Abstract
Stimulation of platelets with strong agonists results in centralization of cytoplasmic organelles and secretion of granules. These observations have led to the supposition that cytoskeletal contraction facilitates granule release by promoting the interaction of granules with one another and with membranes of the open canalicular system. Yet, the influence of the actin cytoskeleton in controlling the membrane fusion events that mediate granule secretion remains largely unknown. To evaluate the role of the actin cytoskeleton in platelet granule secretion, we have assessed the effects of latrunculin A and cytochalasin E on granule secretion. Exposure of platelets to low concentrations of these reagents resulted in acceleration and augmentation of agonist-induced α-granule secretion with comparatively modest effects on dense granule secretion. In contrast, exposure of platelets to high concentrations of latrunculin A inhibited agonist-induced α-granule secretion but stimulated dense granule secretion. Incubation of permeabilized platelets with low concentrations of latrunculin A primed platelets for Ca2+- or guanosine triphosphate (GTP)-γ-S-induced α-granule secretion. Latrunculin A-dependent α-granule secretion was inhibited by antibodies directed at vesicle-associated membrane protein (VAMP), demonstrating that latrunculin A supports soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) protein-dependent membrane fusion. These results indicate that the actin cytoskeleton interferes with platelet exocytosis and differentially regulates α-granule and dense granule secretion.
Introduction
Upon exposure to a strong agonist, platelets undergo a dramatic morphologic transformation that includes shape change and loss of platelet granules. Shape change is accompanied by the appearance of masses of polymerized insoluble cytoplasmic actin filaments and the extension of cytoplasmic pseudopodia. During shape change, platelets lose their discoid shape to form irregular spheres if in solution or to spread if in contact with a surface.1,2 These morphologic alterations result in a dramatic increase in the surface area of the platelet, which is likely to enhance the hemostatic function of the platelet at sites of vascular injury. The membrane required to accommodate this expansion of surface area can be derived from both evagination of the open canalicular system (OCS) and fusion of granules with OCS or plasma membranes. The latter mechanism suggests that granule secretion may be linked to cytoskeletal reorganization. Yet, despite an increasingly detailed understanding of the molecular mechanisms that underlie membrane fusion of platelet granules,3 the role of shape change in controlling granule secretion remains poorly understood. In particular, it is not known how activation-induced reorganization of the actin cytoskeleton affects membrane fusion events required for platelet granule secretion.
The role of the actin cytoskeleton in platelet granule secretion has been evaluated by both electron microscopy and functional studies. Ultrastructural analyses have revealed that the release of granules from activated platelets is unusual in that platelet granules become centralized upon platelet activation prior to release into the OCS,1,4,5 whereas classic exocytosis by nucleated cells occurs both via fusion of granules with granules and with the plasma membrane. The observation that the cytoskeleton directs granule centralization in platelets led to speculation that it provides a contractile force that facilitates the release of granule contents through the OCS.6-8 Platelet function studies using cytochalasins to disrupt the actin cytoskeleton have yielded conflicting results. Cytochalasin B was initially found to augment collagen-induced dense granule secretion from platelets.9 Subsequent studies using similar conditions, however, demonstrated little effect of cytochalasin on collagen-induced dense granule secretion.10 Studies using other agonists such as thrombin, phorbol esters, calcium ionophore, or adenosine diphosphate (ADP) to study the effect of cytochalasins on dense granule secretion have demonstrated either inhibition or augmentation.11-13 A study evaluating α-granule secretion showed no effect of cytochalasin E on thrombin-induced α-granule secretion.14 Differences in experimental design and findings from these functional platelet studies preclude firm conclusions regarding the role of the cytoskeleton in controlling membrane fusion events required for granule secretion.
We sought to evaluate the role of the actin cytoskeleton in platelet membrane fusion in a comprehensive manner using a variety of approaches. In these studies, we used agonists that signal through different pathways to assess the effect of 2 structurally unrelated actin-disrupting agents on both α-granule and dense granule secretion. Agonists and inhibitors were tested at multiple concentrations. We evaluated both the kinetics of granule secretion as well as the degree of secretion. These studies demonstrate that exposure of platelets to low concentrations of actin-disrupting agents accelerate, augment, and decrease agonist concentration requirements for α-granule secretion. In contrast, high concentrations of actin-disrupting agents inhibit α-granule secretion but stimulate dense granule secretion. We also used a permeabilized platelet model to evaluate the role of the actin cytoskeleton on distal mechanisms of granule secretion. These studies demonstrated that disruption of the resting actin cytoskeleton was sufficient to prime permeabilized platelets for granule secretion in response to either Ca2+ or guanosine triphosphate (GTP)-γ-S. This secretory response was inhibited by an antibody directed against vesicle-associated membrane protein (VAMP), a soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) protein required for platelet granule secretion.15,16 Thus, the actin cytoskeleton acts as a functional barrier to SNARE protein-dependent granule secretion.
Materials and methods
Materials
All buffer constituents, magnesium adenosine triphosphate (MgATP), CaCl2, GTP-γ-S, epinephrine, streptolysin-O (SL-O), paraformaldehyde, fluorescein isothiocyanate (FITC)-phalloidin, apyrase, aspirin, Arg-Gly-Asp-Ser (RGDS), and luciferin-luciferase mixture were purchased from Sigma (St Louis, MO). Phorbol 12-myristate 13-acetate (PMA), latrunculin A, and cytochalasin E were purchased from Calbiochem (San Diego, CA). Phosphatidylcholine and phosphatidylserine were purchased from Avanti Polar Lipids (Alabaster, AL). Sepharose 2B and 14C-serotonin were obtained from Amersham Pharmacia Biotech (Piscataway, NJ). N-hydroxysulfo-succinimidobiotin (sulfo-NHS-biotin) and dialysis cassettes were obtained from Pierce (Rockford, IL). Avidin-horseradish peroxidase (HRP) was obtained from Bio-Rad (Hercules, CA). Serine-phenylalanineleucine-leucine-arginine-asparagine (SFLLRN) was synthesized on an Applied Biosystems model 430A peptide synthesizer.
Antibodies
Phycoerythrin (PE)-conjugated AC1.2 anti-P-selectin and anti-LAMP-1 (lysosome-associated membrane protein-1) antibodies were purchased from BD Biosciences (San Jose, CA). Antibody against the P-selectin cytoplasmic tail was described previously.15 Rabbit anti-human β-thromboglobulin antiserum was obtained from Nordic Immunology (Tilburg, The Netherlands). Rabbit polyclonal anti-VAMP-2 antibodies were obtained from StressGene (Victoria, Canada).
Platelet preparation
Blood from healthy donors who had not ingested aspirin in the 2 weeks prior to donation was collected by venipuncture into 0.4% sodium citrate. Approval for these studies was obtained from the Beth Israel Deaconess Medical Center institutional review board. Informed consent was provided according to the Declaration of Helsinki. Citrate-anticoagulated blood was centrifuged at 200g for 20 minutes to prepare platelet-rich plasma. Platelet-rich plasma was then used for ultrastructural studies as described in “Immunonanogold labeling for electron microscopy.” For platelet secretion studies, platelets were purified from platelet-rich plasma by gel-filtration using a Sepharose 2B column equilibrated in PIPES/NaCl buffer (25 mM PIPES (piperazine-N-N′-bis-(2-ethanesulphonic acid)), 137 mM NaCl, 4 mM KCl, 0.1% glucose, pH 6.4) for experiments using intact platelets or in PIPES/EGTA/KCl buffer (25 mM PIPES, 2 mM EGTA (ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid), 137 mM KCl, 4 mM NaCl, 0.1% glucose, pH 6.4) for experiments using permeabilized platelets. Samples were adjusted to pH 7 immediately prior to analysis.
Evaluation of P-selectin expression using flow cytometry
For analysis of P-selectin expression, 20 μL gel-filtered platelets (0.5-1 × 108/mL) were incubated with the indicated agonists. For studies evaluating the kinetics of P-selectin surface expression, platelets were fixed in 0.5% paraformaldehyde at the indicated time following exposure to agonist for 20 minutes. Following this incubation, 10 μL reaction mixture was transferred to 5 μL PE-conjugated AC1.2 anti-P-selectin antibody. Phosphate-buffered saline (PBS; 500 μL) was added to the sample after a 20-minute incubation, and the platelets were analyzed immediately by flow cytometry.
Evaluation of P-selectin expression from permeabilized platelets
For analysis of P-selectin expression from permeabilized platelets,17 20 μL gel-filtered platelets was incubated with either buffer, 2 μM latrunculin A, or 5 mM MgATP as indicated. Samples were subsequently permeabilized with 500 U/mL SL-O in the presence or absence of the indicated concentration of anti-VAMP antibody. Samples were adjusted to pH 6.9 immediately after permeabilization. Following a 15 minute incubation with antibody, CaCl2 or GTP-γ-S was added to the reaction mixture. The amount of CaCl2 required to give a free Ca2+ concentration of 10 μM in the presence of 2 mM EGTA at pH 6.9 was calculated as described previously.17 Following an additional 10-minute incubation after the addition of Ca2+ or 20 minutes after addition of GTP-γ-S, 10 μL reaction mixture was transferred to 5 μL PE-conjugated AC1.2 anti-P-selectin antibody. After a 20-minute incubation, samples were diluted in PBS and analyzed immediately by flow cytometry.
Evaluation of ATP release
A luciferin-luciferase detection system was used to quantify ATP release to monitor dense granule secretion.18 For these experiments, 9 μL platelets (0.5-1 × 108/mL) were stimulated with the indicated agonists. Samples were then incubated with 1 μL luciferin-luciferase (final concentration of 3 mg/mL) at the indicated time following addition of agonists. Chemoluminescence was quantified using a luminometer (TD 20/20; Turner Design, Sunnyvale, CA).
Serotonin release assays
Platelets were loaded with 14C-serotonin for 1 hour at 37°C and washed. Release of 14C-serotonin from 14C-serotonin-loaded platelets following incubation with SFLLRN or PMA was monitored as previously described.19
Evaluation of FITC-phalloidin binding
Flow cytometry
Flow cytometry was performed on gel-filtered platelet samples using a Becton Dickinson FACSCalibur flow cytometer (San Jose, CA). Fluorescent channels were set at logarithmic gain. Particles (104) were acquired for each sample. A 530/30 band pass filter was used for FL-2 fluorescence to measure PE. For evaluation of FITC-phalloidin staining of platelet fractions recovered from Optiprep step gradients, forward scatter was set at E01, side scatter was set at 350, and a 585/42 band pass filter was used for FL1 fluorescence to measure FITC. Data were analyzed using CellQuest software (BD Biosciences) on a MacIntosh PowerPC (Apple, Cupertino, CA).
Electron microscopy
Platelets were fixed in Karnovsky fixative, postfixed in osmium, and stained with uranyl acetate en bloc as previously described.22 Sections (1 μm) were cut and stained with alkaline Giemsa. Representative areas were selected and subsequently thin sectioned. Grids were lightly stained with lead citrate, and samples were studied by transmission electron microscopy (CM-10; Philips, Eindhoven, The Netherlands).
Immunonanogold labeling for electron microscopy
Purified human platelets were fixed in 4% paraformaldehyde and prepared for sectioning as previously described.23 Immunonanogold staining and processing for electron microscopy was performed at room temperature on cryostat sections mounted on glass slides as described previously except for the following modifications. Glass slides with cryostat sections were incubated in primary polyclonal rabbit antibody directed against the cytoplasmic tail of P-selectin, at a dilution of 1:50 in 0.02 M PBS for 60 minutes. Following incubation with the anti-P-selectin cytoplasmic tail antibody, samples were incubated in the secondary antibody affinity-purified Fab′ fragment from goat anti-rabbit immunoglobulin G (IgG) conjugated with 1.4 nm nanogold (Nanoprobes, Stony Brook, NY). Samples were subsequently processed as previously published23 and studied in a CM 10 Philips electron microscope (Philips, Eindhoven, The Netherlands).
The following 4 controls were performed to ensure the specificity of immunostaining: (1) replacement of primary antibody by an irrelevant rabbit IgG, (2) omission of specific primary antibody, (3) omission of the secondary antibody, and (4) omission of the high-quality (HQ) silver enhancement solution.
Subcellular fractionation
Subcellular fractionation of platelet organelles was performed using previously described methods.23,24 Platelet-rich plasma (approximately 1 × 109 platelets/mL) was labeled with 25 μg/mL sulfo-NHS-biotin at 4°C for 1 hour, washed 3 times with buffer, and subjected to nitrogen cavitation for 20 minutes at 100 atmospheres on ice.23 The cavitate was subsequently processed according to a modification of the procedures described by Gogstad et al.24,25 Platelets were removed from the cavitate by centrifugation. The supernatant was collected for analysis. Optiprep (Sigma) was added to the supernatant to a final concentration of 11%. This material (10 mL) was then loaded onto 2-mL beds of 30% Optiprep. The material was subjected to centrifugation at 38 000g for 3 hours at 4°C in a SW-41TI rotor (Beckman, Fullerton, CA). Material at the 11%/30% interface and within the 30% layer was collected, dialyzed, and loaded onto 12-mL step gradients of 11%, 15%, 20%, and 25% and 30% Optiprep. Gradients were subjected to centrifugation at 38 000g for 3 hours at 4°C. Following centrifugation, distinct bands were visualized and collected. Fraction 1 was collected at the 11%/15% interface, fraction 2 was collected at the 15%/20% interface, fraction 3 was collected at the 25%/30% interface, and fraction 4 was collected from within the 30% layer. Fractions were subsequently analyzed by immunoblot analysis as described in “Immunoblot analysis.”
To evaluate the migration of OCS and plasma membranes in the Optiprep step gradients, platelets were labeled with biotin prior to subcellular fractionation. Proteins within fractions from the step gradient were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and stained with HRP-avidin conjugate. Surface labeling is represented by a 65-kDa band that has previously shown to be a dominant protein on the surface of biotin-labeled, resting platelets.26 14C-serotonin was used to evaluate the migration of dense granules in the Optiprep step gradient. In these experiments, 50 μCi (1.85 MBq) 14C-serotonin was added to platelet-rich plasma for 30 minutes. Platelets were subsequently washed and processed as described in “Subcellular fractionation,” and radioactivity in each fraction was quantified by scintillation counting.23 Fractions from the step gradients were also evaluated by immunoblot analysis for reactivity with anti-LAMP-1 and anti-P-selectin antibody to identify lysosomes and α-granules, respectively.
Immunoblot analysis
Platelets were treated with agonists as described in figure legends and subsequently pelleted. Supernatants were then diluted in sample buffer (62.5 mM Tris (tris(hydroxymethyl)aminomethane)-HCl, 2% SDS, 5% β-mercaptoethanol, 10% glycerol, 0.01% bromophenol blue) at 95°C for 5 minutes. Proteins were then separated by SDS-PAGE. Immunoblotting of platelet proteins was performed using β-thromboglobulin, anti-P-selectin cytoplasmic tail, or LAMP-1 antibodies as indicated and visualized using enhanced chemiluminescence.
Results
SFLLRN-versus PMA-induced α-granule secretion
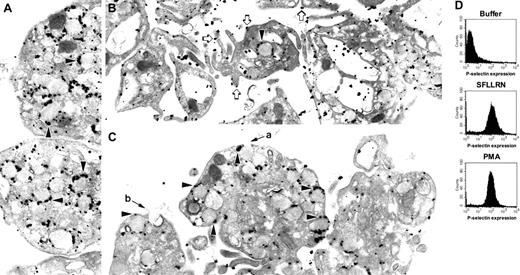
The role of the cytoskeleton in platelet granule secretion is enigmatic. To better understand how the cytoskeleton functions during platelet granule secretion, we compared granule secretion elicited by 2 different platelet agonists (Figure 1). Stimulation of platelets for 10 minutes with the thrombin receptor agonist peptide, SFLLRN, results in dramatic platelet shape change with centralization and loss of α-granules (Figure 1B). In contrast, stimulation of platelets with the phorbol ester PMA for 10 minutes results in only modest shape change (Figure 1C). In the PMA-treated platelets, the α-granules appear to move toward the periphery of the platelet. However, the granule membranes remain largely intact at this time point. In contrast to their different effects on platelet shape change, samples treated with SFLLRN or PMA for 10 minutes demonstrated nearly the same degree of α-granule secretion as monitored by analysis of P-selectin expression by flow cytometry (Figure 1D). To determine whether or not P-selectin remains associated with α-granules following a 10-minute incubation with PMA, we labeled platelets with an antibody directed at the cytoplasmic tail of P-selectin. These studies demonstrated that within this time frame P-selectin did not migrate to the outer surface of the plasma membrane, but rather remained largely associated with α-granule membranes (Figure 1C). In contrast, immunonanogold staining for P-selectin in platelets exposed to SFLLRN demonstrated substantial surface expression of P-selectin (Figure 1B). How could flow cytometry demonstrate increased P-selectin surface expression prior to degranulation of α-granules? Some micrographs of PMA-treated platelets demonstrated pore formation between α-granule and plasma membranes (Figure 1C). Such pores could allow anti-P-selectin antibody access to P-selectin within α-granules. Pores so formed could also allow for the leakage of intragranular contents such as β-thromboglobulin from PMA-stimulated platelets. Morphologically similar fusion pores have previously been observed in 12-O-tetradecanoylphorbol-13-acetate (TPA)-stimulated basophils.27 Stimulation with SFLLRN, in contrast, leads to rapid degranulation and loss of granules.
One possible role of cytoskeletal reorganization on granule secretion is to influence the rate at which granule contents are released. Actin polymerization following stimulation with PMA occurs much more slowly than actin polymerization following stimulation with thrombin receptor agonist peptides.21,28 Cytoskeletal reorganization may accelerate release of granule contents from platelets exposed to SFLLRN compared with release from platelets exposed to PMA. To evaluate this possibility, platelets were treated with either SFLLRN or PMA and monitored for P-selectin expression over time. In platelets treated with SFLLRN, 50% of P-selectin expression occurred within 1 minute following exposure to SFLLRN. P-selectin surface exposure in response to PMA was considerably slower. The time for half-maximal stimulation of SFLLRN-induced α-granule secretion was 5.9-fold lower than that of PMA-induced secretion (Figure 2A). However, the total amount of P-selectin expression elicited by SFLLRN and PMA were similar. Experiments evaluating the time course of loss of the α-granule marker β-thromboglobulin demonstrated a similar result. Release of β-thromboglobulin was observed within 1 minute following stimulation with SFLLRN (Figure 2B). In contrast, release of β-thromboglobulin from PMA-treated platelets was not detected until 5 minutes following exposure to PMA. Similar to experiments in which P-selectin expression was monitored, the degree of release of β-thromboglobulin was similar despite marked differences in the rates of release. It is well established that SFLLRN elicits cytoskeletal reorganization more rapidly than PMA.21,28 We next sought to determine whether rapid α-granule release in response to SFLLRN was a result of rapid actin reorganization or whether these 2 responses were simply epiphenomena.
P-selectin localization in platelets exposed to SFLLRN or PMA. Platelets were exposed to (A) buffer, (B) 100 μM SFLLRN, or (C) 0.2 μM PMA for 10 minutes. Platelets were then processed for immunonanogold staining by using a polyclonal rabbit antibody directed against the cytoplasmic tail of P-selectin. Label is observed primarily on α-granule surfaces (black arrowheads) in resting platelets (A) and platelets exposed to PMA (C). Label is observed mostly on plasma membrane (open arrows) in platelets exposed to SFLLRN (B). Apparent fusion of α-granules with plasma membrane could be visualized in the PMA-treated platelets (a and b). Control samples prepared by using an irrelevant primary antibody or in the absence of primary antibody did not show significant staining. (D) Expression of P-selectin in aliquots of platelet samples also used for electron microscopy was assessed by flow cytometry by using an antibody to the extracellular domain of P-selectin. Magnification of panel A is 25 000 ×;B,21 000 ×; and C, 25 000 ×.
P-selectin localization in platelets exposed to SFLLRN or PMA. Platelets were exposed to (A) buffer, (B) 100 μM SFLLRN, or (C) 0.2 μM PMA for 10 minutes. Platelets were then processed for immunonanogold staining by using a polyclonal rabbit antibody directed against the cytoplasmic tail of P-selectin. Label is observed primarily on α-granule surfaces (black arrowheads) in resting platelets (A) and platelets exposed to PMA (C). Label is observed mostly on plasma membrane (open arrows) in platelets exposed to SFLLRN (B). Apparent fusion of α-granules with plasma membrane could be visualized in the PMA-treated platelets (a and b). Control samples prepared by using an irrelevant primary antibody or in the absence of primary antibody did not show significant staining. (D) Expression of P-selectin in aliquots of platelet samples also used for electron microscopy was assessed by flow cytometry by using an antibody to the extracellular domain of P-selectin. Magnification of panel A is 25 000 ×;B,21 000 ×; and C, 25 000 ×.
Kinetics of SFLLRN- and PMA-induced α-granule secretion from platelets. (A) Platelets were stimulated with either 100 μM SFLLRN (•) or 0.2 μM PMA (□). At the indicated times following exposure to agonist, samples were fixed with paraformaldehyde. Samples were then assayed for P-selectin expression by flow cytometry to monitor α-granule secretion. Error bars indicate the standard deviation of 3 to 7 samples. (B) Platelets were stimulated with either 100 μM SFLLRN or 0.2 μM PMA as indicated. Platelets were then pelleted at the indicated times. The supernatants from stimulated platelets were assayed for β-thromboglobulin (β-TG) by immunoblot analysis as an indicator of release of α-granule contents.
Kinetics of SFLLRN- and PMA-induced α-granule secretion from platelets. (A) Platelets were stimulated with either 100 μM SFLLRN (•) or 0.2 μM PMA (□). At the indicated times following exposure to agonist, samples were fixed with paraformaldehyde. Samples were then assayed for P-selectin expression by flow cytometry to monitor α-granule secretion. Error bars indicate the standard deviation of 3 to 7 samples. (B) Platelets were stimulated with either 100 μM SFLLRN or 0.2 μM PMA as indicated. Platelets were then pelleted at the indicated times. The supernatants from stimulated platelets were assayed for β-thromboglobulin (β-TG) by immunoblot analysis as an indicator of release of α-granule contents.
Regulation of granule secretion by the actin cytoskeleton
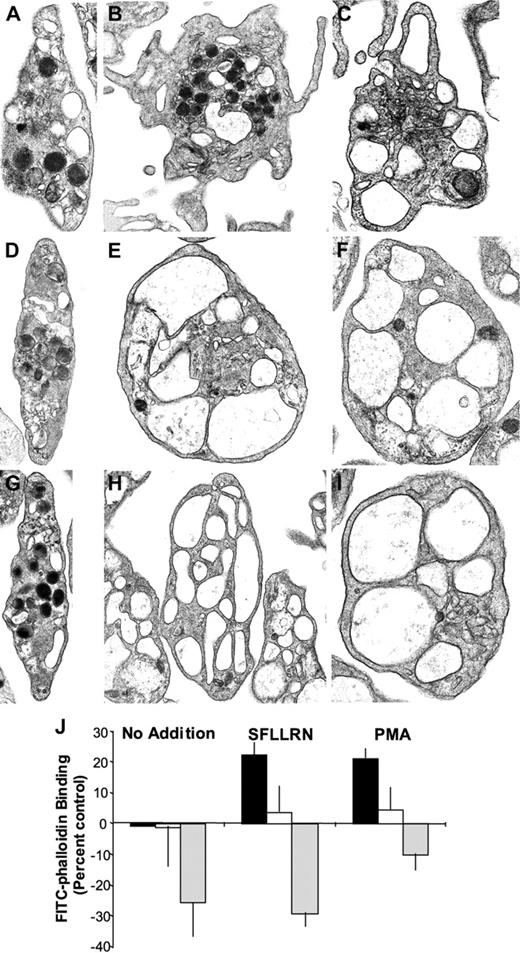
Following platelet activation, the actin cytoskeleton undergoes a dynamic reorganization that involves both disassembly of the resting actin cytoskeleton and actin polymerization.29,30 To further evaluate the role of cytoskeletal reorganization in granule secretion, we incubated platelets in the presence of either latrunculin A or cytochalasin E prior to exposure to agonists. Latrunculin A is a small molecule that binds to monomeric actin, thereby facilitating disassembly of the resting actin cytoskeleton.31 The specificity of latrunculin A has been elegantly demonstrated by the observation that a mutation in actin was sufficient to render yeast resistant to high concentrations of latrunculin A.32 Cytochalasin E is a small molecule that also disrupts the actin cytoskeleton.31 Resting platelets exposed to 4 μM cytochalasin E or latrunculin A demonstrated a morphology similar to that of untreated resting platelets (Figure 3A,D,G). Following stimulation with SFLLRN for 10 minutes, platelets demonstrated granule centralization, loss of granules, and marked pseudopod formation (Figure 3B). In contrast, platelets exposed to either cytochalasin E or latrunculin A demonstrated loss of granules in the absence of pseudopod formation (Figure 3E,H). The OCS was markedly enlarged and expanded in SFLLRN-treated platelets pretreated with actin-disrupting agents. Consistent with previous studies demonstrating that PMA induces actin polymerization slowly,21 formation of pseudopodia was observed along with granule loss in platelets exposed to PMA for 20 minutes (Figure 3C). In the presence of cytochalasin E or latrunculin A, a 20-minute exposure to PMA resulted in degranulation in the absence of pseudopod formation (Figure 3F,I). Enlargement of the OCS was also observed. To further characterize the effects of these actin-disrupting agents on platelets, we evaluated the effects of cytochalasin E and latrunculin A on F-actin formation in resting and activated platelets. Incubation of platelets with 4 μM cytochalasin E did not significantly alter F-actin content in resting platelets (Figure 3J). Cytochalasin E did, however, inhibit activation-induced increases in F-actin content. Incubation of resting platelets with 4 μM latrunculin A resulted in a decrease in F-actin content as evidenced by reduced FITC-phalloidin binding (Figure 3J). F-actin content remained suppressed in latrunculin A-treated platelets even following exposure to SFLLRN or PMA. These studies confirmed the effects of the actin-disrupting agents cytochalasin E and latrunculin A in platelets.
Effects of cytochalasin E and latrunculin A on platelet ultrastructure and F-actin formation. Platelets were incubated in the presence of vehicle (A-C), 4 μM cytochalasin E (D-F), or 4 μM latrunculin A (G-I) for 20 minutes. Actin-disrupting agents had little effect on the morphology of resting platelets. (B) Platelets treated with SFLLRN for 10 minutes demonstrated pseudopod formation. A subpopulation demonstrated granule centralization but most showed only marked degranulation. Pretreatment with (E) cytochalasin E or (H) latrunculin A inhibited pseudopod formation. The OCS becomes expanded and enlarged under these conditions. (C) Platelets treated with PMA for 20 minutes demonstrated degranulation and pseudopod formation. Pretreatment with (F) cytochalasin E or (I) latrunculin A prevented formation of pseudopodia and resulted in enlargement and expansion of the OCS. (J) Platelets were incubated in the presence of vehicle (▪), 4 μM cytochalasin E (□), or 4 μM latrunculin A (▦) for 20 minutes. Platelets were then stimulated with buffer alone, SFLLRN for 10 minutes, or PMA for 20 minutes. Platelets were subsequently fixed and stained with 10 μM FITC-phalloidin in the presence of 0.1% Triton X-100. Fluorescence was then quantified by flow cytometry. Data are expressed as percentage of change in FITC fluorescence compared with samples exposed to buffer alone. Magnification of panels A, D, F, and I is 22 000 ×; B and E, 18 000 ×; C, 26 500 ×; G, 17 000 ×; and H, 15 000 ×.
Effects of cytochalasin E and latrunculin A on platelet ultrastructure and F-actin formation. Platelets were incubated in the presence of vehicle (A-C), 4 μM cytochalasin E (D-F), or 4 μM latrunculin A (G-I) for 20 minutes. Actin-disrupting agents had little effect on the morphology of resting platelets. (B) Platelets treated with SFLLRN for 10 minutes demonstrated pseudopod formation. A subpopulation demonstrated granule centralization but most showed only marked degranulation. Pretreatment with (E) cytochalasin E or (H) latrunculin A inhibited pseudopod formation. The OCS becomes expanded and enlarged under these conditions. (C) Platelets treated with PMA for 20 minutes demonstrated degranulation and pseudopod formation. Pretreatment with (F) cytochalasin E or (I) latrunculin A prevented formation of pseudopodia and resulted in enlargement and expansion of the OCS. (J) Platelets were incubated in the presence of vehicle (▪), 4 μM cytochalasin E (□), or 4 μM latrunculin A (▦) for 20 minutes. Platelets were then stimulated with buffer alone, SFLLRN for 10 minutes, or PMA for 20 minutes. Platelets were subsequently fixed and stained with 10 μM FITC-phalloidin in the presence of 0.1% Triton X-100. Fluorescence was then quantified by flow cytometry. Data are expressed as percentage of change in FITC fluorescence compared with samples exposed to buffer alone. Magnification of panels A, D, F, and I is 22 000 ×; B and E, 18 000 ×; C, 26 500 ×; G, 17 000 ×; and H, 15 000 ×.
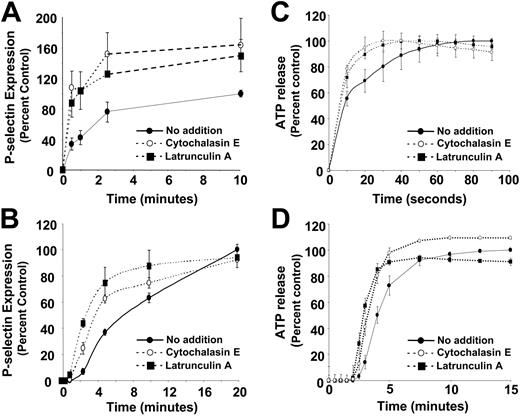
We next sought to determine the effects of disruption of the actin cytoskeleton on the degree and kinetics of granule secretion. Incubation of resting platelets with either cytochalasin E or latrunculin A alone failed to stimulate substantial α-granule secretion. However, incubation of platelets with either of these actin-disrupting agents increased both the degree and rate of SFLLRN-induced P-selectin expression (Figure 4A). Disruption of the cytoskeleton resulted in a 41% ± 18% increase in SFLLRN-induced α-granule secretion. Actin-disrupting agents also accelerated the rate of PMA-induced α-granule secretion (Figure 4B). In addition, exposure of platelets to cytochalasin E resulted in a 2.2- ± 0.7-fold decrease in the EC50 (effective concentration 50%) of SFLLRN in stimulating α-granule release, demonstrating that disruption of the actin cytoskeleton decreases agonist concentration requirements for α-granule secretion. Exposure to either cytochalasin E or latrunculin A resulted in modest acceleration of dense granule secretion in response to SFLLRN and PMA (Figure 4C-D). These reagents failed to augment either SFLLRN- or PMA-induced dense granule release. These results demonstrate that disruption of the actin cytoskeleton can accelerate and augment agonist-induced α-granule secretion and can, to a lesser extent, accelerate dense granule secretion.
Effects of disruption of the actin cytoskeleton on α-granule and dense granule secretion. (A) Platelets were incubated in the presence of either vehicle (•), 4 μM cytochalasin E (○), or 4 μM latrunculin A (▪) for 20 minutes. Platelets were then stimulated with 100 μM SFLLRN and subsequently fixed with paraformaldehyde at the indicated times. Samples were assayed for P-selectin expression by flow cytometry to monitor α-granule secretion. (B) Platelets were incubated in the presence of either vehicle (•), 4 μM cytochalasin E (○), or 4 μM latrunculin A (▪) for 20 minutes. Platelets were then stimulated with 0.2 μM PMA for the indicated times, fixed with paraformaldehyde, and assayed for P-selectin expression. (C) Platelets were incubated in the presence of either vehicle (•), 4 μM cytochalasin E (○), or 4 μM latrunculin A (▪) for 20 minutes. Platelets were then stimulated with 100 μM SFLLRN and assayed for ATP release using a luciferin-luciferase detection system to monitor dense granule release. (D) Platelets were incubated in the presence of either vehicle (•), 4 μM cytochalasin E (○), or 4 μM latrunculin A (▪) for 20 minutes. Platelets were then stimulated with 0.2 μM PMA and assayed for ATP release.
Effects of disruption of the actin cytoskeleton on α-granule and dense granule secretion. (A) Platelets were incubated in the presence of either vehicle (•), 4 μM cytochalasin E (○), or 4 μM latrunculin A (▪) for 20 minutes. Platelets were then stimulated with 100 μM SFLLRN and subsequently fixed with paraformaldehyde at the indicated times. Samples were assayed for P-selectin expression by flow cytometry to monitor α-granule secretion. (B) Platelets were incubated in the presence of either vehicle (•), 4 μM cytochalasin E (○), or 4 μM latrunculin A (▪) for 20 minutes. Platelets were then stimulated with 0.2 μM PMA for the indicated times, fixed with paraformaldehyde, and assayed for P-selectin expression. (C) Platelets were incubated in the presence of either vehicle (•), 4 μM cytochalasin E (○), or 4 μM latrunculin A (▪) for 20 minutes. Platelets were then stimulated with 100 μM SFLLRN and assayed for ATP release using a luciferin-luciferase detection system to monitor dense granule release. (D) Platelets were incubated in the presence of either vehicle (•), 4 μM cytochalasin E (○), or 4 μM latrunculin A (▪) for 20 minutes. Platelets were then stimulated with 0.2 μM PMA and assayed for ATP release.
Actin cytoskeleton in α-granule and dense granule secretion
It has previously been established that platelet α-granule contents are released at lower agonist concentrations than dense granule contents.33,34 The cause of the differential agonist sensitivity is unknown. We compared the effects of disruption of the resting actin cytoskeleton on α-granule and dense granule secretion. Epinephrine is a weak agonist that does not elicit α-granule secretion from untreated platelets. Following treatment of platelets with latrunculin A, however, epinephrine elicited an increase in P-selectin expression from platelets incubated with latrunculin A compared with platelets exposed to epinephrine alone (Figure 5). Incubation with latrunculin A also increased epinephrine-induced release of β-thromboglobulin from platelets (data not shown). Thus, actin disruption potentiated epinephrine-induced α-granule secretion. As observed with stimulation by other weak agonists, granule secretion induced by latrunculin A and epinephrine required autocrine stimulation because it was inhibited by apyrase and aspirin (data not shown). Epinephrine failed to elicit significant dense granule release even in the presence of latrunculin A (Figure 5). Similarly, incubation with latrunculin A stimulated P-selectin expression induced by ADP but failed to stimulate dense granule release in response to ADP as measured by a serotonin release assay (Figure 5). These results suggest that the actin cytoskeleton differentially regulates α-granule and dense granule release in response to weak agonists.
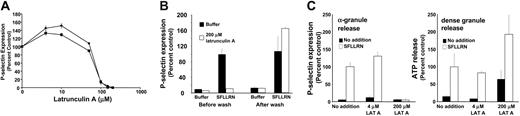
To further assess the differential effects of the actin cytoskeleton on α-granule and dense granule secretion, we evaluated granule secretion following incubation with increasing doses of actin cytoskeleton-disrupting reagents. Incubation of platelets with increasing concentrations of latrunculin A resulted in a biphasic response to SFLLRN-induced α-granule secretion. At relatively low concentrations of latrunculin A, α-granule secretion was augmented. At higher concentrations, however, latrunculin A inhibited α-granule secretion (Figure 6A). If platelets were washed following exposure to high concentrations of latrunculin A, they regained the ability to secrete α-granules in response to SFLLRN (Figure 6B). Thus, inhibition of SFLLRN-induced α-granule secretion by high concentrations of latrunculin A was not secondary to irreversible toxicity. Exposure of dense granules to low concentrations of latrunculin A failed to stimulate dense granule secretion (Figure 6C). Exposure to high concentrations of latrunculin A augmented SFLLRN-induced dense granule release. Furthermore, high concentrations of latrunculin A elicited dense granule secretion in the absence of SFLLRN exposure as monitored by either ATP release (Figure 6C) or 14C-serotonin release (data not shown). These results suggest that, while the resting actin cytoskeleton serves as a barrier to both α-granule and dense secretion, some actin polymerization is required for α-granule secretion but not necessarily dense granule secretion.
Actin cytoskeleton and SNARE protein-dependent granule secretion
Permeabilized cell models have been used to study the sequential steps required for granule secretion.35-38 An ATP-dependent step, termed priming, is required to stimulate secretion in response to an agonist. ATP does not stimulate secretion itself, but rather renders a system competent to secrete granules in response to a trigger such as Ca2+ or GTP-γ-S.17,39 In platelets, exposure to ATP has been proposed to be an absolute requirement for granule release from permeabilized platelets.40 If platelets are permeabilized for 15 minutes or more in the absence of ATP, they will no longer secrete granules in response to Ca2+ (Figure 7).41 Loss of responsiveness is secondary to diffusion of cytosolic ATP from the permeabilized platelet and can be reversed by addition of ATP to the incubation mixture (Figure 7). If the resting cytoskeleton serves as a barrier to granule secretion, we reasoned that disruption of this barrier may substitute for ATP in priming platelets for granule secretion triggered by Ca2+ or GTP-γ-S. In these experiments, platelets were incubated in the presence or absence of 2 μM latrunculin A and then permeabilized with SL-O for 15 minutes to allow diffusion of ATP from the platelet cytosol. Platelets were then exposed to Ca2+ or GTP-γ-S and assayed for P-selectin expression as a marker for α-granule release. P-selectin expression was observed from platelets incubated in the presence but not the absence of latrunculin A (Figure 7). Ca2+- or GTP-γ-S-triggered α-granule secretion from latrunculin A-treated platelets was approximately 50% that of α-granule secretion from platelets incubated with ATP and subsequently exposed to Ca2+ or GTP-γ-S (Figure 7). Latrunculin A-dependent granule secretion was inhibited by either phalloidin oleate or jasplakinolide, 2 reagents that stabilize the actin cytoskeleton (Figure 7C).31 These experiments demonstrate that disruption of the actin cytoskeleton is sufficient to partially prime platelets for Ca2+-or GTP-γ-S-triggered α-granule secretion.
Disruption of the actin cytoskeleton markedly augments α-granule secretion stimulated by weak agonists. Platelets were incubated in the presence (□) or absence (▪) of 4 μM latrunculin A for 15 minutes. Platelets were then incubated with the indicated agonists for 10 minutes and subsequently assayed for α-granule secretion and dense granule secretion. α-Granule secretion was monitored by assaying for P-selectin surface expression by flow cytometry. Dense granule secretion was monitored by assaying for 14C-serotonin release from 14C-serotonin-loaded platelets. Data are expressed as fold increase compared with samples treated with vehicle alone.
Disruption of the actin cytoskeleton markedly augments α-granule secretion stimulated by weak agonists. Platelets were incubated in the presence (□) or absence (▪) of 4 μM latrunculin A for 15 minutes. Platelets were then incubated with the indicated agonists for 10 minutes and subsequently assayed for α-granule secretion and dense granule secretion. α-Granule secretion was monitored by assaying for P-selectin surface expression by flow cytometry. Dense granule secretion was monitored by assaying for 14C-serotonin release from 14C-serotonin-loaded platelets. Data are expressed as fold increase compared with samples treated with vehicle alone.
High concentrations of latrunculin A inhibit α-granule secretion but stimulate dense granule secretion. (A) Platelets were incubated with the indicated concentration of latrunculin A for 20 minutes. Samples were then exposed to either 100 μM SFLLRN for 10 minutes (•) or 0.2 μM PMA for 20 minutes (▪). Platelets were subsequently analyzed for P-selectin expression by flow cytometry. (B) Platelets were incubated in the presence (□) or absence (▪)of200 μM latrunculin A for 20 minutes and divided into 2 aliquots. One aliquot (Before wash) was exposed to either buffer or 100 μM SFLLRN for 10 minutes as indicated. The second aliquot (After wash) was washed to remove latrunculin A and subsequently exposed to buffer or 100 μM SFLLRN for 10 minutes as indicated. Samples were assayed for P-selectin expression by flow cytometry. (C) Platelets were incubated with the indicated concentration of latrunculin A for 20 minutes. Platelets were then exposed to either buffer (▪) or 100 μM SFLLRN (□) for 10 minutes. P-selectin expression was assayed by flow cytometry to monitor α-granule release. ATP release as assayed using a luciferin-luciferase detection system was used to monitor dense granule release. Data are expressed as percentage of granule release compared to samples treated with agonist alone.
High concentrations of latrunculin A inhibit α-granule secretion but stimulate dense granule secretion. (A) Platelets were incubated with the indicated concentration of latrunculin A for 20 minutes. Samples were then exposed to either 100 μM SFLLRN for 10 minutes (•) or 0.2 μM PMA for 20 minutes (▪). Platelets were subsequently analyzed for P-selectin expression by flow cytometry. (B) Platelets were incubated in the presence (□) or absence (▪)of200 μM latrunculin A for 20 minutes and divided into 2 aliquots. One aliquot (Before wash) was exposed to either buffer or 100 μM SFLLRN for 10 minutes as indicated. The second aliquot (After wash) was washed to remove latrunculin A and subsequently exposed to buffer or 100 μM SFLLRN for 10 minutes as indicated. Samples were assayed for P-selectin expression by flow cytometry. (C) Platelets were incubated with the indicated concentration of latrunculin A for 20 minutes. Platelets were then exposed to either buffer (▪) or 100 μM SFLLRN (□) for 10 minutes. P-selectin expression was assayed by flow cytometry to monitor α-granule release. ATP release as assayed using a luciferin-luciferase detection system was used to monitor dense granule release. Data are expressed as percentage of granule release compared to samples treated with agonist alone.
Disruption of the cytoskeleton primes platelets for SNARE protein-mediated α-granule secretion. (A) Platelets were incubated in the presence of vehicle (no addition), 2 μM latrunculin A (LAT A), or 5 mM MgATP (ATP) and permeabilized with SL-O. Following a 15-minute incubation, platelets were exposed to buffer (□) or 10 μM Ca2+ (▪) and assayed for P-selectin expression by flow cytometry. P-selectin expression after exposure to MgATP alone followed by Ca2+ represents 100%. (B) Platelets were incubated in the presence of vehicle (no addition), 2 μM latrunculin A (LAT A), or 5 mM MgATP (ATP) and permeabilized with SL-O. Following a 15-minute incubation, platelets were exposed to buffer (□) or 33 μM GTP-γ-S (▪) and assayed for P-selectin expression by flow cytometry. P-selectin expression after exposure to MgATP alone followed by Ca2+ represents 100%. (C) Platelets were incubated with either vehicle (▪), 8 μM jasplakinolide (▨), or 100 μM phalloidin oleate (□) for 20 minutes. Platelets were then permeabilized in the presence of vehicle (no addition) or 2 μM latrunculin A (LAT A). Following a 15-minute incubation, platelets were exposed to 10 μM Ca2+ and assayed for P-selectin expression. P-selectin expression after exposure to latrunculin A alone followed by Ca2+ represents 100%. (D) Platelets were incubated with either buffer (lane 1), 50μg/mL nonimmune IgG (lane 2), or 50 μg/mL anti-VAMP antibody (lane 3). Samples were permeabilized in the presence of latrunculin A for 15 minutes, exposed to 10 μM Ca2+ (▪) or 33 μM GTP-γ-S (□), and then assayed for P-selectin expression. P-selectin expression after exposure to latrunculin A alone followed by agonist represents 100%.
Disruption of the cytoskeleton primes platelets for SNARE protein-mediated α-granule secretion. (A) Platelets were incubated in the presence of vehicle (no addition), 2 μM latrunculin A (LAT A), or 5 mM MgATP (ATP) and permeabilized with SL-O. Following a 15-minute incubation, platelets were exposed to buffer (□) or 10 μM Ca2+ (▪) and assayed for P-selectin expression by flow cytometry. P-selectin expression after exposure to MgATP alone followed by Ca2+ represents 100%. (B) Platelets were incubated in the presence of vehicle (no addition), 2 μM latrunculin A (LAT A), or 5 mM MgATP (ATP) and permeabilized with SL-O. Following a 15-minute incubation, platelets were exposed to buffer (□) or 33 μM GTP-γ-S (▪) and assayed for P-selectin expression by flow cytometry. P-selectin expression after exposure to MgATP alone followed by Ca2+ represents 100%. (C) Platelets were incubated with either vehicle (▪), 8 μM jasplakinolide (▨), or 100 μM phalloidin oleate (□) for 20 minutes. Platelets were then permeabilized in the presence of vehicle (no addition) or 2 μM latrunculin A (LAT A). Following a 15-minute incubation, platelets were exposed to 10 μM Ca2+ and assayed for P-selectin expression. P-selectin expression after exposure to latrunculin A alone followed by Ca2+ represents 100%. (D) Platelets were incubated with either buffer (lane 1), 50μg/mL nonimmune IgG (lane 2), or 50 μg/mL anti-VAMP antibody (lane 3). Samples were permeabilized in the presence of latrunculin A for 15 minutes, exposed to 10 μM Ca2+ (▪) or 33 μM GTP-γ-S (□), and then assayed for P-selectin expression. P-selectin expression after exposure to latrunculin A alone followed by agonist represents 100%.
We and others have shown that ATP-dependent platelet granule secretion requires SNARE proteins.15,16,23,42,43 We next sought to determine whether membrane fusion that occurs following disruption of the actin cytoskeleton requires SNARE proteins or whether membrane fusion following disruption of the actin cytoskeleton occurs via a SNARE protein-independent pathway. For these studies, we used an antibody directed against VAMP that has previously been shown to inhibit platelet granule secretion.15 Platelets were incubated in the presence of latrunculin A to disrupt the actin cytoskeleton and were subsequently permeabilized in the presence or absence of anti-VAMP antibody. Following a 15-minute incubation, samples were stimulated with either Ca2+ or GTP-γ-S. Both Ca2+-and GTP-γ-S-triggered α-granule secretion from permeabilized platelets was inhibited by anti-VAMP antibodies (Figure 7). These data demonstrate that the actin cytoskeleton serves as a functional barrier to SNARE protein-dependent granule secretion in platelets.
Actin on the granule surface
In nucleated cells, exocytosis generally occurs via fusion of granule membranes directly with plasma membranes or via fusion between granule membranes followed by fusion with the plasma membrane. In platelets, fusion of granule membranes with other granules and with the preformed OCS represents an important mode of granule secretion.44,45 These observations raise the possibility that an F-actin barrier surrounding the granule itself regulates granule secretion. To test the hypothesis that granules are coated with F-actin, we performed subcellular fractionation to isolate fractions enriched in OCS and plasma membranes (fraction 1), lysosomes (fraction 2), α-granules (fraction 3), and dense granules (fraction 4) (Figure 8A). Fractions were exposed to FITC-phalloidin and analyzed by flow cytometry. Staining of OCS and plasma membranes as well as lysosomes was similar to that of synthetic phospholipid micelles devoid of actin (Figure 8B). In contrast, α-granules were readily stained with FITC-phalloidin (Figure 8B). Staining of the α-granule preparation with FITC-phalloidin was inhibited in a dose-dependent manner by latrunculin A (data not shown), confirming the presence of F-actin on the α-granule surface. FITC-phalloidin binding to dense granules was equivocal and not significantly reversed by latrunculin A. These data are consistent with the supposition that platelet α-granules are coated with F-actin.
F-actin coats purified platelet α-granules. (A) Platelets were subjected to nitrogen cavitation and subcellular fractionation as described in “Materials and methods.” Plasma membranes and OCS from biotin-labeled platelets were identified in fraction 1 by staining with HRP-avidin. Lysosomes and dense granules were identified primarily in fractions 2 and 4 by using anti-LAMP-1 antibody. α-Granules were identified in fraction 3 by using antibodies directed at the P-selectin. Dense granules were identified in fraction 4 following subcellular fractionation of 14C-serotonin-labeled platelets. (B) Fractions 1 (OCS and plasma membrane-enriched), 2 (lysosome-enriched), 3 (α-granule-enriched), 4 (dense granule-enriched), phosphatidylcholine (PC) micelles or phosphatidylserine (PS) micelles were incubated in the presence (□) or absence (▪) of 32 μM latrunculin A for 20 minutes. Samples were then incubated with 10 μM FITC-phalloidin for 20 minutes and analyzed by flow cytometry.
F-actin coats purified platelet α-granules. (A) Platelets were subjected to nitrogen cavitation and subcellular fractionation as described in “Materials and methods.” Plasma membranes and OCS from biotin-labeled platelets were identified in fraction 1 by staining with HRP-avidin. Lysosomes and dense granules were identified primarily in fractions 2 and 4 by using anti-LAMP-1 antibody. α-Granules were identified in fraction 3 by using antibodies directed at the P-selectin. Dense granules were identified in fraction 4 following subcellular fractionation of 14C-serotonin-labeled platelets. (B) Fractions 1 (OCS and plasma membrane-enriched), 2 (lysosome-enriched), 3 (α-granule-enriched), 4 (dense granule-enriched), phosphatidylcholine (PC) micelles or phosphatidylserine (PS) micelles were incubated in the presence (□) or absence (▪) of 32 μM latrunculin A for 20 minutes. Samples were then incubated with 10 μM FITC-phalloidin for 20 minutes and analyzed by flow cytometry.
Discussion
Our studies indicate that the resting actin cytoskeleton interferes with agonist-induced granule secretion. This conclusion is based on the observation that disruption of the actin cytoskeleton by 2 structurally independent small molecules, latrunculin A and cytochalasin E, can facilitate platelet granule secretion. Several consequences of this barrier function are evident. The actin cytoskeleton serves to slow the release of both α-granules and dense granules from platelets, as evidenced by the observation that disruption of the actin cytoskeleton results in acceleration of granule secretion. In addition, the actin cytoskeleton inhibits α-granule release in response to weak agonists. For example, the actin cytoskeleton prevents any significant secretion in response to epinephrine or ADP. Upon disruption of the cytoskeleton, either epinephrine or ADP is able to elicit significant secretion of α-granules (Figure 4). Disruption of the actin cytoskeleton also results in augmentation of α-granule secretion induced by strong agonists such as the protease-activated receptor 1 (PAR-1) agonist, SFLLRN. The same concentrations of latrunculin A that elicited these augmenting effects on α-granule secretion failed to influence dense granule release. Higher concentrations of latrunculin A, however, stimulated dense granule release (Figure 5). Finally, disruption of the actin cytoskeleton enhances the sensitivity of platelets to agonists so that they will respond to lower concentrations of agonists. Thus, disruption of the actin cytoskeleton can accelerate, augment, and decrease agonist requirements for platelet granule secretion. The intact actin cytoskeleton may restrict inappropriate release of thrombogenic substances from platelets.
The assertion that the resting actin cytoskeleton opposes granule secretion is further supported by the observation that disruption of this cytoskeleton facilitates granule secretion from permeabilized platelets. Induction of granule secretion from permeabilized platelets can be elicited in the absence of ligand engagement of surface receptors. This strategy enables distal events involved in granule secretion to be experimentally isolated and analyzed. The observation that latrunculin A can, in part, substitute for ATP in rendering permeabilized platelets capable of granule secretion in response to Ca2+ or GTP-γ-S raises the possibility that disruption of the cytoskeleton contributes to ATP-dependent priming. Alternatively, membrane fusion in latrunculin A-treated platelets may proceed through a different mechanism. The fact that anti-VAMP antibodies inhibit both ATP-dependent15,16,23 and latrunculin A-dependent (Figure 7) granule secretion from permeabilized platelets demonstrates that granule secretion following disruption of the actin cytoskeleton requires SNARE proteins.
The results of these studies indicate the complexity of the relationship between cytoskeletal reorganization and platelet granule secretion. Our data demonstrate that, while relatively low doses of actin-disrupting reagents facilitate α-granule secretion, high doses inhibit α-granule secretion. The biphasic response of platelet α-granule secretion to increasing disruption of the actin cytoskeleton suggests that, while the resting actin cytoskeleton opposes granule secretion, some degree of actin polymerization is required for α-granule secretion.46 A similar biphasic response to actin-disrupting agents has previously been observed in pancreatic acinar cells.47 Disruption of actin filaments with high concentrations of latrunculins A and B inhibits insulin-stimulated membrane fusion required for translocation of glucose transporter 4 (GLUT-4) in adipocytes.48,49 In purified yeast vacuoles, actin depolymerization promotes SNARE protein interactions, and subsequent actin polymerization facilitates membrane fusion.50,51 These observations suggest that actin is required in a terminal event leading to membrane fusion.50,51 Unlike its effect on α-granule secretion, we observe that exposure of platelets to high concentrations of latrunculin A does not inhibit agonist-induced dense granule release, but rather stimulates release of dense granules. Thus, activation-induced actin polymerization is not necessarily required for dense granule release. In the absence of a requirement for actin polymerization, disruption of the actin cytoskeleton elicited by high concentrations of latrunculin A is sufficient to enable membrane fusion in the absence of a platelet agonist. The molecular differences between α-granules and dense granules that underlie their differential regulation by the actin cytoskeleton remain to be elucidated.
What is the nature of the cytoskeletal barrier that controls platelet granule secretion? The fact that reagents that target the actin cytoskeleton affect granule secretion under a variety of experimental conditions demonstrate that actin is an important component of this barrier. Actin accounts for approximately 35% of platelet protein, and approximately 40% of actin in the resting platelet is organized into filaments that both fill the cytosol and underlie the plasma membrane.52,53 A ring of cortical actin that lines the cytosolic face of the plasma membrane of neuroendocrine cells is thought to serve as a barrier to secretion and has been shown to dissolve prior to exocytosis.54-56 In platelets, however, granules become centralized following platelet activation and a substantial degree of secretion occurs via fusion of granule membranes with membranes of the OCS.44,45 It is unclear how the resting cortical cytoskeleton could interfere with this pathway of granule release. Whether membranes of the OCS are supported by an underlying actin cytoskeleton is unknown. In some cells, filamentous actin is associated directly with secretory granules.57 It is possible that granule-associated actin serves a barrier function in platelet granule secretion. In support of this model, we find that isolated platelet α-granules are coated with F-actin (Figure 8). F-actin expressed on the surface of the granules may serve as a barrier to membrane fusion. The role of the granular actin coat in secretion, however, remains to be determined. The roles of the membrane skeleton and microtubule system must also be considered in the context of regulation of granule secretion. Future studies will determine whether the cytoplasmic, plasma membrane, and/or granule-associated actin as well as the membrane skeleton or microtubules contribute to the barrier effect of the resting cytoskeleton.
Prepublished online as Blood First Edition Paper, January 25, 2005; DOI 10.1182/blood-2004-04-1392.
Supported by the National Institutes of Health (grants AI33372 and AI44066) (A.M.D.), (grant HL63250) (R.F.). R.F. is a recipient of an American Society of Hematology Junior Faculty Scholar Award and the Burroughs Wellcome Fund Career Award.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.