Abstract
Imatinib mesylate is highly effective in newly diagnosed chronic myeloid leukemia (CML), but BCR/ABL (breakpoint cluster region/abelson murine leukemia)–positive progenitors persist in most patients with CML treated with imatinib mesylate, indicating the need for novel therapeutic approaches. In this study, we have used the murine CML-like myeloproliferative disorder as a platform to characterize the pharmacokinetic, signal transduction, and antileukemic properties of PD166326, one of the most potent members of the pyridopyrimidine class of protein tyrosine kinase inhibitors. In mice with the CML-like disease, PD166326 rapidly inhibited Bcr/Abl kinase activity after a single oral dose and demonstrated marked antileukemic activity in vivo. Seventy percent of PD166326-treated mice achieved a white blood cell (WBC) count less than 20.0 × 109/L (20 000/μL) at necropsy, compared with only 8% of imatinib mesylate–treated animals. Further, two thirds of PD166326-treated animals had complete resolution of splenomegaly, compared with none of the imatinib mesylate–treated animals. Consistent with its more potent antileukemic effect in vivo, PD166326 was also superior to imatinib mesylate in inhibiting the constitutive tyrosine phosphorylation of numerous leukemia-cell proteins, including the src family member Lyn. PD166326 also prolonged the survival of mice with imatinib mesylate–resistant CML induced by the Bcr/Abl mutants P210/H396P and P210/M351T. Altogether, these findings demonstrate the potential of more potent Bcr/Abl inhibitors to provide more effective antileukemic activity. Clinical development of PD166326 or a related analog may lead to more effective drugs for the treatment of de novo and imatinib mesylate–resistant CML.
Introduction
The tyrosine kinase inhibitor imatinib mesylate (Gleevec; Novartis) has recently become the standard of care for the treatment of chronic myeloid leukemia (CML). In the International Randomized Interferon versus STI571 Study (IRIS) trial, imatinib mesylate achieved a complete cytogenetic response rate of 76% in newly diagnosed CML patients, almost 6-fold higher than interferon-α plus arabinosyl cytosine (Ara-C).1,2 Imatinib mesylate was also superior to interferon-α/Ara-C in progression-free survival and in reducing BCR/ABL (breakpoint cluster region/abelson murine leukemia) transcript levels.3 However, the success rate of imatinib mesylate in more advanced stages of CML has been more modest. Imatinib mesylate had a complete cytogenetic response rate of 13% to 24% in accelerated-phase CML2,4,5 and only 7% in CML in blastic phase.6 Further, complete cytogenetic responses in CML blast crisis were generally not durable, particularly in patients with lymphoid blast crisis.6-8 Although resistance to imatinib mesylate is more prevalent in patients with advanced CML, some patients have relapsed on imatinib mesylate while still in chronic phase. Mutations in the ABL kinase domain of BCR/ABL have been found in many patients with CML relapsing on imatinib mesylate,9-12 but Bcr/Abl overexpression,13 clonal evolution,14-17 or other Bcr/Abl-independent factors18-20 likely play a role in some imatinib mesylate failures.21-24 Taken together, these studies indicate that new drugs are still needed for the optimal treatment of CML.
In contrast to the 2-phenylaminopyrimidine kinase inhibitor drugs like imatinib mesylate, the pyrido[2,3-d]pyrimidines (Figure 1) belong to a different group of compounds that were originally characterized as inhibitors of the fibroblast growth factor receptor, epidermal growth factor receptor, platelet-derived growth factor receptor, and c-src protein tyrosine kinases.25-27 Certain members of this class of compounds have been shown to be potent inhibitors of the c-Abl and Bcr/Abl tyrosine kinases. PD180970 inhibited Bcr/Abl tyrosine phosphorylation at an IC50 (inhibitory concentration of 50%) of 170 nM and induced apoptosis of the human CML cell line K562.28 PD180970 also had activity against imatinib mesylate–resistant K562 cells and Ba/F3-P210 cells expressing BCR/ABL kinase domain mutations found in patients with CML resistant to imatinib mesylate.29 Another pyridopyrimidine, PD173955, inhibited proliferation of the Bcr/Abl cell line R10(-) at an IC50 of 2.5 nM, compared with an IC50 of 40 nM for imatinib mesylate.30 Of the 6 pyridopyrimidines examined in that latter study, PD166326 was the most potent inhibitor of CD34+ CML progenitor growth, with an IC50 approximately 4-fold lower than PD173955. In K562 cells, PD166326 inhibited K562 proliferation with an IC50 of 300 pM31 and had activity against several imatinib mesylate–resistant Bcr/Abl mutants.31,32 However, the pharmacokinetics, activity, or tolerability of PD166326 in vivo has not been established.
Chemical structures for the pyrido[2,3-d]pyrimidine PD166326 and the 2-phenylaminopyrimidine imatinib mesylate.
Chemical structures for the pyrido[2,3-d]pyrimidine PD166326 and the 2-phenylaminopyrimidine imatinib mesylate.
To determine the potential of pyridopyrimidines as anti-CML drugs, we have studied the antileukemic activity of PD166326 in the murine bone marrow (BM) retroviral transduction and transplantation model of CML. In this model, mice reconstituted with primary P210BCR/ABL-transduced murine BM cells develop a CML-like illness that mimics many of the central features of the human disease, including responsiveness to imatinib mesylate therapy.33,34 In this report, we find that orally administered PD166326 is well tolerated and promptly reaches concentrations sufficient to inhibit Bcr/Abl kinase activity in vivo. Although PD166326 and imatinib mesylate each prolonged the survival of mice with the CML-like myeloproliferative disorder, PD166326 was superior to imatinib mesylate in controlling the peripheral blood granulocytosis and splenomegaly characteristic of this disease. The activity of PD166326 against mutant forms of BCR/ABL was also investigated in a newly developed murine model of imatinib mesylate–resistant CML.
Materials and methods
Murine bone marrow transduction and transplantation model of CML
Animals were treated according to an established animal protocol approved by the Institutional Animal Care and Use Committee at the University of Texas Southwestern Medical Center. The methodologic details of the murine BM transduction and transplantation model of CML have been extensively described elsewhere.33,35,36 In brief, this model uses a replication-defective BCR/ABL retrovirus to transduce primary murine BM cells, leading to a CML-like disease in lethally irradiated syngeneic recipients. The survival end point was determined by either spontaneous death of the animal or because of elective killing of the animal because of signs of pain or suffering, using established criteria. In experiments with wild-type P210-induced CML, PD16326- and imatinib mesylate–treated mice were electively killed on days 33 to 37 and subjected to complete necropsy to quantitate the extent of leukemia. To compare the white blood cell count WBC count and spleen weights of PD166326-, imatinib mesylate–, and control-treated mice, the median WBC count and spleen weights were calculated and analyzed by the Kruskal-Wallis (nonparametric) and Dunn multiple comparison tests by using the software Prism (GraphPad Software, San Diego, CA). In the murine imatinib mesylate–resistant CML model, the end point was survival, and data were analyzed by the method of Kaplan and Meier.
Drug formulation, administration, and quantitation
PD166326 was synthesized in the Core Synthesis Laboratory at Memorial Sloan-Kettering Cancer Center as previously described.25 Because of limited water solubility, PD166326 was first dissolved in dimethyl sulfoxide (DMSO; Sigma, St Louis, MO), and then water was added with gentle vortexing to achieve a final mixture of 90% water/10% DMSO (vol/vol). The resulting suspension was administered to mice by gavage without further manipulation. The dose range of PD166326 was 25 to 50 mg/kg twice a day (described in greater detail in “Results”). The tyrosine kinase inhibitor imatinib mesylate (Novartis, Basel, Switzerland) was prepared fresh in sterile water as previously described.33 The imatinib mesylate dose level of 200 mg/kg per day was chosen for comparison against PD166326 because it represented a comparable dose of 750 to 833 mg per day in humans,37 the high-dose regimen currently under study in several human trials.38 Moreover, prohibitive weight loss was noted in mice chronically dosed at greater than 200 mg imatinib mesylate/kg a day (data not shown). All drugs were administered in a volume of 250 μL by oral gavage twice a day. Placebo-treated mice received the same regimen of 90% water/10% DMSO alone. For the [3H]thymidine assays, PD166326 and imatinib mesylate were dissolved in pure DMSO.
The analysis of PD166326 in plasma was performed with an automated liquid chromatography–mass spectrometry (LC-MS) system, which consists of a Prospekt-2 automated sample processor (Spark-Holland, Plainsboro, NJ) interfaced with an Agilent 1100 LC-MS (Agilent Technologies, Palo Alto, CA). The Prospekt-2 consists of 2 parts, a high-pressure eluter and an automated cartridge exchanger, which performs automated solidphase extraction with an HD-C18 cartridge (Spark-Holland). The Agilent unit consists of a binary pump, an autosampler, a photodiode array detector, and a mass spectrometer detector SL (MSD-SL) mass spectrometer. Mouse plasma samples were diluted with an equal volume of water for analysis. The high-performance liquid chromatography mobile phase for the analysis of PD166326 consisted of 55% acetonitrile and 45% (vol/vol) 0.1% formic acid at a flow rate of 0.4 mL/minute and was analyzed with a Zorbax Stable Bond C8 column 4 × 80 mm (MAC-MOD Analytical, Chadds Ford, PA). The eluent was monitored with atmospheric pressure chemical ionization positive ion single ion monitoring at m/z 427. PD166326 concentrations were measured from plasma samples from at least 3 mice per dose level, converted from nanogram per milliliter to nanomolar, and expressed as the mean ± standard error. Estimation of PD166326 half-life (t1/2) was determined by using the drug concentrations (Cs) between the 8- and 15-hour time points at steady state according to the formula kelim = ln(C15) - ln(C8)/tinterval, where t1/2 = 0.693/kelim.
Cell lines
The MO7E/P210-derived cell line R10(-) has been previously described.39 R10(-) cells were propagated in Iscoves modified Dulbecco medium (Life Technologies, Grand Island, NY) supplemented with 10% heat-inactivated fetal calf serum (Hyclone, Logan, UT). Parental MO7E cells were propagated in Dulbecco modified Eagle medium supplemented with 20% heat-inactivated fetal calf serum and 10 ng/mL granulocyte-macrophage colony stimulation factor (Immunex Corporation, Seattle, WA), and 2 mM glutamine.
[3H]thymidine proliferation assay
The effect of PD166326 or imatinib mesylate on R10(-) and MO7e proliferation was determined by [3H]thymidine assay as previously described.30 Briefly, cells were plated in triplicate at a density of 104 cells/well in 96-well plates and treated with a range of PD166326 and imatinib mesylate concentrations or control (DMSO). After 48 hours, [3H]thymidine was added to each well for 18 hours, and incorporation was quantitated by using a Packard scintillation counter (Packard Instrument, Downers Grove, IL). After correction for background, count per minute (CPM) values were determined for each sample and normalized relative to control-treated samples. CPM as percentage of control was then plotted versus the log10 concentration of drug. The IC50 values were calculated according to the Hill equation, using the software Prism (GraphPad Software).
Vectors, retroviral generation, and titer
Primary murine BM cells were transduced with a helper-free, replication-defective P210BCR/ABL retrovirus (MSCV/P210-neo) generated by transient transfection of 293T cells with a P210 retroviral construct and an ecotropic retroviral packing construct, as previously described.40 To generate a murine model of imatinib mesylate–resistant CML, murine BM cells were transduced with a retroviral vector expressing 1 of the P210 mutants implicated in human imatinib mesylate–resistant CML. P210/H396P was generated by site-directed mutagenesis and cloned into the EcoRI site of the retroviral vector MIGRI.41 P210/M351T and P210/T315I were constructed by liberating the P210 mutant cDNAs from the vector pSR-α (kindly provided by Brian Druker, Oregon Health and Science University, Portland, OR) as EcoRI fragments and cloning them into MIGRI. The integrity of all P210 mutant constructs was confirmed by restriction enzyme digestion and direct DNA sequencing.
Western immunoblot
Hematopoietic cells from peripheral blood, spleen, and BM were lysed in RIPA buffer (50 mM Tris (tris(hydroxymethyl)aminomethane) pH 7.4, 150 mM NaCl, 1% sodium deoxycholate, 1 mM EDTA (ethylenediaminetetraacetic acid), 1% Triton-X, 0.1% SDS (sodium dodecyl sulfate)) containing 1 mM phenylmethylsulfonyl fluoride, 1% aprotinin (vol/vol), 25 mM sodium fluoride, 1 mM sodium orthovanadate, 1 μg/mL pepstatin, 5 μg/mL leupeptin, and 2 mM Pefabloc SC (Roche Diagnostics, Mannheim, Germany). RIPA lysates were normalized by OD595 (Bio-Rad Protein Assay; Bio-Rad Laboratories, Hercules, CA). For Bcr/Abl expression analysis, cells were lysed directly in 2 × sample buffer, briefly sonicated, and centrifuged for 10 minutes at 12 000g at 4°C to remove insoluble debris. Lysates were normalized by OD595 using parallel RIPA-prepared lysates. Lysates were resolved by SDS/polyacrylamide gel electrophoresis and electrophoretically transferred to a nitrocellulose membrane overnight. Antibodies used in this study were anti-Abl (Oncogene Research Products, Boston, MA), anti-phosphotyrosine (4G10; Upstate Biotechnology, Lake Placid, NY), anti-actin (Santa Cruz Biotechnology, Santa Cruz, CA), anti–Crk-L (Santa Cruz Biotechnology, Santa Cruz, CA), anti–extracellular signal-related kinase (ERK)1/2, anti-pERK, anti-Lyn, and anti-pLyn (all from Cell Signaling Technology, Beverly, MA). Immunoreactivity was detected by enhanced chemiluminescence (Amersham, Piscataway, NJ). The ratios of P210 tyrosine phosphorylationp to total P210 expression, pERK/ERK (sum of ERK1/2), and pCrk-L/Crk-L were generated by densitometric analysis of their respective Western immunoblots using National Institutes of Health (NIH) image software.
Southern blot
Genomic DNA was prepared from murine leukemia cells using a Tris/SDS/proteinase K lysis buffer, followed by 2 rounds of phenol-chloroformisoamyl alcohol extraction. Genomic DNA (10 μg) was digested with BglII to assess the clonality of proviral integration as previously described.33 Samples were resolved on a 0.8% tris-borate-EDTA gel, and complete DNA digestion and equivalent sample loading were confirmed by ethidium bromide staining. Nucleic acids were transferred to a nylon membrane (Zetaprobe; Bio-Rad) by capillary transfer overnight. Membranes were hybridized with a 32P-labeled fragment of the neomycin resistance gene (Radprime DNA labeling system; Gibco BRL, Grand Island, NY) and analyzed by autoradiography.
Results
PD166326 is a more potent inhibitor of Bcr/Abl- and stem cell factor–dependent proliferation than imatinib mesylate
The range of PD166326 concentrations required for activity against Bcr/Abl-expressing cells was determined in R10(-) cells, a cell line derived from P210-MO7e.39 Unlike MO7e cells, R10(-) cells can proliferate in the absence of supplemental cytokine because of the presence of P210.42 By [3H]thymidine incorporation assay, PD166326 had activity against R10(-) cells at concentrations as low as 0.1 nM and completely blocked R10(-) growth at 2.5 nM, for an IC50 of 0.2 nM (Figure 2A).
In parental MO7e cells, PD166326 inhibited stem cell factor (SCF)–dependent proliferation at 5 to 10 nM and completely suppressed SCF-dependent growth at 50 nM, for an IC50 of 12 nM (Figure 2B). Thus, the IC50 of PD166326 for Bcr/Abl-dependent growth was approximately 60-fold lower than the IC50 for c-Kit–mediated growth. Imatinib mesylate, in contrast, had a more similar effect on Bcr/Abl- and c-Kit–dependent proliferation, with an IC50 of 19 nM in R10(-) cells and 82 nM in MO7e cells growing in the presence of SCF (KL, Kit ligand), respectively.43,44 As a result, PD166326 was 95 times more potent in inhibiting Bcr/Abl-dependent growth than imatinib mesylate, but only 6.8 times more potent than imatinib mesylate in blocking c-Kit–mediated proliferation.
PD166326 rapidly achieves therapeutic concentrations in mice after oral administration
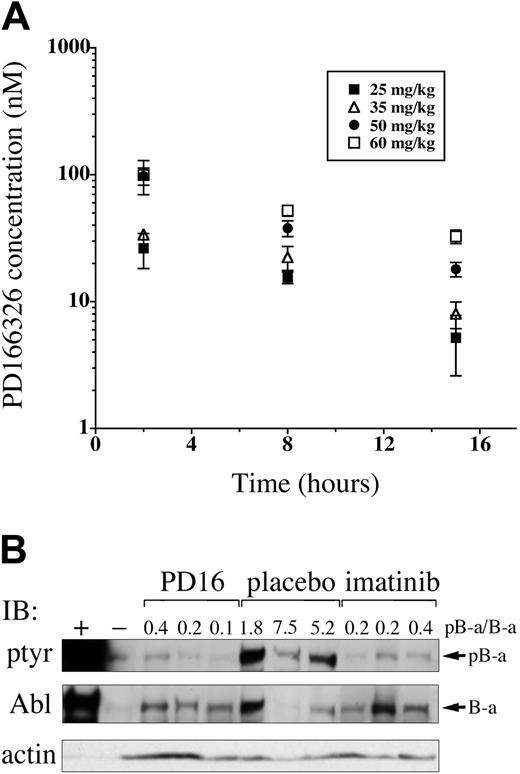
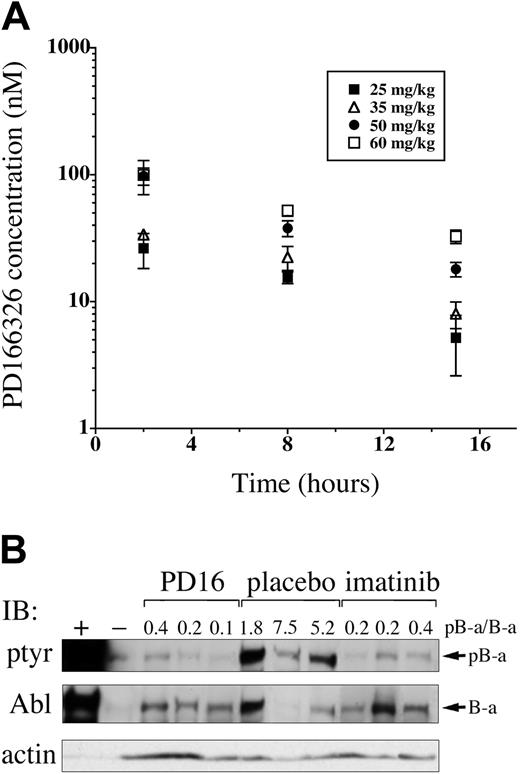
Pyridopyrimidines are potent inhibitors of Bcr/Abl-expressing cell lines in vitro28-32,45 ; however, the pharmacokinetics and in vivo activity of this class of tyrosine kinase inhibitors has not been established. To determine the concentrations of PD166326 achievable in vivo, and to assess tolerability, PD166326 was administered to normal Balb/c mice at a dose of 25 mg/kg, 35 mg/kg, 50 mg/kg, or 60 mg/kg twice a day by oral gavage. Because of limited water solubility, PD166326 was administered as an oral suspension of 90% water and 10% DMSO. After 4 days of treatment, mice were killed, and plasma samples were obtained 2, 8, and 15 hours after the last dose. PD166326 reached concentrations in the expected therapeutic range (Figure 2) within 2 hours after oral administration, with mean PD166326 concentrations of 26 (± 8), 34 (± 3), 98 (± 15), and 100 (± 30) nM at the 25, 35, 50, and 60 mg/kg dose levels, respectively (Figure 3A).
At 15 hours, PD166326 concentrations were still detectable, ranging from 5 (± 3) and 8 nM (± 2) in the 25 mg/kg–and 35 mg/kg–dosed mice to 18 (± 2) and 33 (± 40) nM in the 50 mg/kg–and 60 mg/kg–dosed animals. On the basis of the 8- and 15-hour time points, the half-life of PD166326 in mice was 8.4 hours (range, 6.5-10.3) at steady state. Thus, oral PD166326 reached concentrations within the expected therapeutic range for Bcr/Abl-expressing cells at all dose levels tested. Because additional studies revealed weight loss in mice chronically dosed at 60 mg/kg twice a day (data not shown), 50 mg/kg twice a day was considered the maximum-tolerated dose of PD166326 for subsequent in vivo leukemia studies.
PD166326 is a more potent inhibitor of Bcr/Abl- and stem cell factor–dependent proliferation than imatinib mesylate. (A) The P210-expressing MO7e cell line R10(-) growing in the absence of exogenous cytokine, and (B) parental MO7e cells growing in the presence of stem cell factor (MO7e/KL) were treated with the indicated concentrations of PD166326 (•) and imatinib mesylate (▴) for 48 hours and then analyzed by [3H]thymidine incorporation as described in “Materials and methods.” Results are expressed as the counts per minute (CPM) relative to cells treated with diluent alone (DMSO) versus the concentration (log10 scale) of PD166326 or imatinib mesylate. The calculated IC50 for each drug is shown. The results depicted are representative of multiple independent experiments.
PD166326 is a more potent inhibitor of Bcr/Abl- and stem cell factor–dependent proliferation than imatinib mesylate. (A) The P210-expressing MO7e cell line R10(-) growing in the absence of exogenous cytokine, and (B) parental MO7e cells growing in the presence of stem cell factor (MO7e/KL) were treated with the indicated concentrations of PD166326 (•) and imatinib mesylate (▴) for 48 hours and then analyzed by [3H]thymidine incorporation as described in “Materials and methods.” Results are expressed as the counts per minute (CPM) relative to cells treated with diluent alone (DMSO) versus the concentration (log10 scale) of PD166326 or imatinib mesylate. The calculated IC50 for each drug is shown. The results depicted are representative of multiple independent experiments.
Orally administered PD166326 rapidly reaches concentrations sufficient to inhibit Bcr/Abl tyrosine phosphorylation in vivo. (A) PD166326 was administered to Balb/c mice at the indicated doses (▪, 25 mg/kg; ▵, 35 mg/kg; •, 50 mg/kg; □, 60 mg/kg) twice a day by oral gavage, and plasma PD166326 concentrations were determined at 2, 8, and 15 hours after the last dose at steady state. The error bars indicate the standard error from 3 mice at each dose level. (B) Peripheral blood leukemia cells from mice with the CML-like disease were analyzed by anti-phosphotyrosine (ptyr) or anti-Abl Western immunoblot after a single dose of PD166326 (50 mg/kg), imatinib mesylate (100 mg/kg), or placebo. The ratio of tyrosine-phosphorylated P210 (pB-a) to P210 (B-a) signal was determined by densitometry and is shown above each lane. Ba/F3 cells expressing P210 (+) or not (-) are shown as controls at far left. Anti-actin immunoblot serves as a loading control. Note that the level of P210 expression is not necessarily identical in all animals.33
Orally administered PD166326 rapidly reaches concentrations sufficient to inhibit Bcr/Abl tyrosine phosphorylation in vivo. (A) PD166326 was administered to Balb/c mice at the indicated doses (▪, 25 mg/kg; ▵, 35 mg/kg; •, 50 mg/kg; □, 60 mg/kg) twice a day by oral gavage, and plasma PD166326 concentrations were determined at 2, 8, and 15 hours after the last dose at steady state. The error bars indicate the standard error from 3 mice at each dose level. (B) Peripheral blood leukemia cells from mice with the CML-like disease were analyzed by anti-phosphotyrosine (ptyr) or anti-Abl Western immunoblot after a single dose of PD166326 (50 mg/kg), imatinib mesylate (100 mg/kg), or placebo. The ratio of tyrosine-phosphorylated P210 (pB-a) to P210 (B-a) signal was determined by densitometry and is shown above each lane. Ba/F3 cells expressing P210 (+) or not (-) are shown as controls at far left. Anti-actin immunoblot serves as a loading control. Note that the level of P210 expression is not necessarily identical in all animals.33
PD166326 inhibits P210 tyrosine phosphorylation in primary murine leukemia cells in vivo
Upon activation, P210 becomes autophosphorylated on multiple tyrosines, making P210 tyrosine-phosphorylation a useful indicator of Bcr/Abl kinase activity.46-49 To test the biologic activity of PD166326 in vivo, mice with established CML were given a single oral dose of 50 mg PD166326/kg. Two to 3 hours later, the animals were killed, and peripheral blood leukemia cells were obtained and analyzed by anti-phosphotyrosine and anti-Abl immunoblot. Consistent with previous reports, Bcr/Abl was strongly tyrosine phosphorylated in placebo-dosed CML mice,33,41 with an average ratio of phosphorylated P210 (pB-a) to P210 (B-a) of 4.8 by densitometry (Figure 3B; note the ratio is > 1.0, likely because of multiple tyrosine-phosphorylated residues per Abl molecule). In contrast, a single dose of PD166326 reduced Bcr/Abl tyrosine phosphorylation to the level of detection by anti-phosphotyrosine immunoblot, with an average pB-a/B-a ratio of only 0.24, 20-fold lower than in placebo-treated mice. Consistent with a previous report,49 a single dose of 100 mg imatinib mesylate/kg rapidly suppressed Bcr/Abl tyrosine phosphorylation, with an average pB-a/B-a ratio of 0.23. Therefore, PD166326, like imatinib mesylate, markedly suppressed Bcr/Abl tyrosine phosphorylation in vivo after a single oral dose.
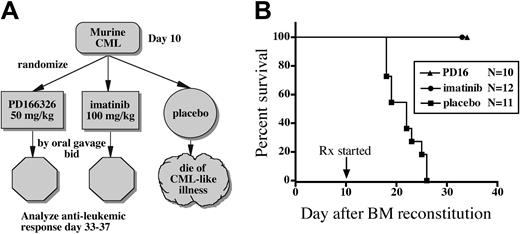
PD166326 provides better control of the murine CML-like illness than imatinib mesylate
To determine the antileukemic activity of PD166326, mice with the CML-like myeloproliferative disorder were treated with PD166326 (50 mg/kg twice a day), imatinib mesylate (100 mg/kg twice a day), or placebo beginning day 10 after BM reconstitution (Figure 4A). Consistent with previous studies,33,35 placebo-treated mice (n = 11) all died from the CML-like disease by day 26, with a median survival of 22 days (Figure 4B). None of the PD166326-treated mice (n = 10) died from leukemia or developed clinical signs of toxicity. Imatinib mesylate–treated mice (n = 12) also all survived the 3 to 4 weeks of treatment and showed no signs of drug toxicity, even though they received a higher-dose imatinib mesylate regimen than our previous study.33 In contrast to imatinib mesylate–and control-treated mice, at least half of the PD166326-treated mice lacked splenomegaly on examination, suggesting that PD166326 was more effective than imatinib mesylate in reducing the leukemic burden. To explore this possibility, PD166326- and imatinib mesylate–treated mice were electively killed on days 33 to 37 to better quantitate the antileukemic response.
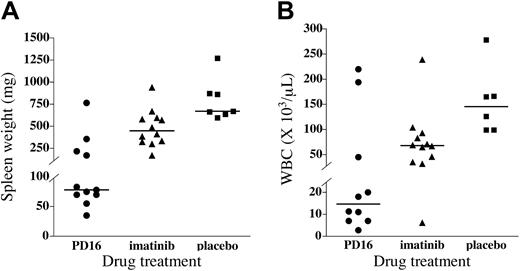
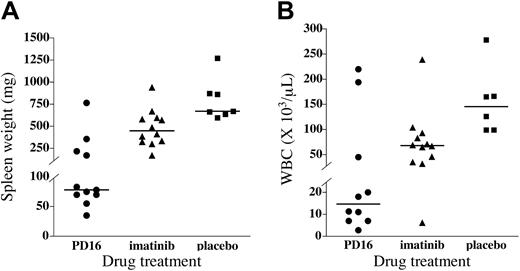
Splenomegaly is a characteristic feature of the CML-like myeloproliferative disorder and is a useful indicator of leukemic burden.33,41,50 Spleens were harvested from drug- and placebo-treated mice, weighed, and subjected to pathologic analysis. Placebo-treated mice had marked splenomegaly, with a median spleen weight of 670 mg (normal range, 50-100 mg33 ).
Consistent with previous results,33 imatinib mesylate–treated mice had less splenomegaly than placebo-treated animals, with a median spleen weight of 449 mg, although this did not reach statistical significance (P > .05). PD166326-treated mice had an even more marked reduction in splenomegaly, with a median spleen weight of only 78 mg, more than 8-fold less than placebo-treated animals (P < .001), and significantly less than imatinib mesylate–treated mice (P < .05; Figure 5A). Two thirds of PD166326-treated mice had spleen weights in the normal range (the average spleen weight was 67 mg for this subgroup), compared with none of the imatinib mesylate–treated mice. As a result, spleens from several PD166326-treated mice showed relative preservation of normal splenic architecture (data not shown), compared with the distorted splenic architecture of placebo-treated CML mice.41,50,51
PD166326 was also effective in reducing the peripheral blood granulocytosis of the murine CML-like disease. Placebo-treated mice had a median peripheral WBC count of 146.0 ×109/L (146 000/μL) at necropsy, comprising almost entirely early and maturing granulocytes, characteristic of the murine CML-like disease.33,41,50 PD166326-treated mice, in contrast, had a median WBC count of only 15.0 ×109/L (15 000/μL), almost 10-fold lower than placebo-treated animals (Figure 5B; P < .01). Imatinib mesylate–treated mice had just more than a 2-fold reduction in peripheral WBC count, with a median WBC count of 68.0 × 109/L (68 000/μL), although this difference did not reach statistical significance (P > .05). Overall, 70% of PD166326-treated CML mice achieved a WBC count fewer than 20.0 × 109/L (< 20 000/μL), compared with only 8% of imatinib mesylate–treated mice. Taken together, these data indicate that the novel tyrosine kinase inhibitor PD166326 has greater antileukemic activity than imatinib mesylate in this murine CML model.
PD166326 prolongs the survival of mice with the CML-like disease. (A) Mice reconstituted with P210-transduced BM cells received PD166326 (PD16), imatinib mesylate, or placebo according to the indicated treatment schema bid indicates twice a day. (B) Survival of the indicated treatment cohort (N) is depicted as the number of days after bone marrow (BM) reconstitution (day 0). The start of drug treatment (Rx) is indicated at the arrow. PD166326-(▴) and imatinib mesylate (•)–treated animals were electively killed on day 33 to 37 for analysis. ▪ indicates mice receiving placebo. The results depicted are from 2 independent experiments.
PD166326 prolongs the survival of mice with the CML-like disease. (A) Mice reconstituted with P210-transduced BM cells received PD166326 (PD16), imatinib mesylate, or placebo according to the indicated treatment schema bid indicates twice a day. (B) Survival of the indicated treatment cohort (N) is depicted as the number of days after bone marrow (BM) reconstitution (day 0). The start of drug treatment (Rx) is indicated at the arrow. PD166326-(▴) and imatinib mesylate (•)–treated animals were electively killed on day 33 to 37 for analysis. ▪ indicates mice receiving placebo. The results depicted are from 2 independent experiments.
Superior control of leukemic burden in PD166326-treated CML mice. (A) Spleen weight and (B) peripheral blood white blood cell (WBC) count of PD166326 (PD16; •)–, imatinib mesylate (▴)–, or placebo (▪)–treated CML mice at necropsy are shown by scatter plot. The median for each treatment group is depicted by the horizontal line. The P values for the differences in median spleen weight are PD166326 versus placebo (P < .001), PD166326 versus imatinib mesylate (P < .05), and imatinib mesylate versus placebo (P < .05). In the WBC count analysis only the PD166326 versus placebo comparison reached statistical significance (P < .01).
Superior control of leukemic burden in PD166326-treated CML mice. (A) Spleen weight and (B) peripheral blood white blood cell (WBC) count of PD166326 (PD16; •)–, imatinib mesylate (▴)–, or placebo (▪)–treated CML mice at necropsy are shown by scatter plot. The median for each treatment group is depicted by the horizontal line. The P values for the differences in median spleen weight are PD166326 versus placebo (P < .001), PD166326 versus imatinib mesylate (P < .05), and imatinib mesylate versus placebo (P < .05). In the WBC count analysis only the PD166326 versus placebo comparison reached statistical significance (P < .01).
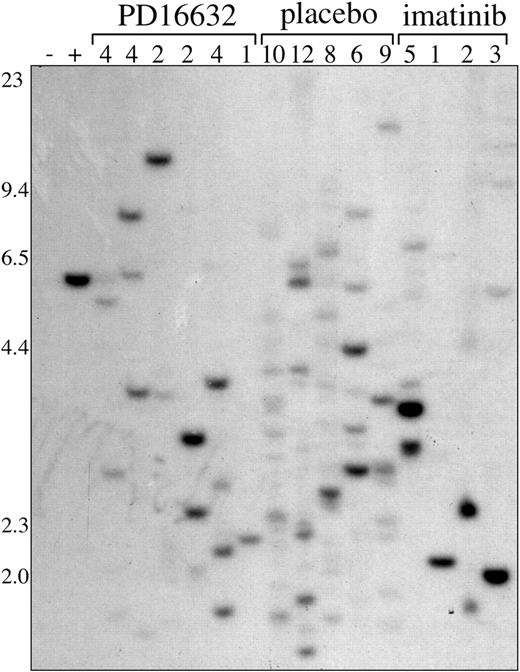
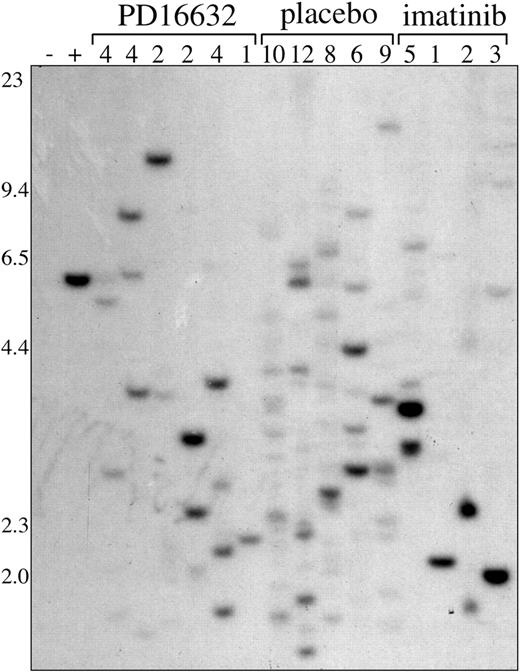
PD166326 and imatinib mesylate are both associated with oligoclonal CML
The CML-like disease generated in this mouse model is polyclonal for the BCR/ABL provirus.33,35 Consistent with these findings, placebo-treated mice had a range of 6 to 12 unique leukemic clones by proviral integration analysis (Figure 6). Leukemic clones containing the BCR/ABL provirus were still detected in PD166326-treated mice after 3 to 4 weeks of therapy, even those with normalization of peripheral WBC count and splenomegaly. However, PD166326-treated CML was markedly oligoclonal by Southern blot, with a range of 1 to 4 unique clones. Interestingly, the monoclonal PD166326-treated animal had a WBC count of only 2.8 × 109/L (2800/μL) and spleen weight of 35 mg at the time of analysis. Imatinib mesylate–treated mice were also oligoclonal compared with placebo-treated mice, with a range of 1 to 5 clones.33 Thus, PD166326 and imatinib mesylate were both associated with oligoclonal leukemia, likely because of the eradication or suppression of less robust leukemic clones, but neither completely eradicated BCR/ABL-containing cells after 3 to 4 weeks of treatment.
PD166326 is a potent inhibitor of leukemia cell protein tyrosine phosphorylation in vivo
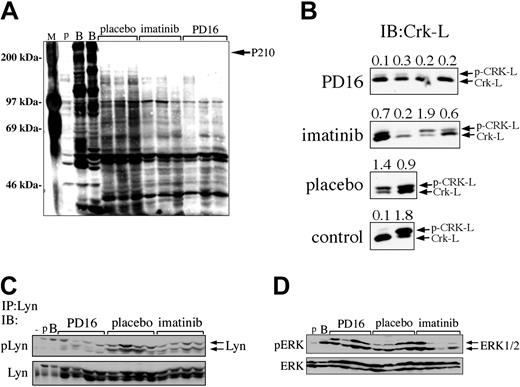
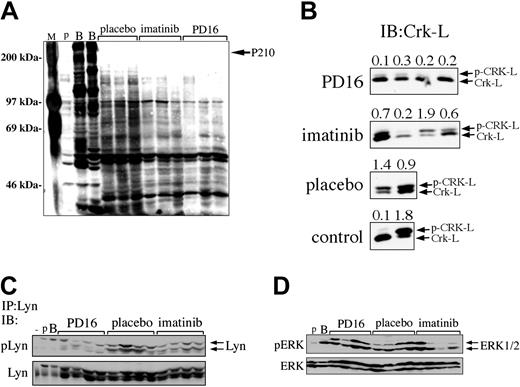
Compared with imatinib mesylate, PD166326 was a more potent inhibitor of Bcr/Abl-dependent proliferation (Figure 2) and more effectively reduced the leukemic burden of CML animals (Figure 5). To determine whether PD166326 was a more potent inhibitor of Bcr/Abl-dependent signaling in vivo, BM protein lysates were prepared from PD166326-, imatinib mesylate–, or control-treated CML mice and analyzed by anti-phosphotyrosine immunoblot. As anticipated, leukemia cells from placebo-treated mice had dozens of constitutively tyrosine-phosphorylated bands (Figure 7A, left). Leukemia cells from PD166326-treated animals, in contrast showed a marked reduction in the degree of constitutive protein tyrosine phosphorylation (Figure 7A, far right), similar to the levels observed in parental Ba/F3 cells (Figure 7A, far left). Consistent with previous results, leukemic cells from imatinib mesylate–treated mice had less protein tyrosine-phosphorylation than placebo-treated leukemia cells,33 but global tyrosine phosphorylation was not suppressed as much as in PD166326-treated cells. These results suggested that the superior antileukemic activity of PD166326 was linked at least in part to its more potent suppression of Bcr/Abl in vivo. These results were confirmed when the migration pattern/phosphorylation status of Crk-L, a prominent Bcr/Abl target,52-54 was used as a surrogate marker of Bcr/Abl kinase activity.55 Consistent with previous results,56 leukemia cells from placebo-treated mice demonstrated a prominent, slower migrating Crk-L form (phosphorylated (p)-Crk-L), in addition to a faster migrating form (unphosphorylated Crk-L), yielding an average p-Crk-L/Crk-L ratio of 1.2 (Figure 7B). In contrast, Crk-L from PD166326-treated leukemia cells was present almost entirely in the faster migrating form, yielding an average p-Crk-L/Crk-L ratio of only 0.23. Leukemia cells from imatinib mesylate–treated animals had a p-Crk-L/Crk-L ratio of 0.8, approximately 3.5-fold higher than PD166326-treated cells. For comparison, parental and P210-Ba/F3 cells had a p-Crk-L/Crk-L ratio of 0.1 and 1.8, respectively (Figure 7B, lower panel).
Oligoclonal CML in mice treated with PD166326 or imatinib mesylate. Genomic DNA was prepared from the spleens of mice with the CML-like illness after 3 to 4 weeks of treatment with the indicated drug or placebo and analyzed for the number of unique BCR/ABL proviral integration sites by Southern blot as described in “Materials and methods.” The number of leukemic clones for each sample is indicated at the top. Genomic DNA from a clonal P210 and parental Ba/F3 cell line are shown at left as controls. DNA size markers are indicated at left.
Oligoclonal CML in mice treated with PD166326 or imatinib mesylate. Genomic DNA was prepared from the spleens of mice with the CML-like illness after 3 to 4 weeks of treatment with the indicated drug or placebo and analyzed for the number of unique BCR/ABL proviral integration sites by Southern blot as described in “Materials and methods.” The number of leukemic clones for each sample is indicated at the top. Genomic DNA from a clonal P210 and parental Ba/F3 cell line are shown at left as controls. DNA size markers are indicated at left.
The effect of PD166326 on leukemic cell signaling in vivo. (A) Bone marrow protein lysates from mice with the CML-like disease on chronic PD166326 (PD16), imatinib mesylate, or placebo therapy were analyzed by anti-phosphotyrosine immunoblot. A protein size marker (M) is shown at far left. (B) Spleen protein lysates from PD16-, imatinib mesylate–, or placebo-treated mice were subjected to anti–Crk-L immunoblot (IB) as a surrogate marker of Bcr/Abl kinase activity in vivo. The positions of the upper (phosphorylated; p-Crk-L) and lower (Crk-L; nonphosphorylated) forms are shown at right. The ratio of p-Crk-L/Crk-L was quantitated by densitometry and is shown at the top of each lane. Parental or P210-Ba/F3 cells are shown in the bottom panel as a control. (C) The same samples in panel B were immunoprecipitated with anti-Lyn antibody and probed with an antibody that recognizes activated (pLyn) or total Lyn protein. (D) The same samples in panel B were probed with an antibody that recognizes activated (pERK) or total ERK1/2. The average pERK/ERK ratio by densitometry was 0.8 for PD16, 0.5 for imatinib mesylate, and 0.6 for placebo samples. Lysates from parental (p) and Bcr/Abl-expressing (B) Ba/F3 cells, or NIH 3T3 cells (-) are shown at far left in each panel as controls. The positions of P210BCR/ABL, Lyn, and ERK1/2 are indicated at the arrows.
The effect of PD166326 on leukemic cell signaling in vivo. (A) Bone marrow protein lysates from mice with the CML-like disease on chronic PD166326 (PD16), imatinib mesylate, or placebo therapy were analyzed by anti-phosphotyrosine immunoblot. A protein size marker (M) is shown at far left. (B) Spleen protein lysates from PD16-, imatinib mesylate–, or placebo-treated mice were subjected to anti–Crk-L immunoblot (IB) as a surrogate marker of Bcr/Abl kinase activity in vivo. The positions of the upper (phosphorylated; p-Crk-L) and lower (Crk-L; nonphosphorylated) forms are shown at right. The ratio of p-Crk-L/Crk-L was quantitated by densitometry and is shown at the top of each lane. Parental or P210-Ba/F3 cells are shown in the bottom panel as a control. (C) The same samples in panel B were immunoprecipitated with anti-Lyn antibody and probed with an antibody that recognizes activated (pLyn) or total Lyn protein. (D) The same samples in panel B were probed with an antibody that recognizes activated (pERK) or total ERK1/2. The average pERK/ERK ratio by densitometry was 0.8 for PD16, 0.5 for imatinib mesylate, and 0.6 for placebo samples. Lysates from parental (p) and Bcr/Abl-expressing (B) Ba/F3 cells, or NIH 3T3 cells (-) are shown at far left in each panel as controls. The positions of P210BCR/ABL, Lyn, and ERK1/2 are indicated at the arrows.
PD166326 also has activity against src protein tyrosine kinases,27,43 which have been implicated in Bcr/Abl transformation,57-61 and are not targeted by imatinib mesylate.43 To investigate the effect of PD166326 on src activation, PD166326-, imatinib mesylate–, and placebo-treated leukemia cells were immunoprecipitated with an antibody against Lyn, a src family member recently implicated in imatinib mesylate–resistant CML.18,20 Primary leukemia cells from PD166326-treated mice showed a marked reduction in constitutive Lyn activation by phospho-specific immunoblot (pLyn) compared with placebo-treated animals (Figure 7C, left). Lyn phosphorylation was also decreased in cells from imatinib mesylate–treated mice, but to a lesser degree than in PD166326-treated mice (Figure 7C, right). Total Lyn protein levels were the same among the 3 groups. ERK1/2 activation (pERK), in contrast, was not decreased in PD166326-treated leukemia cells, with an average pERK/ERK ratio of 0.8 by densitometry, compared with an average pERK/ERK ratio of 0.6 in placebo-treated leukemia cells (Figure 7D, left). Interestingly, ERK1/2 phosphorylation was more consistently decreased in imatinib mesylate–treated CML cells,62 with an average pERK/ERK ratio of 0.5 (Figure 7D, right). Altogether, these results demonstrate that PD166326 is more effective than imatinib mesylate in suppressing the constitutive tyrosine phosphorylation of numerous proteins, including Lyn, in primary Bcr/Abl-expressing leukemia cells.
PD166326 prolongs survival in a murine model of imatinib mesylate–resistant CML. Mice with the CML-like disease induced by the imatinib mesylate–resistant Bcr/Abl mutants H396P (A), M351T (B), or T315I (C) were randomly assigned to treatment with PD166326 (25 mg/kg orally twice a day; ▴), imatinib mesylate (100 mg/kg orally twice a day; •), or placebo (▪) and analyzed for survival according to the method of Kaplan and Meier. The number of animals (N) in each cohort is shown at right. Imatinib mesylate did not prolong the survival of any of the groups. PD166326 prolonged the survival of P210/H396P (P < .001) and P210/M351T (P = .03) animals, but not T315I/P210.
PD166326 prolongs survival in a murine model of imatinib mesylate–resistant CML. Mice with the CML-like disease induced by the imatinib mesylate–resistant Bcr/Abl mutants H396P (A), M351T (B), or T315I (C) were randomly assigned to treatment with PD166326 (25 mg/kg orally twice a day; ▴), imatinib mesylate (100 mg/kg orally twice a day; •), or placebo (▪) and analyzed for survival according to the method of Kaplan and Meier. The number of animals (N) in each cohort is shown at right. Imatinib mesylate did not prolong the survival of any of the groups. PD166326 prolonged the survival of P210/H396P (P < .001) and P210/M351T (P = .03) animals, but not T315I/P210.
PD166326 has activity in vivo against CML induced by imatinib mesylate–resistant BCR/ABL mutations
Previous studies have demonstrated that pyridopyrimidines have activity in vitro against a variety of BCR/ABL mutations reported in CML patients resistant to imatinib mesylate.29,31,32 To determine whether PD166326 has antileukemic activity against mutant forms of Bcr/Abl in vivo, a murine model of imatinib mesylate–resistant CML was established. Instead of wild-type P210, syngeneic mice were reconstituted with BM cells transduced with retroviruses expressing specific P210 kinase domain mutations implicated in human imatinib mesylate–resistant CML. The overall treatment schema remained the same as in the wild-type P210 experiments (Figure 4A), except that mice in the PD166326 arm were dosed at half the maximum-tolerated dose (25 mg/kg orally twice a day), as an additional test of antileukemic potency (the imatinib mesylate regimen remained at 100 mg/kg twice a day). Mice reconstituted with P210/H396P-transduced BM cells all died of the CML-like disease with a median survival of 15.5 days (placebo; Figure 8A).
Imatinib mesylate–treated P210/H396P CML mice had a median survival of 19 days, not significantly different from placebo-treated animals (P = .53, log-rank test). PD166326-treated P210/H396P CML mice, in contrast, survived almost twice as long as placebo-treated animals, with a median survival of 30 days (P < .001). PD166326-treated mice with M351T-induced CML also had increased survival, with a median survival of 27 days, compared with 20 days for placebo-treated mice (Figure 8B; P = .03, log-rank test). As expected, imatinib mesylate did not prolong the survival of P210/M351T animals (median survival, 24 days; P = .42). Consistent with previous findings,10,31,32,45 P210/T315I murine CML was resistant to either PD166326 or imatinib mesylate treatment (Figure 8C), likely because T315 plays an important role in drug contact.63,64 These results demonstrate that PD166326 has significant activity against imatinib mesylate–resistant P210 mutant leukemia cells in vivo but suggest that P210/T315I will pose a significant challenge for even later generation Abl kinase inhibitors.65
Discussion
The long-term success or cure rate of imatinib mesylate for the treatment of CML remains to be fully defined. Several recent studies have suggested that there may be a plateau of response for many patients, with most patients retaining a low level of Bcr/Abl-positive hematopoietic progenitors.66,67 Perhaps imatinib mesylate will durably suppress the emergence of clinically relevant CML in many patients, but for those with overt imatinib mesylate resistance therapeutic options are severely limited. Besides imatinib mesylate–based combinations to prevent imatinib mesylate resistance, the development of more potent tyrosine kinase inhibitors for the treatment of de novo and imatinib mesylate–resistant CML is an important goal for CML therapeutics. Previous in vitro studies by others and us identified the pyridopyrimidine tyrosine kinase inhibitor PD166326 as one of the most potent published inhibitors of Bcr/Abl kinase activity. In this study, we have extended those findings by demonstrating that PD166326 is superior to imatinib mesylate in an in vivo mouse model of CML that mimics many of the central features of the human disease.
PD166326 had profound antileukemic activity in the murine CML-like myeloproliferative disorder, an illness generally considered more clinically aggressive than its human counterpart.68 Approximately two thirds of PD166326-treated CML mice had spleen weights in the normal range, a significantly greater degree of leukemic cytoreduction than imatinib mesylate achieved in this model (Figure 5A). Moreover, PD166326 had potent activity against Bcr/Abl-expressing leukemia cells despite limited aqueous solubility (compared with imatinib mesylate), making it possible that a PD166326 analog with greater bioavailability would possess even greater antileukemic activity. The relatively long half-life of PD166326 in mice (8.4 hours) suggests that once-a-day dosing of PD166326 would be feasible in humans. The superior antileukemic activity of PD166326 is likely explained by its ability to bind the Bcr/Abl kinase domain in either the active or inactive conformation, while imatinib mesylate requires the inactive conformation of Bcr/Abl.63,64 Consistent with this hypothesis, PD166326 was a more potent inhibitor of Bcr/Abl-dependent signaling pathways than imatinib mesylate in vivo (Figure 7A-B) and had considerable activity against CML induced by P210/H396P, a BCR/ABL mutation predicted to favor an active conformation of Bcr/Abl.45,64 Importantly, PD166326 and imatinib mesylate differed in their relative inhibition of Lyn and ERK1/2 signaling, suggesting that PD166326 and other pyridopyrimidine compounds may provide an opportunity to target some signaling pathways distinct from those affected by imatinib mesylate. The increased activity of PD166326 against src family kinases such as Lyn is of particular interest given recent studies that have implicated Lyn in Bcr/Abl-independent mechanisms of imatinib mesylate resistance.18,20
An important question raised by our study and others is how more potent inhibition of Bcr/Abl translates into more effective control of CML. The fact that PD166326 achieved superior leukemic cytoreduction in our mouse model is consistent with a recent report suggesting higher doses of imatinib mesylate may provide better molecular response rates in CML than the standard dose.38 However, it is not yet known whether better inhibition of Bcr/Abl will lead to more effective eradication of Bcr/Abl progenitors or merely achieve better Bcr/Abl suppression. It is reasonable to hypothesize that drugs that achieve the former will have a greater potential for curing CML, while those that accomplish the latter may only postpone progression. How or why quiescent Bcr/Abl hematopoietic progenitors persist during imatinib mesylate therapy has not been fully elucidated,67,69 but this problem may be the biggest hurdle for the pharmacologic cure of CML.70 However, if concomitant inhibition of Bcr/Abl and c-Kit contributes to the quiescence of early CML stem cells, drugs like PD166326 offer a potential advantage over imatinib mesylate. On the basis of IC50, PD166326 was 60-fold better at inhibiting Bcr/Abl-dependent proliferation than it was c-Kit–dependent proliferation (Figure 2). Imatinib mesylate, in contrast, inhibited Bcr/Abl-dependent growth only 4-fold better than it did c-Kit. Thus, compared with imatinib mesylate, PD166326 had an approximate 15-fold better Bcr/Abl/c-Kit inhibitory ratio, raising the possibility that a more favorable ratio might increase CML progenitor vulnerability. In our mouse CML model, BCR/ABL leukemic clones were still detected in all PD166326-treated animals by Southern blot after 3 to 4 weeks of treatment. However, the remarkable reduction in WBC count and splenomegaly in many animals, particularly those achieving monoclonal disease, suggests the possibility that more protracted PD166326 therapy might have “cured” some animals. As novel Abl kinase inhibitors are clinically developed, it will be of considerable interest to determine whether drugs with higher Bcr/Abl/c-Kit inhibitory indices like PD166326 could lead to lower percentages of residual Bcr/Abl-positive hematopoietic progenitors in patients with CML.
On the basis of crystallographic modeling studies, it has been predicted that at least 21 amino acids of the Bcr/Abl kinase domain interact with imatinib mesylate, compared with only 11 for pyridopyrimidines like PD166326.64 Fewer contact points with the Abl kinase domain may provide PD166326 with more flexibility to retain activity against certain imatinib mesylate–resistant Abl kinase domain mutations. Indeed, this study and several others have indicated that pyridopyrimidines, including PD166326, have activity against many of the most prevalent imatinib mesylate–resistant P210 mutations, except for P210 T315I.31,32,71,72 Whether second-generation Abl kinase inhibitors like PD166326 will be able to cure patients with CML with mutant forms of P210 remains to be established, but it is likely that such drugs will be most effective in eradicating mutant forms of P210 early in the pathogenesis of CML, prior to clonal evolution. Besides greater antileukemic activity, drugs with more potent and perhaps broader protein tyrosine kinase inhibition also carry the risk of increased toxicity. In mice, PD166326 was remarkably well tolerated, although incidental tubulointerstitial nephritis was noted in several PD166326-treated mice at autopsy at the 50 mg/kg twice a day dose level but not in mice treated at 25 mg/kg twice a day (data not shown). It will be interesting to determine whether the relatively favorable toxicity profile of imatinib mesylate can be mirrored in other more potent second-generation kinase inhibitors in preclinical and early clinical development.65,73
In summary, we have used a murine model of CML as a platform to characterize the pharmacokinetic, signal transduction, and antileukemic properties of PD166326, one of the most potent members of the pyridopyrimidine class of protein tyrosine kinase inhibitors. Despite suboptimal aqueous solubility, PD166326 had greater antileukemic activity than imatinib mesylate in this CML model and more effectively suppressed Bcr/Abl-dependent signaling. PD166326 also demonstrated activity against mutant forms of Bcr/Abl and src signaling pathways in vivo. Because this mouse CML model has been a useful barometer for the successes and limitations of tyrosine kinase inhibitor–based therapy, we feel our findings provide considerable rationale for the further development of PD166326 or a related analog for human CML studies. Finally, the novel mouse model of imatinib mesylate–resistant CML used in this study should prove useful for designing and testing novel therapeutic approaches for eradicating CML.
Prepublished online as Blood First Edition Paper, January 18, 2005; DOI 10.1182/blood-2004-09-3534.
Supported by Department of Defense (grant CM020016) (R.L.I.) and National Institutes of Health (grant HL61764) (R.L.I.); National Cancer Institute (NCI) (grant CA64593) (B.C.); Mr William H. Goodwin and Mrs Alice Goodwin and the Commonwealth Cancer Foundation for Research, and The Experimental Therapeutics Center of Memorial Sloan-Kettering Cancer Center, New York (B.C.); The Leukemia and Lymphoma Society Translational Research Program (B.C.); The United Leukemia Fund (B.C.); The Westvaco Corporation and MeadWestvaco (B.C.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Brian Druker for generously providing pSRα-P210/M351T and pSRα-P210/T315I and Merrill Egorin and Richard Gaynor for helpful discussions and for reviewing the manuscript prior to submission.

![Figure 1. Chemical structures for the pyrido[2,3-d]pyrimidine PD166326 and the 2-phenylaminopyrimidine imatinib mesylate.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/10/10.1182_blood-2004-09-3534/6/m_zh80100578360001.jpeg?Expires=1768399147&Signature=qNbJA-XbSpf8HZdXva7ikYM~VyFw9ArbFDynB~N7fozk63kHbhRK~2hUtRI8kgVdxrjmPSpSEQG-gJvB2zdPGkITq9ucxNeQvXFGuvUUUPcJM8x3O45ovcsScQukRwcjIjTag2gEBmJhS-4YdS~35-Lzv1flOV8~zljCXHzO6t9WBCM4dURzcUzTaqz29dvH2XZIPhW-wmm3-FS9YoQJ4E16orE~TvFay0stKyRmFlYC0MRg8AQ-9fSRq3-A3gTiGTDyBxD0fSTSvX79QkYo2WTFVB3dsL0j3o518Bm8lGoLQrQ4hDlFirGZ8uSnS5~jGpffNnZPqUR0uSxdpY2rng__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. PD166326 is a more potent inhibitor of Bcr/Abl- and stem cell factor–dependent proliferation than imatinib mesylate. (A) The P210-expressing MO7e cell line R10(-) growing in the absence of exogenous cytokine, and (B) parental MO7e cells growing in the presence of stem cell factor (MO7e/KL) were treated with the indicated concentrations of PD166326 (•) and imatinib mesylate (▴) for 48 hours and then analyzed by [3H]thymidine incorporation as described in “Materials and methods.” Results are expressed as the counts per minute (CPM) relative to cells treated with diluent alone (DMSO) versus the concentration (log10 scale) of PD166326 or imatinib mesylate. The calculated IC50 for each drug is shown. The results depicted are representative of multiple independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/10/10.1182_blood-2004-09-3534/6/m_zh80100578360002.jpeg?Expires=1768399147&Signature=1dZbkcV4pk2xtSIvtpTZWgVYiccGoCfKTNLIA~V1tDjYSm2yZ24AXrwFCyKDT2pFh1TeLyluktwrIIWsxsitjW-JLb1axl2ZNnFNUtesO2D-fXJ9jL0gCOEfYsEkhE49iWmpiAm8qvaFHg1INMXYKm8K7GacgtS79hnuhpm-vjtMOs6rARlYutjLnmLhIlRvPpxZT9Ojw65YhkDcOQTZdDN0VQXek-kyuplZZ6ekDpyuak0IsIln8lVCNybgu59uv~q8--wJ1LtYHwMHShAC-XJ8F8PgPfl9j7noXj3xcmtF6uIj7f8vyXIiG5E4NtwFug5NgVSCTp~bNkTfYuQVsw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



![Figure 1. Chemical structures for the pyrido[2,3-d]pyrimidine PD166326 and the 2-phenylaminopyrimidine imatinib mesylate.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/10/10.1182_blood-2004-09-3534/6/m_zh80100578360001.jpeg?Expires=1768399148&Signature=VcgpytfL8vNidBqZSzsRBluPpGajIT73FhxVbBrAS1nrJmXWR5cbsZLyqXsWWj9vYdZRincVVsp~Beod621HBZHYOryYaYylhzki4OnSr9NuCIlVnEHJahujVfcZCQixbBkeKg-CxZNmgV7YNp88yZgeTjJjjria3cUHHKIQ0f6GRhylJ4jGTNuDFzSWb9bgAAezwz4e1ZRO0al1xQvn9c6E~ZyhugOM~yarSSrA9HaMiqh3J9baNxlJBF0F7KiG7Cro8YNSqZWexqpRhYnvcfBe3icsOBTp8oe-TiSvnqHksscDfuhgeYiB5hF72woRiY2BVv-Nzefvpm3vQi14IQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. PD166326 is a more potent inhibitor of Bcr/Abl- and stem cell factor–dependent proliferation than imatinib mesylate. (A) The P210-expressing MO7e cell line R10(-) growing in the absence of exogenous cytokine, and (B) parental MO7e cells growing in the presence of stem cell factor (MO7e/KL) were treated with the indicated concentrations of PD166326 (•) and imatinib mesylate (▴) for 48 hours and then analyzed by [3H]thymidine incorporation as described in “Materials and methods.” Results are expressed as the counts per minute (CPM) relative to cells treated with diluent alone (DMSO) versus the concentration (log10 scale) of PD166326 or imatinib mesylate. The calculated IC50 for each drug is shown. The results depicted are representative of multiple independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/10/10.1182_blood-2004-09-3534/6/m_zh80100578360002.jpeg?Expires=1768399148&Signature=Axy4a-XGMg1iBf0D7NmQ29ceCs2sKeQmG4jWq8LVHpeuBTfWE4xl78Heu7t8tTfjiEujz37o3fwKOILAqYp9FCyzmZaqWnhzn0PVTYHj-GKKTBpMxVltyHR84yZ2zLdftVmCZVr7YQbDf3vp7ImID0fjbXvOzdo2l5o3qsJRjnAz2ou7sK9SKfoC~NrAVeMM92DHv8YdwdjUqYKJSiwDR81-R8a2Zq8SAZenhe4foC~WiZcr54LFqXfiF1Pul-6DEebxEQAlelJCAbdgoQ47x1QiS7ShXxurt00J-2O~QrN0f1sj~4IU0I25JWO-jvnQOHCNKTnxfQZVOjhI3IvGtQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)