Abstract
During embryonic development, T-lymphoid precursor cells colonize the thymus. Chemoattraction by the fetal thymus is thought to mediate T-precursor cell colonization. However, the molecules that attract T-precursor cells to the thymus remain unclear. By devising time-lapse visualization in culture, the present results show that alymphoid fetal thymus lobes attract T-precursor cells from fetal liver or fetal blood. CD4–CD8–CD25–CD44+ fetal thymocytes retained the activity to specifically re-enter the thymus. The attraction was predominantly due to I-A–expressing thymic epithelial cells and was mediated by pertussis toxin-sensitive G-protein signals. Among the chemokines produced by the fetal thymus, CCL21, CCL25, and CXCL12 could attract CD4–CD8–CD25–CD44+ fetal thymocytes. However, fetal thymus colonization was markedly diminished by neutralizing antibodies specific for CCL21 and CCL25, but not affected by anti-CXCL12 antibody. Fetal thymus colonization was partially defective in CCL21-deficient plt/plt mice and was further diminished by anti-CCL25 antibody. These results indicate that CCL21 is involved in the recruitment of T-cell precursors to the fetal thymus and suggest that the combination of CCL21 and CCL25 plays a major role in fetal thymus colonization.
Introduction
Most T lymphocytes develop in the thymus. Intrathymic T lymphopoiesis is initiated on the colonization of hematopoietic stem cell-derived T-lymphoid precursor cells to the thymus primordium, which occurs in humans at 7 to 8 weeks of gestation1 and in mice at embryonic day 11.5 (E11.5).2 However, the molecular mechanism that controls the seeding of T-precursor cells to the fetal thymus remains elusive. Experiments in a transfilter migration assay have suggested that the fetal thymic rudiment secretes diffusible chemotactic factors that attract T-precursor cells,3 and that this chemoattraction by the fetal thymus is mediated by pertussis toxin (PTX)–sensitive G protein-coupled receptor signals.4 More recent studies have shown that the fetal thymus as early as E12.5 produces transcripts for chemokines including CXCL12 (SDF1), CCL25 (TECK), and CCL21 (SLC).5 However, mice deficient in either CXCL12 or its specific receptor CXCR4 normally accumulate T-precursor cells to their thymic primordium at E11.5 and E12.5, excluding CXCL12 as a chemokine required for the migration of T-precursor cells to the fetal thymus primordium.6 In contrast, mice deficient in the CCL25 receptor, CCR9, exhibit a 3-fold decrease in total thymocyte numbers from E14.5 to E17.5 when compared to wild-type animals.7 However, it is unclear whether CCR9 deficiency impairs colonization of T-precursor cells to the thymus primordium, or the absence of CCR9 causes defects in intrathymic survival or proliferation of immature thymocytes or both. Indeed, it was reported that anti-CCL25 antibody did not prevent thymus colonization.4 The roles of other chemokines, including CCL21, in fetal thymus colonization have not been reported.
The lack of a suitable experimental system has hampered the direct analysis of thymic colonization. Histologic analysis of E11.5 fetal thymus tissues is widely used for examining in vivo thymus colonization,5,6,8 but cannot reveal in sufficient detail the time kinetics and the molecular mechanisms controlling the thymus colonization. Transfilter assays analyzing the migration of T-precursor cells to fetal thymic lobes provide only an indirect measure as to how many precursors successfully colonized the thymus because these lobes are subsequently cultured before further analysis.3,4 In addition to the efficiency of immigration, the number of recovered thymocytes in these experiments is affected by the extent of survival and proliferation of immigrated thymocytes. Thus, T-precursor cell attraction would be better examined by direct real-time visualization of cell migration to the thymus, instead of a static analysis of fixed tissues or indirect evidence from transfilter migration assays.
We report herein a novel method for directly visualizing the recruitment of T-precursor cells to the fetal thymus. Using this in vitro time-lapse visualization technique, we show that alymphoid fetal thymus lobes attract T-precursor cells from fetal liver (FL) or fetal blood (FB), and that the thymus attraction is mediated by PTX-sensitive G protein signals in T-precursor cells. Quantitative polymerase chain reaction (PCR) analysis reveals that several chemokines including CCL21, CCL25, CXCL11, CXCL12, and CX3CL1 are expressed at higher levels in fetal thymus than in nonthymic epithelial/mesenchymal rudiments. We find that among these chemokines, CCL21 is involved in the recruitment of T-cell precursors to the fetal thymus, and CCL21 and CCL25 are essential for the optimal fetal thymus colonization.
Materials and methods
Mice
Reagents
Mouse CCL5, CXCL1, and CXCL10 were obtained from Peprotech (Rocky Hill, NJ). Other chemokines and antichemokine antibodies were obtained from R&D Systems (Minneapolis, MN). Biotinylated anti-EpCAM antibody (G8.8) was a kind gift from Dr Richard Boyd (Monash University, Melbourne, Australia). Anti–pan-cytokeratin antibody was obtained from Dako Cytomation (Carpinteria, CA). All antibodies were pretitrated and used in saturated amounts. PTX was obtained from Sigma (St Louis, MO).
Time-lapse visualization of thymus attraction
Details of cell culture, visualization, and reaggregate preparation are described in the supplemental materials available on the Blood website (see the Supplemental Materials link at the top of the online article) and in previous reports.11-14
CCL21-expressing NIH-3T3 cells
Full-length mouse CCL21 cDNA amplified by reverse transcription-PCR (RT-PCR) from total RNA of adult B6 thymus with primers (5′-ATGGCTCAGATGATGACTCTGAGC-3′ and 5′-TTTTTGAGGCTGGATCACCTTTTTATTC-3′) was cloned with Kozak sequence CCACC into pMRX-IRES-GFP retrovirus vector.15 Retroviruses were packaged in Plat-E cells16 and infected into NIH-3T3 cells by spin infection.13 Green fluorescent protein (GFP)–positive cells were purified by a cell sorter.
Flow cytometry and cell sorting
Immunofluorescence staining, flow cytometric analysis, and cell sorting were performed as described.11 Sorted cells with more than 95% purity were used.
RT-PCR and quantitative analysis
Total cellular RNA-derived cDNA was PCR amplified for 35 cycles, electrophoresed, and visualized with ethidium bromide. For quantitative analysis, real-time RT-PCR was performed by using iQ SYBR Green Supermix and iCycler iQ System (Bio-Rad, Richmond, CA). Amplified signals were confirmed to be single bands by gel electrophoresis and were normalized to the levels of the housekeeping gene, HPRT. Primers are listed in Table S1 (available on the Blood website).
Transwell chemotaxis assay
Cells (2 × 105) were placed in a Corning (Corning, NY) transwell chamber (6.5-mm diameter, 5-μm pore) that was inserted into culture wells containing chemokines. Cells were incubated for 6 hours, counted, and analyzed by flow cytometry.
Immunohistologic analysis
Freshly sliced sections were fixed with acetone, incubated with primary antibodies, and visualized with Alexa 488-conjugated anti–rabbit IgG and Alexa 633-conjugated anti–rat IgG (Molecular Probes, Eugene, OR). Biotin signals were visualized with Alexa 488-streptavidin (Molecular Probes). Confocal images were acquired by TCS SP2 laser scanning microscopy equipped with Ar and He-Ne lasers (Leica, Mannheim, Germany). Immunohistochemical staining was visualized using 3-amino-9-ethylcarbazole (Sigma) as the peroxidase substrate and counterstained with Mayer Hemalum (Merck, Darmstadt, Germany).
Results
Time-lapse visualization of fetal thymus colonization in culture
To examine cellular and molecular mechanisms of fetal thymus colonization by T-cell precursors, we devised a novel time-lapse visualization assay for thymic immigration, in which cell movement was time-lapse recorded using a digital CCD camera (Figure 1A). In this assay system, a deoxyguanosine (dGuo)–treated (ie, alymphoid) fetal thymus lobe was positioned approximately 0.5 mm away from a 10-μL drop of collagen gel containing T-precursor cells. The cell drop and the thymus lobe were both submerged in a culture medium containing collagen gel to minimize liquid swirling during culture. The culture was placed under an inverted microscope, maintained at 37°C in 70% O2 and 5% CO2, and time-lapse monitored for 20 to 24 hours. As shown in Figure 1B-C and in Movie S1 (available on the Blood website), a significant number of FL cells moved toward the thymus lobe. A fraction of the moving cells divided during the movement (arrows in Movie S1). The cell movement detected in this culture system was horizontal, reflecting active cellular movement and not passive movement due to gravity. The cell movement was specifically directed toward the thymus, reflecting directional attraction of the cells to the thymus rather than the enhancement of randomly directed cellular motility. Parallel cultures in the absence of the thymus lobe showed that few FL cells moved out of the originally seeded cell spot (Figure 1B-C). Thus, the cellular movement detected in this novel culture system reflects active attraction of a fraction of FL cells to the alymphoid fetal thymus lobes.
Time-lapse visualization of fetal thymus recruitment of T-precursor cells. (A) Diagram of time-lapse visualization assay in culture. (B) Representative images at indicated time points of the edge of a drop of 1 × 105 fetal liver (FL) cells (shown at the left side of the panels) in the absence (top panels) or presence of dGuo-treated fetal thymus lobe (shown at the right side of the bottom panels). Height and width of each picture at original magnification is 0.96 mm and 1.28 mm, respectively. (C) Means and SEs of the numbers of cells that moved out of the cell spot (□) and that reached the fetal thymus lobe (▨) were determined in microscopically visualized field. Numbers of independent experiments are indicated. (D) dGuo-treated fetal thymus lobes seeded with FL cells as in panels B and C were further cultured for 18 days under conventional organ culture conditions. Cells were 3-colored stained for CD3, CD4, and CD8. Shown are representative results (n = 5) of CD3 profiles (solid lines) and control staining profiles (dotted lines) of the cells within indicated CD4/CD8 subpopulations (as shown in dot plots). (E) Indicated FL cell fractions (1 × 105) and total fetal blood (FB) cells (5 × 105) from E14.5 embryos were examined for fetal thymus recruitment by time-lapse visualization assay. Bars are shown as in panel C. (F) Indicated thymocyte subpopulations (1 × 105) were examined for fetal thymus recruitment. Bars are shown as in panel C. Asterisks denote significantly smaller numbers than the cell numbers attracted from DN1 E14.5 fetal thymocytes (*P < .05; **P < .01; ***P < .001). Significance was evaluated individually for shaded bars and open bars. (G) dGuo-treated fetal thymus lobes or fetal salivary gland lobes were examined for the attraction of E14.5 fetal thymocytes. Bars are shown as in panel C. Thymus lobes or salivary gland lobes were cultured further for 9 days under conventional organ culture conditions. Cells were analyzed by flow cytometry for forward scatter (FSC) and side scatter (SSC) and for CD3, CD4, and CD8, as in panel D. Shown are representative results of 4 independent experiments. Numbers in quadrants indicate the frequency of cells within that box.
Time-lapse visualization of fetal thymus recruitment of T-precursor cells. (A) Diagram of time-lapse visualization assay in culture. (B) Representative images at indicated time points of the edge of a drop of 1 × 105 fetal liver (FL) cells (shown at the left side of the panels) in the absence (top panels) or presence of dGuo-treated fetal thymus lobe (shown at the right side of the bottom panels). Height and width of each picture at original magnification is 0.96 mm and 1.28 mm, respectively. (C) Means and SEs of the numbers of cells that moved out of the cell spot (□) and that reached the fetal thymus lobe (▨) were determined in microscopically visualized field. Numbers of independent experiments are indicated. (D) dGuo-treated fetal thymus lobes seeded with FL cells as in panels B and C were further cultured for 18 days under conventional organ culture conditions. Cells were 3-colored stained for CD3, CD4, and CD8. Shown are representative results (n = 5) of CD3 profiles (solid lines) and control staining profiles (dotted lines) of the cells within indicated CD4/CD8 subpopulations (as shown in dot plots). (E) Indicated FL cell fractions (1 × 105) and total fetal blood (FB) cells (5 × 105) from E14.5 embryos were examined for fetal thymus recruitment by time-lapse visualization assay. Bars are shown as in panel C. (F) Indicated thymocyte subpopulations (1 × 105) were examined for fetal thymus recruitment. Bars are shown as in panel C. Asterisks denote significantly smaller numbers than the cell numbers attracted from DN1 E14.5 fetal thymocytes (*P < .05; **P < .01; ***P < .001). Significance was evaluated individually for shaded bars and open bars. (G) dGuo-treated fetal thymus lobes or fetal salivary gland lobes were examined for the attraction of E14.5 fetal thymocytes. Bars are shown as in panel C. Thymus lobes or salivary gland lobes were cultured further for 9 days under conventional organ culture conditions. Cells were analyzed by flow cytometry for forward scatter (FSC) and side scatter (SSC) and for CD3, CD4, and CD8, as in panel D. Shown are representative results of 4 independent experiments. Numbers in quadrants indicate the frequency of cells within that box.
To examine the developmental potential of FL cells that successfully entered the dGuo-treated fetal thymus, the thymus lobes were recovered from the coculture and further cultured for additional 18 days under conventional fetal thymus organ culture conditions. It was found that CD4+CD8+, CD4+CD8–, and CD4–CD8+ thymocytes were generated in the dGuo-treated fetal thymus lobes that were horizontally seeded with FL cells (Figure 1D). The CD4+CD8– and CD4–CD8+ populations included phenotypically mature T cells expressing CD3 at high levels (Figure 1D). In contrast, neither immature nor mature thymocytes could be recovered from cultures of dGuo-treated fetal thymus lobes that had not been seeded with FL cells (data not shown). These results indicate that FL cells that are attracted to the thymus lobes contain T-precursor cells that are capable of developing into mature T cells.
FL, FB, and fetal thymocytes contain cells that are attracted to fetal thymi
T-lymphoid precursor cells produced in the FL gain access to the thymus primordium by way of blood circulation.17,18 The presence of T-precursor cells that were capable of seeding the fetal thymus was therefore determined in FL and FB, using time-lapse visualization assay. Among E14.5 FL cells, cells that were attracted to the fetal thymus lobes were exclusively contained in the TER119–CD45+ nonerythroid population (Figure 1E), which contains leukocyte progenitor cells including T-precursor cells.12,19,20 E14.5 FB also contained cells that were attracted to the fetal thymus lobes (Figure 1E). The relative frequency of cells that were attracted to the thymus lobe was smaller in FB than in FL (Figure 1E), in agreement with previously reported frequency of T-precursor cells in FB and FL at E14.5.18 Thus, the potential to migrate to the fetal thymus was detected in FL and FB cells.
Interestingly, E14.5 fetal thymocytes contained cells that retained the activity to move toward the fetal thymus (Figure 1F; Movie S2). Cell numbers that reached the thymus lobe were more than 3-fold and significantly (P < .05) higher in fetal thymocytes than in FL cells (Figure 1E-F), suggesting that fetal thymocytes are enriched for T-precursor cells that still are capable of re-entering the thymus. Similar to the moving FL cells, a fraction of moving thymocytes was found to divide during the movement (arrows in Movie S2). E14.5 fetal thymocytes were significantly more attracted to the dGuo-treated fetal thymus lobes than to dGuo-treated nonthymic fetal rudiments such as salivary gland lobes (Figure 1G; Movies S2-S3), and fetal thymocytes that were attracted to the fetal thymus differentiated into mature T cells in the fetal thymus environment (Figure 1G). These results indicate that fetal thymocytes contain T-precursor cells that are capable of specifically re-entering the thymus.
The E14.5 fetal thymocytes mainly consist of the most immature CD4–CD8–CD25–CD44+ “double negative 1” (DN1) thymocytes and their progeny CD4–CD8–CD25+CD44+ DN2 thymocytes. Between these 2 subpopulations, DN1 rather than DN2 thymocytes were responsible for the thymus re-entry activity (Figure 1F). Further developed CD4–CD8–CD25+CD44–DN3 thymocytes, which are generated at E15.5, and CD4+CD8+ (double-positive [DP]) thymocytes, which appear by E17.5 and dominate in adult thymus, exhibited significantly lower thymus re-entry activity than DN1 thymocytes (Figure 1F). These results indicate that in addition to FL cells and FB cells, DN1 fetal thymocytes are enriched for T-precursor cells that specifically move to the fetal thymus
I-A+ thymic epithelial cells are primarily responsible for attraction of T-precursor cells
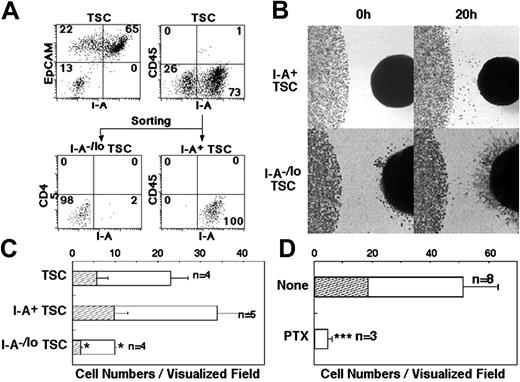
The thymus microenvironment is made up of different stromal cells, which exert separate functions.21 However, the nature of the stromal cells that attract T-precursor cells to the thymic microenvironment remains to be defined. For this purpose, dGuo-treated fetal thymic stromal cells (TSCs) were fractionated by flow cytometry and examined for E14.5 fetal thymocyte-attracting activity. I-A+CD45– TSCs, which were mostly EpCAM+ epithelial cells, and other I-A–/low TSCs were isolated (Figure 2A) and reaggregated to form a thymus lobe–like cell mass (Figure 2B). It was found that I-A+ TSCs were significantly more efficient in attracting fetal thymocytes than I-A–/low TSCs (Figures 2B-C; Movies S4-S5). Thus, among the TSCs, the I-A–expressing thymic epithelial cells (TECs) are primarily responsible for the attraction of T-precursor cells.
Cellular and molecular characterization of fetal thymus recruitment. (A) TSCs dissociated from dGuo-treated E14.5 thymus lobes were stained for I-A, CD45, and EpCAM (top panels). Numbers indicate the frequency of cells within each quadrant. TSCs were sorted into CD45–I-A+ cells and CD45–I-A–/low cells (bottom panels). (B) Representative images at indicated time points of the edge of a drop of fetal thymocytes (1 × 105) in the presence of reaggregate of CD45–I-A+ TSCs (top panels) and CD45–I-A–/low TSCs (bottom panels). Note that many fibroblast-like spikes spread out of the reaggregate of CD45–I-A–/low TSCs during culture. Original magnification is as indicated in Figure 1B. (C) Means and SEs of the numbers of E14.5 fetal thymocytes that moved out of the cell spot (□) and that reached the reaggregate of indicated cells (▨) were determined in visualized field. Numbers of independent experiments are also indicated. CD45–I-A–/low TSCs attracted a significantly smaller number of cells than CD45–I-A+ TSCs (P < .05). (D) E14.5 fetal thymocytes were treated with phosphate-buffered saline (None) or 100 ng/mL PTX at 37°C for 2 hours. Means and SEs of the numbers of cells that moved out of the cell spot (□) and that reached the dGuo-treated fetal thymus (▨) were determined in visualized field. Numbers of independent experiments are also indicated. PTX significantly inhibited the numbers of cells that were attracted to the fetal thymus (P < .001).
Cellular and molecular characterization of fetal thymus recruitment. (A) TSCs dissociated from dGuo-treated E14.5 thymus lobes were stained for I-A, CD45, and EpCAM (top panels). Numbers indicate the frequency of cells within each quadrant. TSCs were sorted into CD45–I-A+ cells and CD45–I-A–/low cells (bottom panels). (B) Representative images at indicated time points of the edge of a drop of fetal thymocytes (1 × 105) in the presence of reaggregate of CD45–I-A+ TSCs (top panels) and CD45–I-A–/low TSCs (bottom panels). Note that many fibroblast-like spikes spread out of the reaggregate of CD45–I-A–/low TSCs during culture. Original magnification is as indicated in Figure 1B. (C) Means and SEs of the numbers of E14.5 fetal thymocytes that moved out of the cell spot (□) and that reached the reaggregate of indicated cells (▨) were determined in visualized field. Numbers of independent experiments are also indicated. CD45–I-A–/low TSCs attracted a significantly smaller number of cells than CD45–I-A+ TSCs (P < .05). (D) E14.5 fetal thymocytes were treated with phosphate-buffered saline (None) or 100 ng/mL PTX at 37°C for 2 hours. Means and SEs of the numbers of cells that moved out of the cell spot (□) and that reached the dGuo-treated fetal thymus (▨) were determined in visualized field. Numbers of independent experiments are also indicated. PTX significantly inhibited the numbers of cells that were attracted to the fetal thymus (P < .001).
Fetal thymus attraction of T-precursor cells is inhibited by PTX
To examine the molecular basis of the attraction of T-precursor cells by fetal thymus lobes, fetal thymus colonization was examined in the presence of PTX, an inhibitor of Gi protein-mediated signals.22 As indicated in Figure 2D, E14.5 fetal thymocytes pretreated with 100 ng/mL PTX showed markedly reduced movement toward the fetal thymus lobes. None of the PTX-treated cells contacted the thymus lobes. These results indicate that the thymic attraction of T-precursor cells is mediated by chemoattracting factors via the interaction with G protein-coupled receptors.
Chemokine mRNAs expressed by fetal TSCs
To explore the possible roles of chemokines in the recruitment of T-precursor cells to the fetal thymus, we next examined the mRNA levels of chemokines expressed in I-A–expressing TECs (Figure 3A). All of the 36 chemokines so far identified in the mouse genome were examined by RT-PCR. Chemokine mRNA expression levels were examined in E14.5 embryos using isolated I-A+ TECs, whole dGuo-treated fetal thymus lobes, and dGuo-treated salivary gland lobes. The mRNAs of 21 chemokine species were detected in either I-A+ TECs or whole fetal thymus lobes (Figure 3A arrows). Quantitative RT-PCR for the mRNA levels of these 21 chemokine species showed that the expression of 10 chemokines (CCL5, CCL19, CCL21, CCL25, CXCL10, CXCL11, CXCL12, CXCL14, CXCL16, and CX3CL1) was significantly higher in I-A+ TECs and whole fetal thymus lobes than in salivary gland lobes (Figure 3B). Among these chemokines, 7 (CCL5, CCL19, CCL21, CCL25, CXCL10, CXCL11, and CX3CL1) were prominently detected in I-A+ TECs and whole fetal thymus lobes rather than in salivary gland lobes (Figure 3A large arrows). Additionally, chemokines such as CCL6 and CXCL15 were detected at higher levels in whole fetal thymus lobes than in I-A+ TECs (Figure 3A-B), suggesting that nonepithelial stromal cells in fetal thymus may produce these chemokines.
Expression of chemokine mRNAs by fetal thymi. (A) Semi-quantitative RT-PCR for mRNAs in I-A+ TSCs, whole dGuo-treated fetal thymus lobes, and dGuo-treated salivary gland lobes from E14.5 fetal mice. Adult TSC debris was used as positive control (PC), except adult thymocytes for CCL3, CCL9, CXCL11, CXCL16, and CX3CL1, E14.5 fetal skin for CXCL15, E14.5 fetal gut for CCL28, and adult spleen cells for CXCL7. DW indicates distilled water. Large arrows indicate molecular species that are prominently expressed at higher levels in I-A+ TSCs and whole dGuo-treated fetal thymus lobes than in dGuo-treated salivary gland lobes. Small arrows indicate molecular species that are detected in I-A+ TSCs and whole dGuo-treated fetal thymus lobes irrespective of the detected levels in dGuo-treated salivary gland lobes. (B) Real-time RT-PCR quantification of mRNA levels of chemokines indicated by arrows in panel A. Signal levels relative to HPRT levels were normalized to the levels in salivary gland. Means and SEs are indicated.
Expression of chemokine mRNAs by fetal thymi. (A) Semi-quantitative RT-PCR for mRNAs in I-A+ TSCs, whole dGuo-treated fetal thymus lobes, and dGuo-treated salivary gland lobes from E14.5 fetal mice. Adult TSC debris was used as positive control (PC), except adult thymocytes for CCL3, CCL9, CXCL11, CXCL16, and CX3CL1, E14.5 fetal skin for CXCL15, E14.5 fetal gut for CCL28, and adult spleen cells for CXCL7. DW indicates distilled water. Large arrows indicate molecular species that are prominently expressed at higher levels in I-A+ TSCs and whole dGuo-treated fetal thymus lobes than in dGuo-treated salivary gland lobes. Small arrows indicate molecular species that are detected in I-A+ TSCs and whole dGuo-treated fetal thymus lobes irrespective of the detected levels in dGuo-treated salivary gland lobes. (B) Real-time RT-PCR quantification of mRNA levels of chemokines indicated by arrows in panel A. Signal levels relative to HPRT levels were normalized to the levels in salivary gland. Means and SEs are indicated.
CCL21, CCL25, and CXCL12 can attract DN1 fetal thymocytes
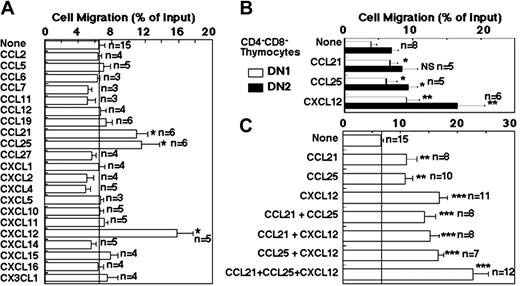
We then examined the chemotactic activity of E14.5 fetal thymocytes to the chemokines expressed in fetal thymus lobes, including the 10 chemokines that were detected at significantly higher levels in whole fetal thymus lobes than in salivary gland lobes. We found distinct responses of the E14.5 fetal thymocytes to 100 nM CCL21, CCL25, or CXCL12 in a 6-hour culture (Figure 4A). Similar but lower responses were detected for these 3 chemokines in a conventional 90-minute culture (data not shown). Other chemokines tested showed no responses at a range of concentrations between 10 and 100 nM (Figure 4A and data not shown). CCL25 and CXCL12 attracted both DN1 thymocytes and DN2 thymocytes, whereas the chemotaxis of E14.5 fetal thymocytes to CCL21 was significantly detected only in DN1 thymocytes but not in DN2 thymocytes (Figure 4B). The attraction of fetal thymocytes by CCL21, CCL25, and CXCL12 was additive rather than synergistic, and the combination of these 3 chemokines most strongly attracted E14.5 fetal thymocytes (Figure 4C). These results indicate that among the chemokines prominently produced by the fetal thymus, DN1 fetal thymocytes are responsive to CCL21, CCL25, and CXCL12.
Transwell chemotaxis of E14.5 fetal thymocytes to chemokines. (A) Freshly isolated E14.5 fetal thymocytes were examined for chemotactic responses to indicated recombinant chemokines (100 nM) in 6 hours. The responses are expressed as percentage of migrated cells within total input cells. (B-C) A low concentration (20 nM) of chemokines was used for the 6-hour transwell chemotaxis of E14.5 fetal thymocytes. Migrated cells were stained for CD44 and CD25 to identify the subset of DN1 and DN2 thymocyte subpopulations in panel B. Means and SEs are indicated. Numbers next to bars indicate the numbers of independent experiments. Asterisks denote significant responses (*P < .05; **P < .01; ***P < .001).
Transwell chemotaxis of E14.5 fetal thymocytes to chemokines. (A) Freshly isolated E14.5 fetal thymocytes were examined for chemotactic responses to indicated recombinant chemokines (100 nM) in 6 hours. The responses are expressed as percentage of migrated cells within total input cells. (B-C) A low concentration (20 nM) of chemokines was used for the 6-hour transwell chemotaxis of E14.5 fetal thymocytes. Migrated cells were stained for CD44 and CD25 to identify the subset of DN1 and DN2 thymocyte subpopulations in panel B. Means and SEs are indicated. Numbers next to bars indicate the numbers of independent experiments. Asterisks denote significant responses (*P < .05; **P < .01; ***P < .001).
Antibody neutralization of CCL21 and CCL25, but not CXCL12, inhibits fetal thymus attraction of T-precursor cells
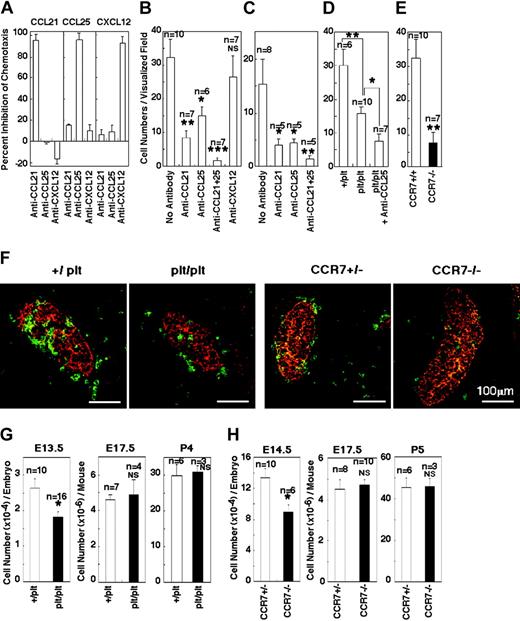
To examine the roles of CCL21, CCL25, and CXCL12 in fetal thymus attraction, time-lapse visualization cultures were carried out in the presence of antibodies specific for these chemokines. The antibodies used were capable of specifically neutralizing the activities of CCL21, CCL25, and CXCL12 in conventional chemotaxis assay (Figure 5A). As shown in Figure 5B for E14.5 fetal thymocytes and in Figure 5C for FL progenitor cells, the fetal thymus attraction was partially but significantly inhibited by anti-CCL21 or anti-CCL25 antibody. The combination of anti-CCL21 and anti-CCL25 antibodies markedly reduced the attraction to the fetal thymus (Figure 5B-C). On the other hand, the anti-CXCL12 antibody did not significantly affect fetal thymus colonization (Figure 5B) even at the concentration that showed nearly complete neutralization of CXCL12 as much as 20 nM (Figure 5A). These results suggest that CCL21 and CCL25 but not CXCL12 are involved in fetal thymus colonization.
Roles of CCL21, CCL25, and CXCL12 in fetal thymus colonization. (A) Specific neutralization of CCL21-, CCL25-, and CXCL12-mediated chemotaxis by anti-CCL21, anti-CCL25, and anti-CXCL12 antibodies. Antibody specific for CCL21 (20 μg/mL), CCL25 (10μg/mL), or CXCL12 (20 μg/mL) was added to transwell chemotactic culture of E14.5 fetal thymocytes with CCL21, CCL25, or CXCL12 (20 nM). Shown are means and SEs of percentage inhibition of chemotaxis in 2 to 3 independent experiments. (B-C) Means and SEs of the numbers of E14.5 fetal thymocytes (B) or TER119–CD45+FcRlow E14.5 fetal liver cells (C) that reached dGuo-treated B6 fetal thymus lobe in the microscopically visualized field were determined in the absence or presence of indicated antibodies at the concentration used in the experiments in panel A. Asterisks denote significant reduction of fetal thymus attraction (*P < .05; **P < .01; ***P < .001). NS indicates not significant. (D) Means and SEs of the numbers of E14.5 fetal thymocytes that reached dGuo-treated fetal thymus lobe from +/plt or plt/plt mice were determined in the absence or presence of anti-CCL25 antibody (*P < .05; **P < .01). (E) E14.5 fetal thymocytes from CCR7-deficient mice were examined for the movement to dGuo-treated B6 fetal thymus lobe (**P < .01). (F) Colonization of hematopoietic cells in E11.5 thymus of plt/plt mice and CCR7-deficient mice. Frozen sections of indicated fetal mice were stained for CD45 (green) and pan-cytokeratin (red). (G-H) Cellularity of thymocytes in plt/plt mice (G) and CCR7-deficient mice (H) at indicated gestational ages. P4 and P5 indicate postnatal ages 4 and 5 days, respectively. Means and SEs of the numbers of thymocytes per mouse are shown (*P < 0.05; NS, not significant).
Roles of CCL21, CCL25, and CXCL12 in fetal thymus colonization. (A) Specific neutralization of CCL21-, CCL25-, and CXCL12-mediated chemotaxis by anti-CCL21, anti-CCL25, and anti-CXCL12 antibodies. Antibody specific for CCL21 (20 μg/mL), CCL25 (10μg/mL), or CXCL12 (20 μg/mL) was added to transwell chemotactic culture of E14.5 fetal thymocytes with CCL21, CCL25, or CXCL12 (20 nM). Shown are means and SEs of percentage inhibition of chemotaxis in 2 to 3 independent experiments. (B-C) Means and SEs of the numbers of E14.5 fetal thymocytes (B) or TER119–CD45+FcRlow E14.5 fetal liver cells (C) that reached dGuo-treated B6 fetal thymus lobe in the microscopically visualized field were determined in the absence or presence of indicated antibodies at the concentration used in the experiments in panel A. Asterisks denote significant reduction of fetal thymus attraction (*P < .05; **P < .01; ***P < .001). NS indicates not significant. (D) Means and SEs of the numbers of E14.5 fetal thymocytes that reached dGuo-treated fetal thymus lobe from +/plt or plt/plt mice were determined in the absence or presence of anti-CCL25 antibody (*P < .05; **P < .01). (E) E14.5 fetal thymocytes from CCR7-deficient mice were examined for the movement to dGuo-treated B6 fetal thymus lobe (**P < .01). (F) Colonization of hematopoietic cells in E11.5 thymus of plt/plt mice and CCR7-deficient mice. Frozen sections of indicated fetal mice were stained for CD45 (green) and pan-cytokeratin (red). (G-H) Cellularity of thymocytes in plt/plt mice (G) and CCR7-deficient mice (H) at indicated gestational ages. P4 and P5 indicate postnatal ages 4 and 5 days, respectively. Means and SEs of the numbers of thymocytes per mouse are shown (*P < 0.05; NS, not significant).
Role of CCL21 in fetal thymus colonization
The role of CCL21 in fetal thymus colonization was further examined by using (1) mice deficient in CCL21 or its receptor, CCR7, and (2) cells ectopically expressing CCL21. A mouse strain harboring the plt/plt mutation lacks functional genes that encode the CCR7 ligands, CCL19 and CCL21.9,23 Fetal thymus lobes from plt/plt mice attracted significantly lower numbers of fetal thymocytes than fetal thymus lobes from control +/plt mice (Figure 5D), indicating that plt/plt fetal thymuses are partially deficient in attracting fetal thymocytes. Also, CCR7-deficient fetal thymocytes were partially defective in moving toward B6 fetal thymus lobes (Figure 5E). The in vitro attraction by plt/plt fetal thymus lobes was further reduced in the presence of anti-CCL25 antibody (Figure. 5D), suggesting that the combination of CCL21 and CCL25 plays a major role in fetal thymus colonization.
Immunofluorescence analysis of E11.5 fetal thymus showed that in vivo fetal thymus colonization was partially impaired in both plt/plt mice and CCR7-deficient mice (Figure 5F). In plt/plt mice, the number of fetal thymocytes was still reduced at E13.5; however, this defect was restored to the normal level by E17.5 (Figure 5G). Similarly, the number of fetal thymocytes in CCR7-deficient mice was still reduced at E14.5 but was recovered to the normal level by E17.5 and the newborn period (Figure 5H). Thus, colonization by fetal thymocytes in vivo was partially defective in plt/plt mice and CCR7-deficient mice, indicating the role of CCR7 signals in fetal thymus colonization. The recovery of the number of fetal thymocytes by E17.5 suggests that the colonized T precursor cells proliferate in the thymus to compensate for the reduced number of recruited thymocytes.
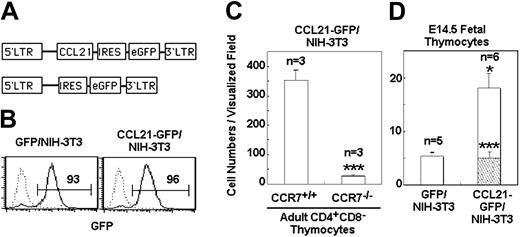
We then examined the ability of cells ectopically expressing CCL21 to attract fetal thymocytes (Figure 6). To this end, a retrovirus expressing either GFP alone or GFP along with CCL21 was infected into NIH-3T3 cells that were negative for endogenous CCL21 expression (Figure 6A). GFP+ cells were purified by fluorescence-activated cell sorting (Figure 6B) and were aggregated. The expression of functional CCL21 by the aggregated mass of CCL21-transduced NIH-3T3 cells was ascertained by demonstrating the attraction of wild-type CD4+CD8– thymocytes but not CCR7-deficient CD4+CD8– thymocytes (Figure 6C). The CCL21-expressing NIH-3T3 cells exhibited significant attraction of E14.5 fetal thymocytes (Figure 6D). Thus, ectopically expressed CCL21 cells attracted immature fetal thymocytes, resembling fetal TECs.
Attraction of fetal thymocytes to CCL21-transfected NIH-3T3 cells. (A) Diagram of pMRX-CCL21-IRES-GFP and pMRX-IRES-GFP constructs. (B) Retroviruses expressing either GFP alone or CCL21 along with GFP were infected into NIH-3T3 cells. GFP+ cells sorted by flow cytometry were reanalyzed for GFP expression. Numbers indicate frequency of cells within marked area. (C) CCL21-GFP–infected NIH-3T3 cells were examined for the production of functional CCL21. Reaggregated CCL21-GFP–infected NIH-3T3 cells (5 × 105) were examined for the attraction of adult CD4+CD8– thymocytes from wild-type or CCR7-deficient mice. Means and SEs of attracted cell numbers are indicated (***P < .001). (D) Reaggregates of either CCL21-GFP–infected NIH-3T3 cells or GFP-infected NIH-3T3 cells were examined for the attraction of E14.5 fetal thymocytes. Means and SEs of the numbers of cells that moved out of the cell spot (□) and reached the cell reaggregate (▧) were determined in visualized field (*P < .05; ***P < .001).
Attraction of fetal thymocytes to CCL21-transfected NIH-3T3 cells. (A) Diagram of pMRX-CCL21-IRES-GFP and pMRX-IRES-GFP constructs. (B) Retroviruses expressing either GFP alone or CCL21 along with GFP were infected into NIH-3T3 cells. GFP+ cells sorted by flow cytometry were reanalyzed for GFP expression. Numbers indicate frequency of cells within marked area. (C) CCL21-GFP–infected NIH-3T3 cells were examined for the production of functional CCL21. Reaggregated CCL21-GFP–infected NIH-3T3 cells (5 × 105) were examined for the attraction of adult CD4+CD8– thymocytes from wild-type or CCR7-deficient mice. Means and SEs of attracted cell numbers are indicated (***P < .001). (D) Reaggregates of either CCL21-GFP–infected NIH-3T3 cells or GFP-infected NIH-3T3 cells were examined for the attraction of E14.5 fetal thymocytes. Means and SEs of the numbers of cells that moved out of the cell spot (□) and reached the cell reaggregate (▧) were determined in visualized field (*P < .05; ***P < .001).
Expression of CCL21 and CCL25 proteins in fetal thymi
Finally, we examined the expression of CCL21 and CCL25 proteins in the fetal thymus primordium. Immunohistologic analysis of fetal thymus sections showed that CCL21 and CCL25 proteins were expressed in the fetal thymus at E12.5 (Figure 7A), whereas the fetal thymus primordium at E11.5 stained positively for CCL21 but not CCL25 (Figure 7B).
Expression of CCL21 and CCL25 proteins in fetal thymus. Frozen sections of B6 embryos at E12.5 (A) and E11.5 (B) were stained with the indicated antibodies. Left and right boxes in panel A show a set of multicolor images of identical sections.
Expression of CCL21 and CCL25 proteins in fetal thymus. Frozen sections of B6 embryos at E12.5 (A) and E11.5 (B) were stained with the indicated antibodies. Left and right boxes in panel A show a set of multicolor images of identical sections.
Discussion
The time-lapse visualization assay devised in the present study revealed that alymphoid fetal thymus attracts a fraction of cells from FL and FB. The fetal thymus specifically attracts T-precursor cells because (1) thymus-recruited cells subsequently developed into mature T cells in the thymus and (2) TER119+ erythroid cells or CD45– nonleukocytic cells in FL were not attracted to the thymus. The present study did not examine whether the fetal thymus also attracted leukocytic cells other than T-precursor cells, such as dendritic cells and their precursor cells. Nonetheless, the present results directly indicate that the fetal thymus secretes factors that specifically recruit T-lymphoid precursor cells. Our results also show that fetal thymocytes are enriched for the cells that specifically re-enter fetal thymus. The frequency of cells that are attracted by fetal thymus is significantly higher in fetal thymocytes than in FL cells. The capability to move toward the fetal thymus was detected within DN1 thymocytes, whereas further developed DN2, DN3, and DP thymocytes displayed a significantly lower activity than DN1 thymocytes to move toward the thymus. Thus, time-lapse visualization of re-entry by fetal thymocytes offers a specific and efficient detection of the attraction of T-precursor cells to the fetal thymus.
Our results indicate that the T-precursor cell-attracting activity of the fetal thymus is mainly due to TECs. Consistent with this notion, lack of the FoxN1 transcription factor causes a deficiency in the colonization of the fetal thymus primordium by T-precursor cells,5,8 indicating that the FoxN1-dependent thymic epithelium plays an essential role in attracting T-precursor cells to the thymus. Our results also show that fetal thymus attraction is mediated by PTX-sensitive G protein-coupled signals in T-precursor cells. Thus, chemokines secreted by TECs are the most likely candidates for the molecules involved in this developmental event. Among the 36 chemokines so far identified in the mouse, we found that 10 (CCL5, CCL19, CCL21, CCL25, CXCL10, CXCL11, CXCL12, CXCL14, CXCL16, and CX3CL1) were expressed at significantly higher levels in fetal TECs and whole thymus lobes than in salivary gland lobes. Among these chemokine species, 3 (CCL21, CCL25, and CXCL12) showed DN1 fetal thymocyte-attracting activity. Moreover, fetal thymus attraction was partially but significantly inhibited by the anti-CCL21 or anti-CCL25 antibody, whereas the anti-CXCL12–neutralizing antibody did not significantly affect the movement of T-precursor cells. The combination of anti-CCL21 and anti-CCL25 antibodies markedly inhibited fetal thymus attraction. Thus, the present results suggest that among the chemokines so far identified in mouse, CCL21 and CCL25 are the most important chemokines for the colonization of the fetal thymus by T precursor cells.
The present results further show that in vitro T-precursor cell attraction by the thymus from CCL21-deficient plt/plt mice is partially but significantly lower than the attraction by wild-type thymus. Also, in vivo fetal thymus colonization detected at E11.5 was partially reduced in plt/plt mice and CCR7-deficient mice. This is in agreement with the observation that the absolute numbers of fetal thymocytes were reduced at E13.5 and E14.5 in both plt/plt mice and CCR7-deficient mice. Thus, our results indicate that CCL21 plays a significant, although not exclusive, role in fetal thymus colonization by T precursor cells. However, normal numbers of fetal thymocytes were recovered in these mice by E17.5, suggesting that the small number of thymus-colonizing cells in plt/plt mice and CCR7-deficient mice could efficiently proliferate within the thymus environment and thus compensate for the impaired attraction of T-precursor cells. Our results also show that CCL21 protein is prominently expressed in the E11.5 fetal thymus primordium. Fibroblasts that ectopically expressed CCL21 were capable of attracting immature fetal thymocytes, and the CCL21-mediated chemotaxis of fetal thymocytes was specific for DN1 immature thymocytes, resembling fetal thymus attraction of T-precursor cells. These results demonstrate the role of CCL21 in the optimal recruitment of T-precursor cells to the fetal thymus.
It is well established that CCL21 is involved in attracting mature naïve T cells and dendritic cells to peripheral lymph nodes9,24 and to inflammatory sites.25,26 On the other hand, the role of CCL21 in regulating lymphocyte traffic during development has been unclear. We have previously shown that another CCR7 ligand, CCL19, can attract mature T cells out of fetal thymus organ cultures and that the newborn supply of peripheral T cells is impaired in CCR7-deficient mice.11 Our results showing the role of CCL21 in the recruitment of T-precursor cells to the fetal thymus reveal an earliest function of CCR7 ligands in ontogeny. The role of CCR7 ligands in fetal thymus colonization is consistent with a recent report showing that CCR7 transcripts are detected in interleukin 7 receptor-positive (IL-7R+) lymphoid precursor cells in FB.18 Moreover, we have recently found that a small fraction of CD45+TER119– E14.5 FL cells express CCR7 on their cell surface, which is detected by use of a CCL19-IgG fusion protein (C.L., T.U., and Y.T., unpublished results, 2004).
It has been shown that CCR7 is a common receptor for both CCL21 and CCL19.27 Our results also show that like plt/plt embryos, CCR7-deficient embryos have a decreased thymocyte cellularity, suggesting that CCL21 signal in fetal thymus attraction is mediated by CCR7. It is therefore interesting to note that unlike CCL21, CCL19 displays no or only marginal activity in inducing the chemotaxis of immature fetal thymocytes, even at a wide range of concentrations (10-100 nM; Figure 4 and data not shown). Thus, CCL21 and CCL19 may trigger different CCR7 signals in T-precursor cells.
Concerning the role of CCL25, the present results indicate that the antibody neutralization of CCL25 partially but significantly inhibits fetal thymus attraction. However, it has been previously noted in a transfilter migration assay that the anti-CCL25 antibody failed to inhibit thymus colonization by T-precursor cells.4 The difference with our results may be explained by the possibility that the inhibitory effect of an anti-CCL25 antibody may not have been detected because immigrated thymocytes could have subsequently proliferated to restore normal thymus cellularity. The possibility that CCL25 is partially involved in fetal thymus attraction as suggested in the present study is consistent with previous results showing that the number of fetal thymocytes is partially reduced in mice deficient in the CCL25 receptor, CCR9.7
Our results further show that the combination of neutralizing antibodies specific for CCL21 and CCL25 markedly inhibits fetal thymus attraction and the partially reduced attraction by CCL21-deficient plt/plt fetal thymus is further diminished by the addition of anti-CCL25 antibody. Altogether, the present results suggest that the combination of CCL21 and CCL25 plays a major role in fetal thymus recruitment. However, our results also show that the E11.5 thymus primordium stains positive for CCL21 but not CCL25 (Figure 7), whereas both CCL21 and CCL25 are detected in E12.5 fetal thymus5 (Figure 7). Thus, CCL21 and CCL25 may play unequal roles in fetal thymus attraction rather than simply sharing a redundant role in this phenomenon. By crossing CCR7-deficient mice with CCR9-deficient mice, we are currently investigating whether the combination of CCL21 and CCL25 signals is indispensable for fetal thymus colonization.
It was previously shown that the fetal thymus primordium of foxn1-deficient nude mice expresses CCL21, but not CCL25 or CXCL12,5 and can attract lymphoid precursors to the perithymic area but not further to the inner epithelial cluster.5,8 Thus, CCL21 may be involved in the initial step of attraction of precursors to the perithymic area, and the subsequent migration into the inner region may be regulated by CCL25. This possibility of a 2-step migration8 is in agreement with our results showing that the thymus primordium sequentially expresses CCL21 and then CCL25.
Our results show that the CCL25-producing fetal thymus does not attract adult DP thymocytes (Figure 1F), whereas adult DP thymocytes are shown to respond to CCL25.28-30 However, previous reports used 100 to 300 nM CCL25 to detect the chemotaxis of DP thymocytes,28-30 whereas fetal DN thymocytes show CCL25 responses at 10 to 20 nM (Figure 4). Thus, the fetal thymus may secrete CCL25 at a concentration lower than that for attracting adult DP thymocytes, but higher than that for attracting fetal DN thymocytes.
Concerning the role of CXCL12 in thymocyte migration, our results show that CXCL12 can exhibit chemotactic activity for fetal thymocytes including immature DN1 thymocytes. Also, it has been recently demonstrated that CXCL12 is essentially involved in the localization of developing thymocytes within the postnatal thymus.31 On the other hand, our results show that the attraction by CXCL12-producing fetal thymus is not significantly affected by the antibody neutralization of CXCL12. The lack of inhibition by the anti-CXCL12 antibody is likely due to the attraction mediated by CCL21 and CCL25. Parallel to these observations, it was previously shown that the mice deficient in CXCL12 or the CXCL12 receptor, CXCR4, normally accumulate lymphoid precursor cells to the thymic primordium at E11.5 and E12.5.6 Thus, we think that CXCL12 signals are not required for the fetal thymus colonization by T-precursor cells but are involved in the intrathymic migration of developing thymocytes.
Fetal thymus colonization initially occurs by transmigration through the capsule before the thymus vascularization, which is first detected around E15 (L.P. and G.A.H., unpublished results, 2002). Nonetheless, it remains unclear how chemokines produced by the fetal thymus primordium attract T precursor cells. As suggested by the role of chemokines in the homing of mature T cells to peripheral lymph nodes, locally produced chemokines may coordinate with adhesion molecules for the specific accumulation of target cells into the tissue. Thus, chemokines produced by the fetal thymus primordium may locally trigger the arrest of T-precursor cells as they pass in the vicinity of the thymus primordium between E11.5 and E15. Alternatively, it is possible that chemokines may generate a systemic gradient attracting T-precursor cells remotely localized in FL or FB. Nevertheless, the entire process of fetal thymus colonization is likely controlled by an orchestrated activity of various molecules including chemokines, adhesion molecules, and other molecules such as matrix metalloproteinases expressed by different types of cells (eg, TECs and mesenchymal cells).
In conclusion, our results reveal that CCL21 expressed by the fetal thymus is involved in the recruitment of T-precursor cells and suggest that the combination of CCL21 and CCL25 plays a major role in fetal thymus colonization. Moreover, we have presented a novel time-lapse visualization technique for analyzing T-precursor cell recruitment to the fetal thymus. Together, these results provide direct evidence of the functional involvement of chemokines in the fetal thymus colonization by T-precursor cells.
Prepublished online as Blood First Edition Paper, September 9, 2004; DOI 10.1182/blood-2004-04-1369.
Supported by Ministry of Education, Culture, Sports, Science and Technology grants-in-aid for scientific research, Tokyo; the core-to-core program of the Japan Society for the Promotion of Science, Tokyo; the Mochida Memorial Foundation for Medical and Pharmaceutical Research, Tokyo; the Uehara Memorial Foundation, Tokyo; and the University of Tokushima, Tokushima, Japan.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr R. Boyd for discussion and the supply of anti-EpCAM antibody, and Drs M. Itoi, T. Amagai, H. Kawamoto, M. Miyasaka, N. Iwanami, and D. Gray for discussion and technical support.