Abstract
Tyrosine kinases phosphorylate proteins on tyrosine residues, producing a biologic signal that influences many aspects of cellular function including cell growth, proliferation, differentiation, and death. Constitutive or unregulated activity through mutation or overexpression of these enzymes is a common pathologic feature in many acute and chronic leukemias. Inhibition of tyrosine kinases represents a strategy to disrupt signaling pathways that promote neoplastic growth and survival in hematologic malignancies and likely in other neoplasias as well. This review focuses on tyrosine kinases that have been implicated in the pathogenesis of hematologic diseases other than chronic myelogenous leukemia and discusses the evidence for the use of small molecules to target these kinases.
Introduction
Tyrosine kinases are enzymes that transfer phosphate from adenosine triphosphate (ATP) to tyrosine residues in specific substrate proteins. There are approximately 100 tyrosine kinases in mammalian cells, which can be divided into 2 large subfamilies, receptor and nonreceptor tyrosine kinases. Receptor tyrosine kinases are highly conserved transmembrane enzymes that respond to ligand binding at the cell surface and transmit messages throughout the cell via phosphorylation of target proteins. Nonreceptor tyrosine kinases may be found in the cytoplasm or nucleus, and like receptor tyrosine kinases, are also often involved in the regulation of differentiation, growth, and cell death.
Abnormal activation of tyrosine kinases or the signaling pathways they control is thought to play a critical role in the neoplastic process of many human malignancies (Table 1). In normal cells, ligand binding tightly regulates kinase activation. However, mutation or aberrant expression may result in constitutive activation of kinase activity, setting off an intracellular cascade of signaling events that may promote unregulated cell growth or other features of transformed cells. Mutations resulting in constitutive autophosphorylation due to conformational change or overexpression due to gene duplication are likely to be pathophysiologically central to the neoplastic clone. Overexpression due to up-regulation at the RNA or protein synthesis level could still be potentially worthy of targeting, but perhaps less so than mutations. Selective inhibition of these activated tyrosine kinases by small-molecule inhibitors represents a rational strategy to disrupt signaling pathways that promote neoplastic growth and survival.
Therapy with imatinib mesylate (formerly STI571) in chronic myelogenous leukemia (CML) exemplifies the paradigm of successful targeted therapy through tyrosine kinase inhibition. Imatinib mesylate binds to the ATP-binding site in the kinase domain of the BCR/ABL tyrosine kinase, thus preventing ATP binding and activation of the kinase. Therapy with imatinib mesylate results in durable and complete cytogenetic response in the early stages of CML.40-42 However, in accelerated phase and blast crisis CML (whether lymphoid or myeloid) as well as Philadelphia chromosome–positive acute lymphoblastic leukemia (ALL), response rates are not as impressive and are short-lived.43-48 This review focuses on the use of tyrosine kinase inhibitors in other hematologic malignancies and highlights the difficulties inherent in this therapeutic strategy.
Mutated tyrosine kinases in chronic myeloproliferative disorders
As noted, the successful targeting of BCR/ABL by imatinib mesylate has intensified the search for tyrosine kinases that play similar pivotal roles in the molecular pathogenesis of other malignancies. Chromosomal translocations that generate fusion genes involving tyrosine kinase receptors have been identified in a small fraction of myeloproliferative disorders (MPDs). These fusion genes represent ideal targets for tyrosine kinase inhibition because of their presumed central role in the neoplastic process of these disorders.
PDGFR-β
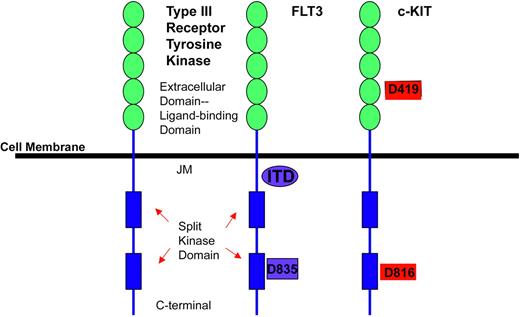
Platelet-derived growth factor receptor β (PDGFR-β) belongs to the type III receptor tyrosine kinase family characterized by a transmembrane domain, a juxtamembrane domain, a split kinase domain containing a kinase insert region and a C-terminal tail (Figure 1).49 Ligand binding to the receptor initiates the signaling cascade by inducing receptor dimerization, leading to activation of the kinase domain, and resulting in autophosphorylation. The autophosphorylated tyrosine residues then act as intracellular docking sites for second messengers involved in mitogenesis, cytoskeletal rearrangements, and chemotaxis.49,50
Schematic depicting structure of receptor tyrosine kinase type III family. Structure of receptor tyrosine kinase type III family, on the left, shows 5 immunoglobulin-like ligand-binding domains (JM indicates the juxtamembrane domain) and two split kinase domains. FLT3 receptor has mutations identified in the JM as well as in the second split kinase domain at codon 835. c-KIT has a mutation in the immunoglobulin extracellular domain at a highly conserved aspartate residue at codon 419 as well as in the split kinase domain at codon 816, which is frequently seen in mast cell leukemia or mastocytosis.
Schematic depicting structure of receptor tyrosine kinase type III family. Structure of receptor tyrosine kinase type III family, on the left, shows 5 immunoglobulin-like ligand-binding domains (JM indicates the juxtamembrane domain) and two split kinase domains. FLT3 receptor has mutations identified in the JM as well as in the second split kinase domain at codon 835. c-KIT has a mutation in the immunoglobulin extracellular domain at a highly conserved aspartate residue at codon 419 as well as in the split kinase domain at codon 816, which is frequently seen in mast cell leukemia or mastocytosis.
PDGFR-β rearrangements are associated with chronic myelomonocytic leukemia (CMML), an MPD characterized by dysplastic monocytosis, bone marrow fibrosis, eosinophilia, and progression to AML. In 1994, Golub et al demonstrated that the chromosomal abnormality t(5;12)(q31-q33;p13) found in rare cases of CMML resulted in a fusion gene linking TEL (ETS variant gene 6 [ETV6]), a transcription factor, with PDGFR-β on chromosome 5.7 Since the initial description, multiple PDGFR-β rearrangements have been identified, which characterize other cases of CMML (Table 2).8-12,51-53 These chromosomal rearrangements result in fusion proteins such that PDGFR-β activity is ligand independent and constitutively activated. The breakpoint of the PDGFR-β contains the transmembrane and intracellular domains of the kinase, but the fusion gene partner replaces its extracellular ligand-binding domain and likely functions to induce ligand-independent dimerization, and ultimately, constitutive activation of the kinase.54 The fusion protein ETV6-PDGFR-β alone causes hematopoietic cell lines to become growth factor independent55 and causes a CMML-like disorder in transgenic mice, implicating it in the molecular pathogenesis of the disorder.56,57
Imatinib mesylate inhibits PDGFR-β suggesting it is a rational therapy for patients with MPDs associated with activated PDGFR-β receptors. In preclinical models, imatinib mesylate inhibited cell lines expressing ETV6-PDGFRB58 as well as the RAB5-PDGFR-β fusion proteins.59 In mouse models of CMML transformed by ETV6-PDGFR-β, imatinib mesylate treatment resulted in statistically significant prolonged survival compared to controls.60
Given these results, imatinib mesylate was studied in patients with PDGFR-β translocations. In an updated report by Apperley et al, 9 patients with chromosomal translocations involving PDGFR-β (5q33) were treated with 200 to 800 mg imatinib mesylate daily.50,61 Five of the 9 patients had the ETV6-PDGFRB fusion gene. All patients had leukocytosis and eosinophilia. At a median follow-up of 14 months, all patients responded rapidly. All but 1 patient had a complete cytogenetic response and 2 patients attained a complete molecular remission as defined by polymerase chain reaction (PCR) negativity for the ETV6-PDGFRB transcript.61 Similarly, a patient with CMML and the RAB5-PDGFRB fusion gene also responded to imatinib mesylate treatment following relapse after stem cell transplantation.59 In contrast, imatinib mesylate has not been effective in patients with CMML without a PDGFR-β gene rearrangement.62
PDGFR-α
In the report by Apperley et al, eosinophilia was a prominent clinical feature in patients with PDGFR-β gene rearrangements. The authors speculated that PDGFR-β activation is linked to the eosinophilia seen in these patients.50 DeAngelo and colleagues treated 16 patients with hypereosinophilic syndrome (HES), a rare disorder characterized by sustained overproduction of eosinophils in the bone marrow, peripheral eosinophilia, and tissue infiltration with resultant organ damage; 11 of these patients had symptomatic disease and were treated with imatinib mesylate 100 to 400 mg/d.25 Nine patients had normal cytogenetics, one patient had a t(1;4)(q44; q12), and one patient had multiple cytogenetic abnormalities. Ten of 11 patients achieved a complete hematologic remission after a median treatment duration of 4 weeks, one of which was transient. This patient was later unresponsive to escalated doses of imatinib mesylate. These observations led Cools and colleagues to closely examine known imatinib mesylate targets for possible involvement in the pathogenesis of HES. One patient in this clinical series was noted to have a t(1;4) chromosomal translocation. This focused attention on the PDGFR-α locus, known to be located at chromosome 4q12. In an elegant example of molecular detective work, Cools et al found that the kinase domain of PFGFR-α was fused to a previously uncharacterized gene also found on chromosome 4, and subsequently named FIP1-like1 (FIP1L1).25 Further analysis of patient samples found that 9 of 16 patients contained the same novel fusion gene, 8 of whom were men. Interestingly, this fusion gene is not the result of a chromosomal translocation, but rather an interstitial deletion.
Sequence analysis of the PDGFR-α kinase domain in the patient with clinical resistance to imatinib mesylate demonstrated that the fusion protein had acquired a substitution of isoleucine for tyrosine at codon 674 (T674I) in the ATP-binding region, an analogous position to the common T315I mutation found in BCR/ABL that confers clinical resistance to imatinib mesylate.63 This verifies that the fusion gene is the target for imatinib mesylate in HES. The inhibitory concentration of 50% (IC50) of imatinib mesylate in BA/F3 cell lines transformed with the novel fusion gene is 3.2 nM; thus FIP1L1-PDGFRA is more sensitive to imatinib mesylate than BCR/ABL, explaining why patients with HES responded to imatinib mesylate at doses of 100 mg/d, well below the established 400 mg/d used in patients with CML.25 Only 60% of the patients who responded to imatinib mesylate in this series had the novel fusion gene, suggesting that the remaining 40% of responders possibly contain other mutated tyrosine kinases yet to be identified.
An interesting molecular overlap has been identified between the rare disorders of HES and systemic mast cell disease (SMCD). The clinical observation that patients with HES who respond to imatinib mesylate tend to have elevated tryptase levels, combined with the evidence that 3 patients with SMCD and a peripheral eosinophilia had complete responses to imatinib mesylate,26 led investigators to search for evidence of the FIP1LI-PDGFRA fusion gene in these patients. Using fluorescent in situ hybridization (FISH), the novel fusion gene was found all 3 responding patients.26 Thus, in this fraction of patients with SMCD, the therapeutic target of imatinib mesylate may not be related to c-KIT as previously thought but rather to FIP1LI-PDGFR-α.
Other mutated kinases in chronic MPDs
Classic translocations resulting in novel fusion genes have been identified in several chronic MPDs and may be additional potential targets for small-molecule tyrosine kinase inhibitors. The 8p myeloproliferative syndrome, a rare MPD with associated eosinophilia that rapidly transforms into acute leukemia, has been linked to translocations involving chromosome 8.39 The classic translocation, t(8;13)(p11;q12), which was first described in this disorder, juxtaposes the fibroblast growth factor receptor (FGFR) to a zinc finger motif,64 generating a fusion protein with constitutive activity.65 In addition, other fusion proteins, such as TEL-ABL, or TEL-JAK2, or BCR-JAK2, are also potential targets for tyrosine kinase inhibition. In one brief report, a patient with an aggressive MPD characterized by a t(9;12) translocation (TEL-ABL) was treated with imatinib mesylate 600 mg daily and had a considerable, although transient, clinical response to this treatment.66 Therefore, patients with disorders characterized by these rare translocations may derive benefit from treatment with small-molecule inhibitors and this is an active area of investigation.
Mutated tyrosine kinases in acute leukemias
FLT3
Recently, the Fms-like tyrosine kinase 3 (FLT3) receptor has been identified as a potential therapeutic target in acute myelogenous leukemia (AML). The FLT3 receptor also belongs to the type III class of receptor tyrosine kinases (Figure 1)49 and was cloned in 1991.67 It plays an important role in normal hematopoiesis as well as leukemogenesis. FLT3 is expressed on stem cell progenitors as well as in 70% to 100% of patients with AML, but it is uncommon in B-cell ALL, T-cell ALL, and CML.68-70 Its endogenous ligand is FLT3 ligand, a growth factor for immature myeloid cells and stem cells.71
Mutations in FLT3 were first reported in 1996 when internal tandem duplications (ITDs), repeats of 5 to 40 or more amino acids in the juxtamembrane region, were discovered in patients with AML.23 Subsequent studies have demonstrated that these FLT3-ITDs are found in approximately 25% of all cases of AML, 3% to 5% of myelodysplastic syndromes (MDSs), and infrequently in ALL.24,72,-77 FLT3 mutations are more common in patients with AML with normal cytogenetics and t(15;17).72-74 The mutation is associated with increased peripheral blood leukocyte counts and a worse prognosis compared to patients without the mutation.73,78,79 In patients with t(15;17), FLT3 mutations are associated with a higher white blood cell count and an increased induction death rate and therefore a lower complete remission rate, but no increase in relapse rate.80 The loss of the wild-type FLT3 allele in conjunction with a FLT3-ITD has been shown to confer an even poorer prognosis.81
Mutations within the FLT3 activation loop of the kinase have also been identified. Approximately 7% of patients with AML have a substitution of aspartic acid at codon 835, most commonly for a tyrosine residue (D835Y),82 but other substitutions have been reported.83 In 2 patients, a 6–base pair (bp) insertion between codons 840 and 841 in the activation loop of FLT3 has been reported.84 Interestingly, although infants with mixed lineage leukemia (MLL) do not have FLT3-ITD mutations, a significant fraction has point mutations in the activation loop. These mutations may be at either codon 835 or 836.85 Infants with MLL gene translocation also tend to express wild-type FLT3 receptors at exceptionally high levels.86
The result of either an FLT3-ITD mutation or an activating loop mutation is constitutive activation of the kinase. When wild-type and mutant FLT3 genes are transfected into interleukin 3 (IL-3)–dependent cell lines, including 32D and BA/F3 cells, the mutant FLT3-transfected cells (FLT3-ITD) become independent of growth factor.87 In contrast, the wild-type FLT3-transfected cells have minimal proliferation despite stimulation with FLT3 ligand.87 Furthermore, the cells expressing mutant FLT3-ITD demonstrate constitutive activation of signal transducer and activator of transcription 5 (STAT5) and mitogen-activated protein (MAP) kinases,87 the signaling pathways of FLT3.88 When FLT3-ITD mutants identical to those identified from primary human leukemia samples were transduced into primary mouse bone marrow cells using a retrovirus, an MPD but not overt leukemia developed.89 This suggests that FLT3-ITD is sufficient to induce a proliferative signal similar to that of BCR/ABL in chronic-phase CML but not sufficient to cause acute leukemia unless paired with other cellular events. The need for a second cooperating mutation has been confirmed in a murine model using promyelocytic leukemia-retinoic acid receptor α (PML-RARA) transgenic mice transduced with an activated FLT3 allele.90 Introduction of the mutated FLT3 allele into the background of the PML-RARA mouse resulted in a more rapid transformation to leukemia, with a range of 62 to 299 days versus 8.5 months in mice without the mutated FLT3 allele.90
These preclinical studies demonstrated that the FLT3-ITD mutation is a potential therapeutic target in AML. The first studies to validate this concept used the compounds herbimycin a, AC1296, and AG1295.91-93 These compounds inhibited FLT3-ITD–transformed cells and prolonged the development of leukemia phenotypes in mice with FLT3-ITD–induced MPDs.
Newer FLT3 inhibitors have been developed that have shown promise in preclinical models and have moved to clinical trials in humans. At least 4 compounds are currently under development (Table 3). All have been found to be active in preclinical in vitro and animal models of FLT3-ITD disease.94,98,103,110,112 CEP-701 (Cephalon, West Chester, PA) is a novel indolocarbazole derivative that inhibits the autophosphorylation of wild-type and constitutively activated FLT3 in vitro with an IC50 of 2 to 3 nM.99 Results of the phase 1/2 trial using single-agent CEP-701 in patients with relapsed, refractory, or poor-risk AML and activating FLT3 mutations have recently been reported.99 The first 3 patients were treated with 40 mg by mouth twice a day, but ex vivo analysis showed incomplete inhibition of FLT3 autophosphorylation and no response was seen. The next 14 patients were treated with 60 mg twice a day, 3 of whom were escalated to 80 mg twice a day. Of these 14 patients, 4 had a decrease in peripheral blood leukemic blasts to less than 5%, with improvement in absolute neutrophil counts, and one patient had a decrease in bone marrow blasts to less than 5%. Grade 3 and 4 toxicities included febrile neutropenia, which occurred in 11 patients. More common less severe grade 1 and 2 side effects included nausea, emesis, and fatigue.
MLN518 (CT53518) is a piperazinyl quinazoline. In a phase 1 trial, 40 patients with AML or myelodysplasia were treated with escalating doses of the compound.104 The dose-limiting toxicity of MNL518 was reversible, generalized weakness, which occurred in 3 of 9 patients treated at doses of 525 mg or more. This toxicity correlated with plasma concentrations more than 2000 nM, well above the level associated with inhibition of FLT3 autophosphorylation. Stabilization of peripheral blood counts, for longer than 5 months was seen in 2 patients with wild-type FLT3. In one patient with FLT3-ITD, the bone marrow blast count decreased from 80% to 15% with reductions of the peripheral blood blast count over the first 288 days of therapy. The phase 2 study of this drug will evaluate response at the maximum tolerated dose of 525 mg twice daily dose in patients with relapsed or refractory AML with confirmed FLT3-ITD. Of note, unlike CEP-701, MLN518 is not active against the D835Y mutation in the activation loop of FLT3.103
PKC412 (Novartis Pharmaceuticals, Basel, Switzerland), an N-benzolystaurosporine, originally developed as a vascular endothelial growth factor receptor (VEGFR) and protein kinase C (PKC) inhibitor, is one of the more developed FLT3 inhibitors. A phase 1 trial of PKC412 in patients with advanced solid malignancies showed it to be a well-tolerated oral therapy. The most frequent treatment-related toxicities were nausea, vomiting, fatigue, and diarrhea.95 A phase 2 trial of PKC412 at 75 mg by mouth 3 times a day was undertaken in patients with AML that expressed either a FLT3-ITD or an activation loop mutation.96,97 Patients had to have relapsed or refractory disease or not be candidates for cytotoxic chemotherapy. Of the first 14 patients treated, 12 had a more than 50% reduction in peripheral blasts compared to baseline including 2 patients who cleared blasts by day 29. Five patients had reduction in bone marrow blasts by more than 50%, of whom one had less than 5% blasts with normal peripheral counts on day 96 of treatment. Furthermore, a decrease in FLT3 autophosphorylation relative to total FLT3 protein occurred in 75% of patients' blast samples obtained 24 hours after the start of PKC412 compared to baseline, indicating the target was inhibited. A phase 2 trial was also undertaken in patients with wild-type FLT3 and no significant responses beyond transient hematologic improvement were seen.113
SU5416, SU5614, and SU11248 (Sugen, San Francisco, CA), which are indolinones, also have FLT3 inhibitor activity, whereas SU6668 has no significant effect on FLT3 (IC50 > 50 μM).106,110 A phase 1 study of SU11248 has been completed in patients with AML.111 Five patients had FLT3 gene mutations (3 ITDs and 2 activating loop mutations). The investigators reported a decrease in peripheral blast counts in some patients following a single dose of SU11248; however, data on response rates are not yet available.
The initial results of these early FLT3 inhibitor studies indicate that these compounds have biologic activity against AML. However, few patients have achieved a complete or durable remission with single-agent therapy. Diverse factors may contribute to the rather modest activity of FLT3 inhibitors in AML. Incomplete kinase inhibition has been seen in some cases. However, primary resistance was also observed in the presence of complete kinase inhibition, perhaps because FLT3 mutations are a late event in the pathogenesis of AML, and thus may not be essential to leukemogenesis per se.99 This is in contrast to BCR-ABL, which is thought to initiate CML. In addition, the patients studied were heavily pretreated. In this setting, a low response rate is not unexpected, in analogy to myeloid blast crisis of CML, where patients pretreated for blastic transformation responded less well to imatinib mesylate than patients without prior therapy.45 Nonetheless, similar to imatinib mesylate, these agents are well tolerated with few side effects and their toxicity profiles are well suited to combination with cytotoxic chemotherapy. To have a significant clinical impact, it is apparent that FLT3 inhibitors will need to be combined with chemotherapy or even other targeted therapy, much in the way that all-trans-retinoic acid (ATRA) is for acute PML.
c-KIT
c-KIT is another receptor tyrosine kinase in the type III subfamily (Figure 1). It is expressed on hematopoietic progenitor cells, mast cells, germ cells, and the pacemaker cells of the gut.114 c-KIT is expressed in a variety of human malignancies and is mutated and constitutively activated in gastrointestinal stromal cell tumor (GIST), mastocytosis/mast cell leukemia, and AML. Activating mutations can occur in many different exons of the c-KIT gene and activation of signaling pathways leads to proliferation, differentiation, migration, and survival of hematopoietic stem cells, mast cells, melanocytes, and germ cells.28,115
Valine substituted for aspartic acid at codon 816 (D816V mutation) in the activation loop of the kinase catalytic domain is the most common activating mutation in c-KIT (Figure 1). It is predominantly found in systemic mastocytosis or mast cell leukemia but has also been detected in patients with MPDs and some cases of AML.49,114 This mutation results in a 10-fold increase in the specific activity and a 9-fold increase in ATP affinity.114 Although imatinib mesylate inhibits wild type c-KIT, the D816V mutation is resistant to imatinib mesylate.116 This is likely related to the increased affinity of the mutated c-KIT for ATP or to conformational changes in the activation loop of the receptor that inhibits binding of imatinib mesylate.
Recently, investigators demonstrated that PKC412 inhibits BA/F3 cell lines stably transformed by the c-KIT mutations, D816Y and D816V, with an IC50 of 20 and 30 nM, respectively.117 These results were extended clinically when a 48-year-old woman with mast cell leukemia and a D816V mutation underwent treatment with PKC412. Treatment with PKC412, 100 mg twice daily, resulted in clinical improvement, with a reduction of mast cells and a decrease in myeloblasts from 5% to 10% in the marrow at diagnosis to less than 5% after 2 months of therapy. Further follow-up and larger studies are warranted to determine the true efficacy of PKC412 or other tyrosine kinase inhibitors in diseases characterized by D816V c-KIT mutations.
Two isoforms of mutated c-KIT have been identified in AML, both associated with the cytogenetic abnormalities of t(8;21) or inv(16).28,29 One involves aspartate 816 previously mentioned; the other involves a highly conserved aspartic acid residue at position 419, located in the extracellular domain (Figure 1).28 The D816 mutation is identical to that in mastocytosis and thus is not amenable to targeted therapy with imatinib mesylate. It is unclear if the other mutation is amenable to targeting with imatinib mesylate, as it has not been reported to be an activating mutation.28 Targeting of c-KIT mutations in AML is further inhibited by the fact that less than 8% of AML patients have c-KIT mutations.28
Overexpression of tyrosine kinase as target
c-KIT
Although c-KIT is rarely mutated, it is expressed in approximately 60% to 80% of cases of AML and “overexpressed” in a fraction of those cases.118-120 The addition of stem cell factor (SCF), the endogenous ligand for c-KIT, results in the proliferation of KIT+ AML blast cell lines such as MO7E.121 Because a significant percentage of patients with AML overexpress wild-type c-KIT, and imatinib mesylate has been shown to be selective inhibitor of c-KIT, the use of imatinib mesylate in c-KIT+ AML has been proposed. The rationale for treatment is based on the hypothesis that high levels of receptor in vivo might confer a growth advantage in the marrow because of the presence of abundant KIT ligand present in stromal cells. In a phase 2 pilot study, 21 patients with relapsed or refractory c-KIT+ AML were treated with imatinib mesylate 600 mg/d.122 Five responses were seen; 2 patients had a complete hematologic remission, 2 had partial responses (PRs), and another patient had no evidence of leukemia. Western blotting of primary blasts taken from patients confirmed that c-KIT was activated, but mutational analysis did not identify any previously identified c-KIT mutations. Furthermore, activity of imatinib mesylate did not correlate with decreased c-KIT phosphorylation. Therefore, it is difficult to determine the cause for the modest activity of imatinib mesylate in this disease.
As noted, the small molecules SU5416 and SU6668 inhibit c-KIT, as well as VEGFR-2, FGFR, FLT3, and PDGFR.107 In preclinical models using M07E cells, a human myeloid leukemia cell line, SU5416 and SU6668 inhibited tyrosine autophosphorylation of c-KIT in a dose-dependent manner. In addition, when used on leukemic blasts from c-KIT+ patients, both compounds inhibited SCF-induced phosphorylation of c-KIT and induced apoptosis.107 In Europe, SU5416 has been tested in patients with refractory c-KIT+ AML.108,123 In this trial 43 patients with AML whose leukemic blasts overexpressed c-KIT were treated with twice-weekly infusions of 145 mg/m2 SU5416. Of 25 evaluable patients, only one patient had a complete response, defined as less than 5% blasts in the bone marrow, absence of circulating blasts in the peripheral blood, but without normalization of blood counts, and 7 patients had a PR (defined as reduction of blasts in blood or bone marrow by at least 50%), which lasted 1 to 5 months. None of the patients who responded had FLT3-ITDs but the presence of FLT3 activating loop mutations was not evaluated and targeting of c-KIT was not demonstrated in ancillary studies.
VEGF
Similar to c-KIT in AML, the VEGFRs are tyrosine kinases that are overexpressed in hematologic malignancies. Their ligand, VEGF, is responsible for many endothelial cell functions, which are regulated through the receptors: VEGFR-1 (FLT1), VEGFR-2, and VEGFR-3 (FLT4).124 VEGF has been implicated in tumor angiogenesis and may play a role in the pathophysiology of hematologic malignancies by regulation of bone marrow angiogenesis through autocrine and paracrine mechanisms. Murine models using homozygous gene knockouts suggest differential roles for VEGFR-1 and VEGFR-2; VEGFR-2 expression is important in angiogenesis and vasculogenesis, whereas VEGFR-1 may be more important for endothelial organization and remodeling.125,126
Microvessel density is an indirect way of measuring angiogenesis and is elevated in the bone marrow of patients with MDS and AML and decreases following chemotherapy in responsive disease.127,128 Immunohistochemical analysis of bone marrow biopsies reveal that changes in microvessel density parallels changes in VEGF and VEGFR-2 expression in patients with AML implicating VEGF signaling in angiogenesis.129 Furthermore, cellular VEGF levels appear to correlate inversely to survival in patients with AML.130 As a result, a number of receptor tyrosine kinase inhibitors are being used in hematologic malignancies to block angiogenesis through VEGF inhibition. These include SU5416, SU11248, PKC412, PTK787, and others.131,132
In the large European study using SU5416 in patients with c-KIT+ AML, correlative studies were performed to assay VEGF inhibition.108 The investigators evaluated VEGF mRNA expression, measured by PCR and found that patients who responded to SU5416 expressed high levels of VEGF mRNA prior to treatment. In contrast, the nonresponders did not have high VEGF expression prior to treatment. Decreased microvessel density in the bone marrow of responders correlated with VEGF expression. Although these results are intriguing, it remains unclear whether the clinical activity seen with this compound is due to VEGF or c-KIT or FLT3 inhibition, or if these results are an epiphenomenon.
Furthermore, large studies have not been able to demonstrate a clear relationship between changes in microvessel density and clinical response133 and the best assay for measuring changes in angiogenesis remains unclear. Recently, circulating endothelial cells (CECs) have been shown to be a promising, noninvasive surrogate marker for angiogenesis in preclinical studies using murine models of cancer, as well as in several clinical studies of angiogenesis inhibitors.134-136
Problems in the development of tyrosine kinase inhibitors as therapeutic agents
The most important issue pertaining to tyrosine kinase inhibition is whether a putative target is relevant in the pathogenesis of the disease in question. Mutation-independent overexpression, as seen with VEGF and c-KIT, may well represent an epiphenomenon and may not be important in the neoplastic pathophysiology. As a result, inhibition of the overexpressed target might have little clinical benefit. Furthermore, a mutation may be a later mutational event in the pathophysiology of the disease and inhibition of this “secondary” target may only inhibit the proliferative thrust, but not delete the leukemic clone. Ancillary studies from the clinical trial of CEP-701 demonstrated that despite more than 85% FLT3 inhibition in 2 patients there was no clinical impact on disease.99 Other studies suggest that FLT3 mutational status changes from diagnosis to relapse and different FLT3 mutation clones may be dominant from one stage of disease to another.137,138 If an FLT3-ITD is a later event, then there will be FLT3–, or wild-type leukemic clones, and thus FLT3 inhibition may preferentially select for these clones causing resistance. Key to further development of these inhibitors will be the identification and verification of their therapeutic targets given their broad specificity.
Resistance remains one of the greatest challenges facing the development of tyrosine kinase inhibitors. Imatinib mesylate resistance in patients with CML serves as a cautionary note for other drugs under development. The mechanism of action of these small-molecule inhibitors is thought to be similar; that is, blocking of ATP binding to the kinase domain.139,140 Thus, mechanisms of imatinib mesylate resistance in CML may be analogous to resistance to other small-molecule inhibitors. In patients with CML, point mutations in BCR/ABL have been identified at the time of resistance that are not always detectable in samples taken prior to the initiation of therapy.63,141-144 These point mutations predominantly occur in the kinase domain, and more than a dozen different mutations have been reported.141-143,145 This same mechanism of resistance has been demonstrated in HES as well as with FLT3 inhibitor resistance, in vitro.146 Data are emerging from the FLT3 inhibitor clinical trials, which will help to determine clearly the mechanisms of resistance to these small molecules.
Strategies to overcome resistance may include the combination of small molecules with chemotherapy or even other signal transduction inhibitors. For example, the development of FLT3 inhibitors in AML will probably require a combination of small molecules with chemotherapy for full activity. Preclinical studies demonstrate that the schedule of administration and drug combinations are important factors for efficacy.100 Giving a tyrosine kinase inhibitor prior to chemotherapy could prevent DNA synthesis, thereby making the leukemic blasts relatively resistant to cell cycle–active chemotherapy agents. In addition, inhibitors with different binding specificities could be combined. In vitro studies have demonstrated that different FLT3 mutations have varying sensitivities to the different FLT3 inhibitors.147 This suggests that molecular analysis of relapse samples may be useful in determining which inhibitor to use or how best to combine inhibitors.
Conclusion
Tyrosine kinase inhibition as a therapeutic strategy has been proven successful by the treatment of chronic phase CML with imatinib mesylate. This rationale has been translated successfully to other chronic hematologic malignancies such as CMML and HES that may arise from a mutation of a single tyrosine kinase. This strategy is more complicated when extended to advanced diseases such as AML. In some cases, tyrosine kinases are not mutated but overexpressed resulting in increased autocrine signaling through these receptors. Targeting this mechanism of signal transduction activation has not resulted in overwhelming benefit or response, as exemplified by c-KIT, wild-type FLT3, and VEGF inhibition. Furthermore, if an FLT3 inhibitor works it will be important to determine if efficacy is only due to inhibition of activating FLT3 mutations or due to inhibition of other targets. Ancillary studies may help to determine which targets are inhibited, but there is no substitute for clinical trials in this regard. Furthermore, given the low frequency of complete remissions with single-agent therapy, FLT-3 inhibitors will need to be combined with cytoreductive chemotherapy. As the use of tyrosine kinases in chronic and acute leukemias has expanded, new targets such as the FIP1L1-PDGFR-α rearrangement in HES and SMCD have been discovered. The search is on to identify other novel targets. Molecularly targeted therapy remains a promising area of development that will continue to expand, as we understand more about the molecular pathogenesis of hematologic malignancies.
Prepublished online as Blood First Edition Paper, September 9, 2004; DOI 10.1182/blood-2003-11-3896.