse T-cell defects. One of the most profound of these defects is the inability of patients with SzS to produce TH1 cytokines as well as IL-12.
In the present study we have demonstrated that although patients with SzS have the capacity to produce IL-12 and TNF-α when their PBMCs are stimulated with bacterial products such as LPS or SAC in conjunction with IFN-γ or with soluble CD40L, they fail to produce significant levels of these cytokines when their PBMCs are stimulated with anti-CD3. These results imply that peripheral APCs in patients with SzS are able to produce IL-12 and TNF-α as a consequence of direct stimulation but manifest defects when such cytokine production is to be elicited by antigen activation of CD4+ T cells. This is most likely due to the fact that most CD4+ T cells in the peripheral blood of such patients are tumor cells and consequently unable to transmit TCR/CD3 signals and express CD40L. As such, this study not only provides an explanation for some of the immune abnormalities associated with SzS but also raises the possibility that the poor TH1 T-cell responses and antitumor immunity in patients with SzS may be ameliorated by treatment with recombinant CD40L.
Prepublished online as Blood First Edition Paper, August 17, 2004; DOI 10.1182/blood-2004-03-1055.
Supported in part by National Institutes of Health (NIH) grants CA81022 and CA89442 and grants from the Leukemia and Lymphoma Society, Oncosuisse, the Leenaards Foundation, the Swiss National Science Foundation, and the Louis Jeantet Foundation.
Two of the authors (L.E.F., S.C.) have declared a financial interest in a company whose potential product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank K. Schuler-Grosdemange, G. Radlgruber, and S. Everetts for their technical assistance, K. Alevizopoulos for help with cloning megaCD40L, Apoxis for providing pure recombinant megaCD40L, and B. Denardo, P. Bromley, and W. H. Macey for help in collecting clinical samples.