Abstract
Although there is no compelling evidence that vitamin C has antitumor activity in humans, clinical trials are testing the hypothesis that ascorbic acid (AA) will enhance the efficacy of arsenic trioxide (As2O3) in myeloma. In vitro, AA cytotoxicity depends on its interaction with free transition metal ions in culture media leading to the generation of H2O2 and other reactive oxygen species (ROSs). Therefore, to circumvent the extracellular in vitro pro-oxidant effects of AA, we loaded HL60, U266, and RPMI-8226 cells with vitamin C by incubation with dehydroascorbic acid (DHA). Loading cells in this manner resulted in prominent, dose-dependent protection of As2O3-treated cells as measured by viability, colony formation, and apoptosis assays. Glutathione depletion enhanced cell sensitivity to the cytotoxic effects of As2O3 and vitamin C loading provided protection. AA was found to generate cytotoxic concentrations of H2O2 in culture medium without cells and copper/iron chelators inhibited this reaction. However, AA did not generate H2O2 in simple buffer or human plasma. Direct incubation with AA resulted in increased intracellular ROSs, whereas DHA incubation decreased it. These results clarify an apparent paradox and indicate that vitamin C loading in HL60, U266, and RPMI-8226 cells ameliorates As2O3 cytotoxicity.
Introduction
Arsenic is an environmental toxin but it has also been used as a therapeutic agent for more than 2400 years. In the 1930s, patients with chronic myelogenous leukemia were treated with arsenic and the use of arsenic in leukemia resurfaced when reports from China provided evidence that arsenic induced remissions in acute promyelocytic leukemia (APL).1,2 Subsequently the efficacy of arsenic trioxide (As2O3) in the treatment of APL was confirmed in randomized clinical trials.3,4 Currently clinical trials are testing arsenic in the treatment of other hematologic malignancies including lymphoma and myeloma (for a review see Murgo5 ).
Arsenic appears to exert its effects on tumor cells by several mechanisms.6-9 The activity of arsenic in APL is in part due to degradation of the oncoprotein promyelocytic leukemia/retinoic acid receptor α (PML-RARα) and induction of differentiation.2,10-12 The therapeutic effects of arsenic are also dependent on its ability to induce growth inhibition, cell cycle arrest, and activation of apoptotic pathways. Some of the apoptotic effects of arsenic are attributed to its ability to cause the collapse of the inner mitochondria transmembrane potential and release of cytochrome c,13-16 the down-regulation of B-cell lymphoma 2 (Bcl-2),12,17 and the activation of caspases, which ultimately lead to cell death.17-19 Numerous studies indicate that arsenic induces the generation of reactive oxygen species (ROSs) that contribute significantly to cell killing.14,15,20 Antioxidant molecules such as glutathione (GSH) reduce the cytotoxic effects of arsenic largely by quenching ROSs,21-24 and cells containing low levels of GSH are more sensitive to arsenic.24 Catalase suppresses arsenic-induced apoptosis.13 These observations suggest that down-regulating antioxidant molecules, or the biochemical pathways that generate them, could be therapeutically useful in altering the cytotoxicity of arsenic. Paradoxically, vitamin C, a key antioxidant molecule,25-27 was reported to augment the toxicity of arsenic in vitro.24,28-31 These studies suggested that the pro-oxidant properties of vitamin C in vitro increase ROSs that enhance the cytotoxicity of arsenic.24,28 Based on these reports, clinical trials have been designed to test the use of vitamin C to augment arsenic activity in the treatment of myeloma.32 Recent studies in rats using sodium arsenite, however, indicate that vitamin C ameliorated arsenic-induced toxicity.33-36
The pro-oxidant characteristics of vitamin C in vitro are well documented and the generation of ROSs by ascorbic acid (AA) in vitro is dependent on free transition metal ions, such as iron and copper, in tissue culture media.26,37 There is little evidence that free transition metals are available in vivo to enable the reactions by which AA can produce ROSs in vitro.26,27,37 In vivo, iron and copper are generally sequestered by transition metal binding proteins, including ferritin, transferrin, lactoferrin, and caeruloplasmin.26,38 The production of ROSs by AA in vitro is believed to be largely a tissue culture phenomenon.26,37 In the studies that reported an increase in arsenic cytotoxicity with vitamin C, AA was directly added to the tissue culture media.24,28-31
There are 2 known mechanisms that cells use to transport and accumulate vitamin C. All cells transport dehydroascorbic acid (DHA), the oxidized form of vitamin C, via the facilitative glucose transporters.39,40 The reduced form of vitamin C, ascorbic acid (AA), is transported by sodium-dependent AA transporters,41 which are widely expressed at the mRNA level; however, only specialized cells appear to transport AA directly.42-44 Sodium-dependent transport is critical for development as shown by knockout studies.45 Most cells appear to rely on AA oxidation to DHA for the transport and accumulation of vitamin C.46-48
To circumvent the in vitro pro-oxidant properties of AA, we loaded HL60 myeloid leukemia cells, U266 cells, and RPMI-8226 myeloma cells with vitamin C using DHA and then tested their response to arsenic. HL60 and U266 cell lines were used previously and reported to be sensitized to arsenic toxicity with vitamin C.24,28 Contrary to those findings, we found that intracellular vitamin C protected these cancer cells from arsenic cytotoxicity.
Patient, materials, and methods
Reagents and cell lines
HL60 cells were obtained from American Type Culture Collection (ATCC, Manassas, VA) and maintained in Iscove modified Dulbecco medium (IMDM). U266 and RPMI-8226 cells were maintained in RPMI supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 100 IU/mL penicillin, and 100 μg/mL streptomycin in an atmosphere of 5% CO2 at 37°C. The cells used in the experiments were in the logarithmic phase of growth and their viability was greater than 90%. Cell viability was determined by trypan blue exclusion. AA; N-acetyl-l-cysteine (NAC); 2′,7′-dichlorofluorescein diacetate (H2DCFDA); ascorbate oxidase; diethylenetriaminepentaacetic acid (DTPA); As2O3; 2,3-dimercaptopropanol (DMP); catalase; dl-buthionine-sulfoximine (BSO); maleic acid diethyl ester; and cytochalasin B and E were obtained from Sigma Chemical (St Louis, MO). Methylcellulose-based media (MethoCult) was purchased from Stem Cell Technologies (Vancouver, BC, Canada). Ascorbic acid l-(1-14C) specific activity 4.75 to 8.2 mCi/mM (175.75-303.4 MBq/mM) was obtained from DuPont NEN (Boston, MA). The Amplex Red H2O2/peroxidase assay kit was purchased from Molecular Probes (Eugene, OR). The TUNEL (terminal deoxynucleotidyl transferase [TdT]–mediated deoxyuridine triphosphate [dUTP] nick-end labeling) enzyme and TUNEL label were obtained from Roche (Indianapolis, IN).
Uptake of AA and DHA and loading cells with vitamin C
Cell loading with vitamin C was as described previously.39,40 In brief, cells were washed, suspended in incubation buffer (IB; pH 7.4; 15 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 135 mM NaCl, 5 mM KCl, 1.8 mM CaCl2, and 0.8 mM MgCl2), and incubated at 37°C for 30 minutes. DHA was prepared by incubating AA with ascorbate oxidase (60 nmoles/unit) in IB (pH 5.5) at room temperature for 3 minutes and then stored on ice. Cells (3 × 106/mL) were loaded with vitamin C by incubation with DHA for 1 hour at 37°C in IB (pH 7.4). Loaded cells were washed once in phosphate-buffered saline (PBS) and suspended in tissue culture media containing the indicated concentrations of As2O3. For uptake studies l-(1-14C) AA (specific activity 6.0 mCi/mmol [222 MBq/mmol]) was included in the preparation of DHA from AA. Briefly, cells were washed and suspended in IB (pH 7.4). DHA was prepared by incubating 0.04 to 2.2 μCi (0.00148-0.0814 MBq) l-(1-14C) AA (specific activity 6.0 mCi/mmol [222 MBq/mmol]) at a final concentration of 0.025 to 1.5 mM AA and ascorbate oxidase for 3 minutes and then stored on ice. For the AA uptake analysis, ascorbate oxidase was omitted and 0.1 mM dithiothreitol (DTT) was added to prevent oxidation of AA to DHA. Cells were incubated with DHA or AA for various time periods, washed with Ca2+- and Mg2+-free PBS, solubilized in 10 mM Tris-HCl (pH 8.0) containing 0.2% sodium dodecyl sulfate (SDS), and counted by liquid scintillation spectrometry to determine the cell-associated radioactivity. Cell volume and cell-associated radioactivity were used to estimate the intracellular concentration of vitamin C.
Cell volume determination
Cell volume was estimated as described previously with 30% correction for trapping extracellular radioactivity.39,49 Briefly, 5 million cells were incubated for 60 minutes at room temperature in 200 μL of incubation buffer containing 1 mM 3-oxy-methyl glucose (OMG), a nonmetabolizable glucose analog, and 5 μCi (0.185 MBq) of 3H-labeled OMG to achieve equilibrium between the intracellular and extracellular concentrations of OMG. After incubation, 2 μL of 2 mM cytochalasin B was added to cells for 5 minutes to prevent the efflux of trapped OMG during washing. Cells were then washed 3 times with cold PBS containing 20 μM of cytochalasin B to remove unincorporated radioactivity. After lysis in 10 mM Tris-HCl (pH 8.0) containing 0.2% SDS, the incorporated radioactivity was determined by liquid scintillation spectrometry. The amount of radioactivity trapped within cells is directly proportional to the intracellular volume. The internal volume of HL60 and U266 cells was estimated to be 0.6 and 1.0 μL per 106 cells, respectively. The cell volume was used to calculate the intracellular concentration of vitamin C based on the amount of 14C vitamin C uptake.
ROS determination
Intracellular ROSs were monitored by flow cytometry using H2DCFDA. This dye diffuses readily into cells and it is trapped intracellularly when hydrolyzed by esterases. ROSs produced by cells oxidize H2DCFDA to the fluorescent compound 2′,7′-dichlorofluorescein (DCF) and fluorescence intensity is proportional to the amount of ROSs produced. Cells were treated with the indicated concentrations of AA or DHA for 1 hour, washed in PBS, and then incubated in growth media containing 80 μM H2DCFDA for 1 hour at 37°C. At the end of the incubation, cells were washed once with PBS and suspended in cold PBS, and the fluorescence was monitored by flow cytometry.
Depletion of GSH
Cells were depleted of GSH as previously described.50 Briefly, 3 × 105 cells/mL were incubated with 0.2 mM BSO for 16 hours, followed by 1-hour incubation with 1 mM diethyl maleate and washing in PBS.
Treatment of cells with of As2O3
As2O3 was prepared as a 0.5-mM stock solution in PBS and stored at room temperature in the dark. Cells were treated with different concentrations of As2O3 for the time indicated and cell viability was determined by trypan blue exclusion 24 or 48 hours after treatment. Cells were washed in PBS prior to plating in methylcellulose to remove excess As2O3 and colonies were counted as indicated in the figure legends.
Methylcellulose colony assays
MethoCult containing 1% methylcellulose, 10% fetal bovine serum, 2 mM l-glutamine, 100 IU/mL penicillin, and 100 μg/mL streptomycin in IMDM was prepared according to the manufacturer's instructions. Methylcellulose was dispensed into tubes using a 16-gauge blunt needle, 3-mL/tube. Cells treated under different conditions with vitamin C and As2O3 for 48 hours were washed in PBS and serially diluted in fresh media (1:1-1:100). Cells (0.3 mL) were added to 3 mL methylcellulose and gently vortexed to suspend the cells evenly. Several dilutions were plated and the one giving rise to approximately 300 colonies/well was chosen for later experiments. Cells were plated in 6-well plates using a 16-gauge blunt needle, 1.1 mL cells/well. The colonies were counted at 10 days (HL60) or 14 days (U266) after plating using a Labovert FS stereoscopic inverted microscope (Leitz, Wetzlar, Germany). For HL60 cells, a 50-cell colony was the minimum counted whereas a 25-cell colony was used for U266 cells.
Determination of H2O2 generation in cell-free media
Amplex red reagent (10-acetyl-3,7-dihydroxyphenoxazine) was used for the detection of H2O2. In the presence of horseradish peroxidase, Amplex red reacts with H2O2 in a 1:1 stoichiometry to produce the red-fluorescent oxidation product resorufin. The fluorescence was measured with a fluorescence microplate reader using excitation at 535 nm and detection at 590 nm. The concentration of H2O2 generated in the cell-free system was determined by comparing the fluorescence to a standard curve generated with known concentrations of H2O2. Experiments were done in a 96-well plate in a total volume of 0.1 mL. Reactions containing 50 μM Amplex red reagent, 0.1 U/mL horseradish peroxidase, and the indicated concentration of AA, DHA, As2O3, copper, or chelator were incubated in IMDM media, 50 mM phosphate buffer (pH 7.4), or plasma for 1 hour at 37°C in the dark.
Plasma isolation
With appropriate informed consent, peripheral blood was obtained from a healthy volunteer in Becton Dickinson (San Jose, CA) green-top sterile tubes containing 143 United States Pharmacopeia (USP) units of sodium heparin and mixed thoroughly. The tubes were centrifuged for 30 minutes at 1700g and the plasma was transferred to a new tube and recentrifuged. The plasma was collected and used within 30 minutes of its isolation.
Detection of apoptosis
Apoptotic cells were labeled using a TUNEL assay according to the manufacturer's instructions. Positively TUNEL-stained cells were counted using an Olympus BX50 fluorescent microscope (Olympus, Hamburg, Germany). The annexin V–fluorescein isothiocyanate (FITC)/propidium iodine (PI) apoptosis detection kit (Pharmingen, San Diego, CA) was also used to determine the frequency of apoptosis in HL60 cells using flow cytometry analysis.
Statistical analyses
Data are presented graphically as the mean ± standard deviation (SD). Statistical analysis was done using Sigmaplot software (Jandel Scientific, Chicago, IL).
Results
HL60 and U266 cells transport the oxidized form of vitamin C, DHA, but not the reduced form, AA
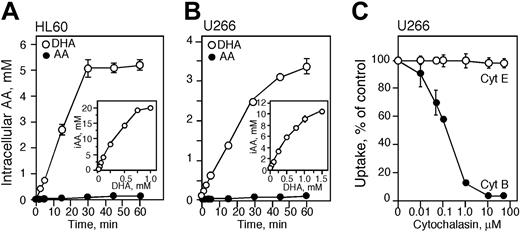
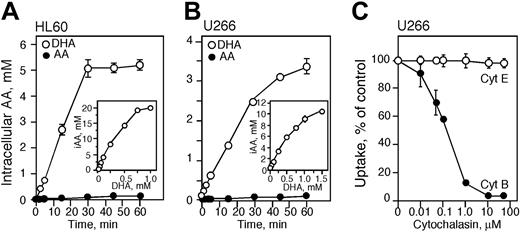
We previously reported that HL60 cells transport DHA and not AA40,51 and those observations are confirmed in the present studies. HL60 cells were incubated with 250 μM 14C-labeled AA or DHA for various time points and the intracellular concentration of vitamin C was measured. There was no significant accumulation of vitamin C in cells treated with 250 μM AA for up to 60 minutes (Figure 1A). Incubation with 250 μM DHA resulted in efficient accumulation after 30 minutes. Incubation with 25, 50, or 100 μM DHA for 60 minutes at 37°C resulted in an intracellular concentration of vitamin C of approximately 1, 2, and 4 mM, respectively (Figure 1A). The intracellular concentration of vitamin C increased in cells incubated with higher concentrations of DHA, and exposure to 750 and 1000 μM DHA for 1 hour resulted in an intracellular concentration of approximately 20 mM vitamin C (Figure 1A).
To determine the preferred form of vitamin C transported by U266 cells, U266 cells were incubated with 14C-labeled AA or DHA for various time points. U266 cells, like HL60 cells, efficiently transported DHA, the oxidized form of vitamin C, but not AA (Figure 1B). Incubation of U266 cells with 50, 100, and 250 μM DHA increased the intracellular concentration of vitamin C to approximately 1, 2, and 4 mM, respectively (Figure 1B). Incubation with 1 and 1.5 mM DHA for 1 hour resulted in intracellular AA concentrations of approximately 9 and 10 mM, respectively. To confirm that U266 cells transport DHA via the facilitative glucose transporters, cells were incubated for 60 minutes with cytochalasin B (a glucose transporter inhibitor) and then 100 μM 14C-labeled DHA was added for 10 minutes. The accumulation of vitamin C decreased with increasing concentrations of cytochalasin B reaching 50% inhibition at approximately 0.2 μM and complete inhibition at 10 μM. Cytochalasin E, a structurally similar molecule, was used as a control and had no effect on the transport of 14C-labeled DHA (Figure 1C).
Transport and accumulation of vitamin C in HL60 and U266 cells. HL60 (A) and U266 (B) cells were incubated for the time indicated with 250 μM dehydroascorbic acid (DHA; ○) or ascorbic acid (AA; •) at room temperature. Intracellular accumulation of vitamin C in HL60 and U266 cells treated with DHA for 60 minutes at 37°C (A-B insets). (C) Inhibition of DHA transport in U266 cells treated with cytochalasin E (Cyt E; ○) and B (•). (A-C) Data are expressed as the mean ± SD of 3 independent experiments. iAA indicates intracellular AA.
Transport and accumulation of vitamin C in HL60 and U266 cells. HL60 (A) and U266 (B) cells were incubated for the time indicated with 250 μM dehydroascorbic acid (DHA; ○) or ascorbic acid (AA; •) at room temperature. Intracellular accumulation of vitamin C in HL60 and U266 cells treated with DHA for 60 minutes at 37°C (A-B insets). (C) Inhibition of DHA transport in U266 cells treated with cytochalasin E (Cyt E; ○) and B (•). (A-C) Data are expressed as the mean ± SD of 3 independent experiments. iAA indicates intracellular AA.
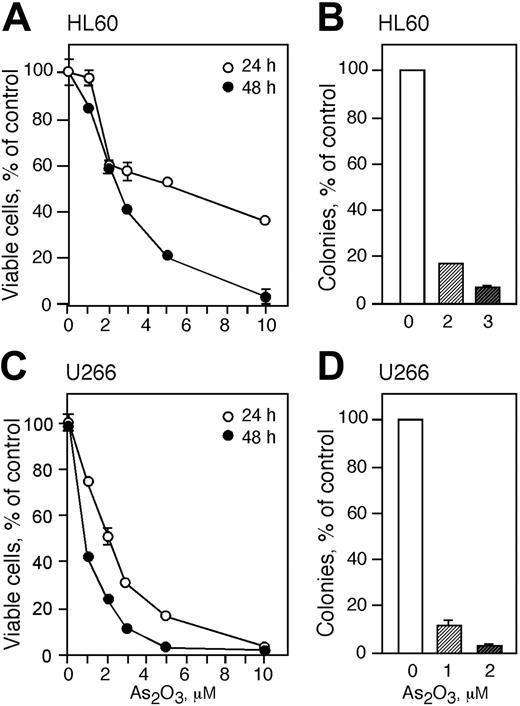
Arsenic trioxide (As2O3) is cytotoxic to HL60 and U266 cells
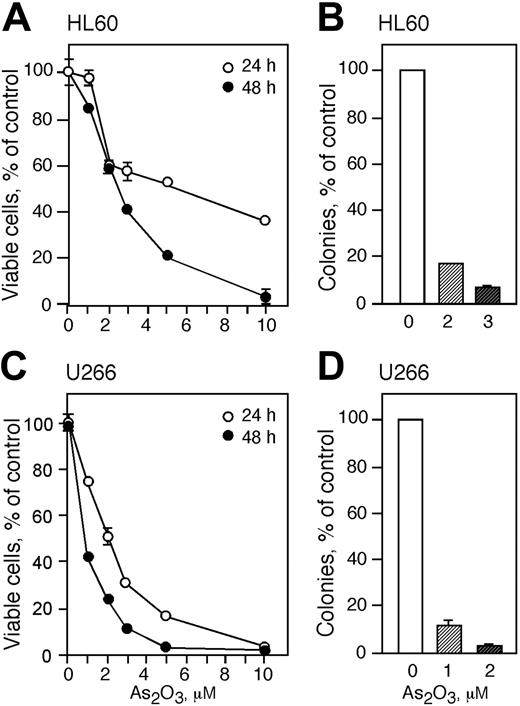
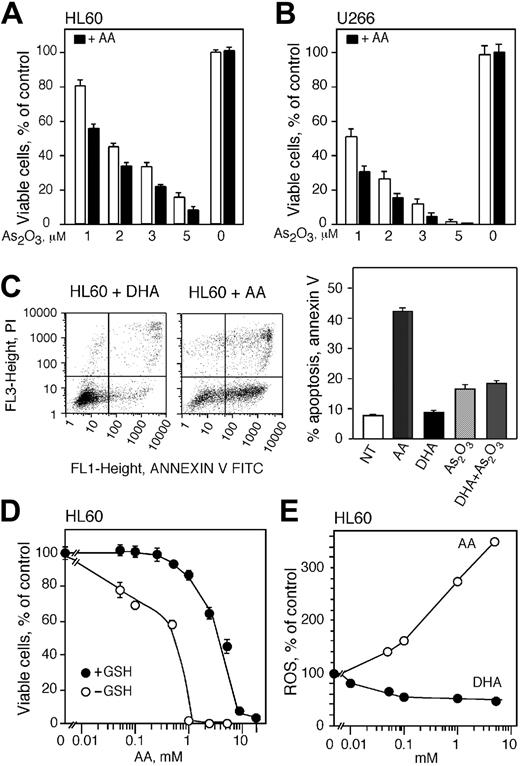
Cells were treated with increasing concentrations of As2O3 for 24 and 48 hours and viable cells were monitored by trypan blue exclusion and proliferative potential was measured by colony formation in methylcellulose (Figure 2). HL60 cells were more resistant to As2O3 than U266 cells and treatment with 1 μM As2O3 for 24 hours did not result in a change of viable cell numbers (Figure 2A). Treatment with 2 and 3 μM As2O3 for 24 hours decreased cell viability to approximately 60% whereas higher concentrations (5 and 10 μM) resulted in viable cell counts of 55% and 40%, respectively. Exposing HL60 cells to 1, 2, 3, and 5 μM As2O3 for 48 hours reduced viable cell counts to 85%, 60%, 40%, and 20%, respectively. Cell viability was reduced to zero in cells treated with 10 μM As2O3 for 48 hours. Since 2 and 3 μM As2O3 decreased the cell viability to about 50%, we used these concentrations to further study the cytotoxicity of As2O3 in HL60 cells. Treatment of HL60 cells with 2 or 3 μM As2O3 reduced the cloning capacity to about 18% and 5%, respectively (Figure 2B).
U266 cells were more sensitive than HL60 cells to As2O3 toxicity and viability in cells treated for 24 hours with 1, 2, 3, 5, and 10 μM As2O3 decreased to 75%, 50%, 30,% 18%, and 0%, respectively (Figure 2C). Exposing U266 cells to As2O3 concentrations of 1, 2, and 3 μM for 48 hours lowered the viability to 40%, 20%, and 10%, respectively. We subsequently used 1 and 2 μM As2O3 for the rest of the studies with U266 cells. Treatment of U266 cells with 1 or 2 μM As2O3 reduced the colony-forming capacity of the cells to about 10% and 3% of control, respectively (Figure 2D). There was no colony formation in cells treated with higher concentrations of As2O3.
As2O3 is cytotoxic to HL60 and U266 cells. HL60 (A) and U266 (C) cells were treated with 1, 2, 3, 5, and 10 μMAs2O3 for 24 (○) and 48 (•) hours and viability was determined by trypan blue exclusion. Cloning efficiency of HL60 (B) and U266 (D) cells treated with 1 to 3 μM As2O3 or were left untreated. (A-D) Data are expressed as the mean ± SD of 3 independent experiments.
As2O3 is cytotoxic to HL60 and U266 cells. HL60 (A) and U266 (C) cells were treated with 1, 2, 3, 5, and 10 μMAs2O3 for 24 (○) and 48 (•) hours and viability was determined by trypan blue exclusion. Cloning efficiency of HL60 (B) and U266 (D) cells treated with 1 to 3 μM As2O3 or were left untreated. (A-D) Data are expressed as the mean ± SD of 3 independent experiments.
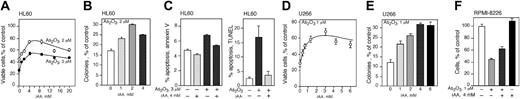
Vitamin C protects HL60, U266, and RPMI-8226 cells from As2O3 cytotoxicity
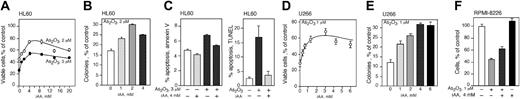
We tested the effect of vitamin C on As2O3 cytotoxicity by increasing the intracellular concentration of vitamin C in HL60 and U266 cells and then exposing them to various concentrations of As2O3. To avoid the artifactual generation of extracellular ROSs in the tissue culture media caused by direct addition of AA,37 we loaded cells with vitamin C by incubation with DHA.52,53 HL60 and U266 cells were incubated with DHA for 1 hour in amounts to achieve the desired intracellular concentration of vitamin C. Cells were then suspended in media containing the indicated concentration of As2O3 and after 48 hours cell viability and cloning efficiency was determined. Vitamin C loading protected both cell lines from arsenic toxicity in a concentration-dependent manner (Figure 3A-E). The viability of HL60 cells treated with As2O3 improved with increasing concentrations of vitamin C. Maximum protection was achieved at approximately 4 mM vitamin C with increased viability of approximately 1-fold at 2 and 3 μM As2O3 (Figure 3A). Vitamin C loading also protected U266 cells from the toxicity of 1 μMAs2O3 (Figure 3D). Maximum cell protection was achieved at 3 mM intracellular vitamin C with a 1-fold increase in viability. In both cell lines, higher concentrations of vitamin C did not increase protection from arsenic toxicity (Figure 3A,D). Vitamin C loading also protected U266 and HL60 cells from arsenic toxicity as measured by the capacity to proliferate and form colonies in methylcellulose (Figure 3B,E). U266 and HL60 cells were maximally protected from 1 and 2 μM As2O3 with 4 and 2 mM intracellular vitamin, respectively (Figure 3B,E). We found that 4 mM intracellular AA inhibited arsenic-induced apoptosis in HL60 cells as measured by annexin V/FITC assay. Using this assay, cells treated with As2O3 showed an approximately 7-fold increase in apoptosis (Figure 3C left panel). Additionally, As2O3 induced a 6-fold increase in apoptosis in HL60 using a TUNEL assay, whereas cells loaded with 4 mM vitamin C and treated with 3 μM As2O3 were almost completely protected compared with cells treated with As2O3 alone (Figure 3C right panel). The myeloma cell line RPMI-8226 also showed amelioration of cytotoxicity by vitamin C loading. DHA treatment had no effect on RPMI-8226 cell viability (Figure 3F). RPMI-8226 cells incubated with 1 μM As2O3 had 46% viability whereas cells loaded with 4 mM AA showed 62% viability (Figure 3F).
Intracellular AA (iAA) protects HL60, U266, and RPMI-8226 cells from As2O3 cytotoxicity. Cell viability of HL60 (A) and U266 (D) cells loaded with vitamin C using DHA and exposed to As2O3 for 48 hours. Cloning efficiency of HL60 (B) and U266 (E) cells loaded with vitamin C and treated with 2 and 1 μMAs2O3, respectively. (C) HL60 cells were loaded with 4 mM iAA, treated with 3 μMAs2O3 for 24 or 48 hours, and apoptotic cells were stained using annexin V–FITC assay (left panel) or a TUNEL assay (right panel), respectively. (F) Cell viability of RPMI-8226 cells loaded with 4 mM AA and exposed to 1 μMAs2O3 for 48 hours. (A-F) Data are expressed as the mean ± SD of 3 independent experiments.
Intracellular AA (iAA) protects HL60, U266, and RPMI-8226 cells from As2O3 cytotoxicity. Cell viability of HL60 (A) and U266 (D) cells loaded with vitamin C using DHA and exposed to As2O3 for 48 hours. Cloning efficiency of HL60 (B) and U266 (E) cells loaded with vitamin C and treated with 2 and 1 μMAs2O3, respectively. (C) HL60 cells were loaded with 4 mM iAA, treated with 3 μMAs2O3 for 24 or 48 hours, and apoptotic cells were stained using annexin V–FITC assay (left panel) or a TUNEL assay (right panel), respectively. (F) Cell viability of RPMI-8226 cells loaded with 4 mM AA and exposed to 1 μMAs2O3 for 48 hours. (A-F) Data are expressed as the mean ± SD of 3 independent experiments.
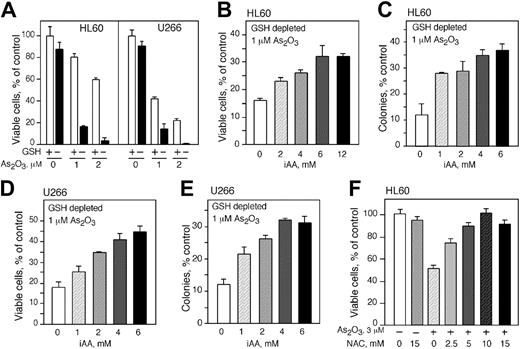
Vitamin C protects GSH-depleted cells from As2O3 toxicity
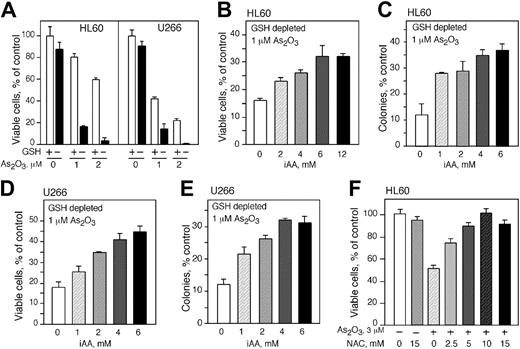
Although the importance of GSH for the survival of As2O3-treated cells has been described previously, the ability of vitamin C to rescue these cells and replace GSH as an antioxidant has not been delineated. We depleted GSH from the cell lines and loaded them with vitamin C before As2O3 treatment. Depletion of GSH had no significant effect on cell viability; however, depletion of GSH from HL60 and U266 cells increased their susceptibility to As2O3 toxicity (Figure 4A). The viability of HL60 cells treated with 1 and 2 μMAs2O3 decreased from 80% and 60% to approximately 20% and 5%, respectively (Figure 4A). Similarly, the viability of U266 cells treated with 1 and 2 μMAs2O3 declined from 40% and 25% to 20% and 3%, respectively, in GSH-depleted cells (Figure 4A). HL60 cells depleted of GSH and loaded with vitamin C using DHA became more resistant to As2O3. Increasing concentrations of vitamin C improved cell survival and provided protection in a concentration-dependent manner. Maximum protection was seen at approximately 6 mM intracellular vitamin C and higher concentrations of intracellular vitamin C did not improve the level of protection (Figure 4B). Clonogenicity studies confirmed the viability assays. GSH-depleted HL60 cells loaded with vitamin C and treated with As2O3 produced substantially more colonies compared with cells treated with As2O3 alone. Optimal protection was found in cells containing 4 mM intracellular vitamin C, a 2-fold difference compared with the control. There was no increase in clonogenicity with higher concentrations of vitamin C (Figure 4C). Comparable results were obtained with U266 cells (Figure 4D-E). GSH-deprived cells were more resistant to As2O3 when they contained vitamin C. Cell viability was 1.5-fold higher in U266 cells containing 6 mM intracellular vitamin C and treated with 1 μM As2O3 compared with cells treated with As2O3 alone (Figure 4D). Also, an approximately 2-fold increase in colony-forming capacity was seen in cells containing 4 mM intracellular vitamin C and treated with 1 μM As2O3 compared with cells treated with As2O3 alone (Figure 4E). Another antioxidant, NAC, which may act to increase the intracellular concentration of GSH in cells, protected HL60 cells from the cytotoxicity of As2O3 (Figure 4F). Incubation of cells with 2.5, 5, 10, and 15 mM of NAC provided increasing protection, and 10 mM NAC completely abrogated the toxicity of 3 μMAs2O3 (Figure 4F).
Intracellular AA (iAA) protects HL60 and U266 cells depleted from GSH and treated with As2O3. As2O3 toxicity in HL60 (A) and U266 (D) cells with (□) or without (▪) GSH. Cell viability (B,E) and colony formation (C,F) of HL60 and U266 cells depleted of GSH, loaded with vitamin C, and treated with 1 μMAs2O3. Cell viability was determined 48 hours after treatment. (A-F) Data are expressed as the mean ± SD of 3 independent experiments.
Intracellular AA (iAA) protects HL60 and U266 cells depleted from GSH and treated with As2O3. As2O3 toxicity in HL60 (A) and U266 (D) cells with (□) or without (▪) GSH. Cell viability (B,E) and colony formation (C,F) of HL60 and U266 cells depleted of GSH, loaded with vitamin C, and treated with 1 μMAs2O3. Cell viability was determined 48 hours after treatment. (A-F) Data are expressed as the mean ± SD of 3 independent experiments.
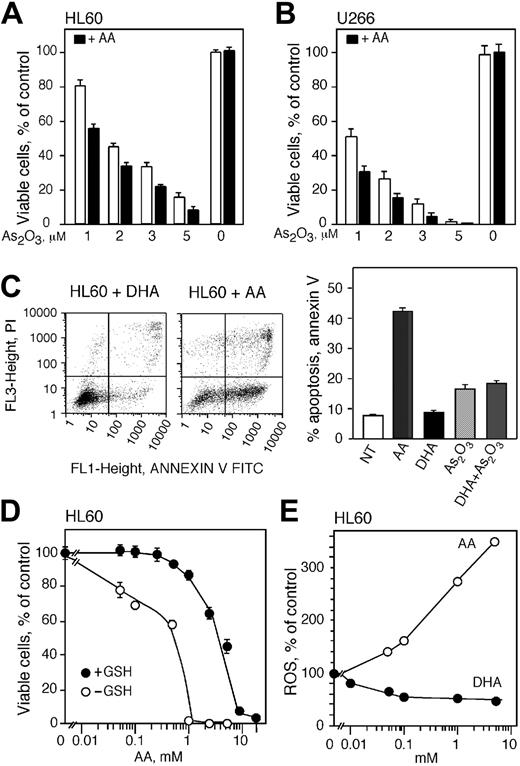
Extracellular vitamin C increases the toxicity of As2O3 in vitro by generating ROSs
As reported by others,24,28 the addition of ascorbic acid to cell cultures increased the toxicity of As2O3 (Figure 5). The viability of HL60 and U266 cells decreased when cells were treated with 100 μM AA and As2O3 compared with cells treated with As2O3 alone (Figure 5A-B). The viability of HL60 and U266 cells declined from 80% to 57% and 50% to 30%, respectively, in cells treated with 100 μM AA and 1 μM As2O3 compared with cells treated with As2O3 alone (Figure 5A-B). A comparable decrease in cell viability was detected in cells treated with 100 μM AA and 2, 3, and 5 μM As2O3. Addition of DTT to cell cultures incubated with AA did not change the cell viability (data not shown). We found that cells pretreated with DHA accumulate AA that protects against As2O3-induced cytotoxicity. To determine the effect of DHA when incubated in the media as for the AA treatments, HL60 cells were incubated for 20 hours with 1 mM DHA or AA and apoptosis was measured using annexin V/FITC assay (Figure 5C). DHA treatment did not induce apoptosis, however, AA at the same concentration resulted in a 40% increase in apoptosis (Figure 5C right panel). Additionally, we found that DHA in the media incubated simultaneously with As2O3 did not enhance the cytotoxicity of As2O3 (Figure 5C, right panel).
AA is known to generate ROSs in culture media without cells,37 and high concentrations of AA generate quantities of ROSs that can kill cells in the absence of other agents. We examined the independent toxicity of AA by incubating HL60 cells with AA and assessing cell viability 48 hours after treatment. There were no detectable changes in cell viability in cells treated directly with AA up to 0.5 mM AA (Figure 5D). Higher concentrations of AA (2.5 and 5 mM) reduced cell viability to approximately 60% and 40%, respectively. At very high concentrations of AA (10 and 20 mM) the entire population of cells was killed (Figure 5D). Decreasing the antioxidant capacity of the cells by depleting intracellular GSH greatly sensitized HL60 cells to the oxidative stress generated by extracellular AA in culture media (Figure 5D). The viability of GSH-depleted HL60 cells treated with 0.05 and 0.1 mM AA for 48 hours was reduced to 80% and 70%, respectively. In cells treated with 1 mM AA the viability was reduced to zero compared with 90% viability in cells containing GSH (Figure 5D). These studies indicate that GSH protects cells from ROSs generated by AA in vitro and cells containing low levels of GSH are more susceptible to ROSs generated by AA in the culture media.
Extracellular ascorbic acid generates ROSs and augments the cytotoxicity of As2O3. Cell viability of HL60 (A) and U266 cells (B) treated with 1, 2, 3, and 5 μMAs2O3 with (▪) or without (□) 100 μM extracellular ascorbic acid for 48 hours. (C, left) Annexin V (FL1) and PI (FL3) flow cytometry analysis of apoptotic HL60 cells treated with AA or DHA for 20 hours. (Right) Percentage of apoptotic cells after treatment with either AA, DHA, As2O3, or the combination of DHA and As2O3.NT indicates cells not treated. (D) Cell viability of HL60 cells containing (•) or depleted of (○) GSH and treated with increasing concentrations of ascorbic acid in culture media. Cell viability was determined 48 hours after treatment. (E) Production of ROSs in HL60 cells loaded with vitamin C using DHA (•) and in cells where ascorbic acid was added in culture media (○). Data are expressed as the mean ± SD of 3 (A-B) and 2 (C-E) independent experiments.
Extracellular ascorbic acid generates ROSs and augments the cytotoxicity of As2O3. Cell viability of HL60 (A) and U266 cells (B) treated with 1, 2, 3, and 5 μMAs2O3 with (▪) or without (□) 100 μM extracellular ascorbic acid for 48 hours. (C, left) Annexin V (FL1) and PI (FL3) flow cytometry analysis of apoptotic HL60 cells treated with AA or DHA for 20 hours. (Right) Percentage of apoptotic cells after treatment with either AA, DHA, As2O3, or the combination of DHA and As2O3.NT indicates cells not treated. (D) Cell viability of HL60 cells containing (•) or depleted of (○) GSH and treated with increasing concentrations of ascorbic acid in culture media. Cell viability was determined 48 hours after treatment. (E) Production of ROSs in HL60 cells loaded with vitamin C using DHA (•) and in cells where ascorbic acid was added in culture media (○). Data are expressed as the mean ± SD of 3 (A-B) and 2 (C-E) independent experiments.
We measured intracellular accumulation of ROSs in order to explain the difference in survival seen in cells treated with AA or loaded with vitamin C using DHA (Figure 5E). HL60 cells were incubated with DHA for 60 minutes and ROS was measured as described. Incubation of HL60 cells with increasing concentrations of DHA decreased the amount of intracellular ROSs progressively with 50% reduction of ROSs at 0.1 mM DHA (Figure 5E), corresponding to an intracellular vitamin C concentration of 4 mM. The ROS level did not decline further in cells treated with up to 5 mM DHA. Exposure of cells to AA in culture media for 60 minutes increased the accumulation of cellular ROSs significantly (Figure 5E). Incubation with 0.1 mM AA raised the level of ROSs by 60% compared with untreated cells. Greater concentrations of AA elevated ROSs even further and 1 and 5 mM AA increased ROSs by approximately 1.8- and 2.6-fold, respectively (Figure 5E).
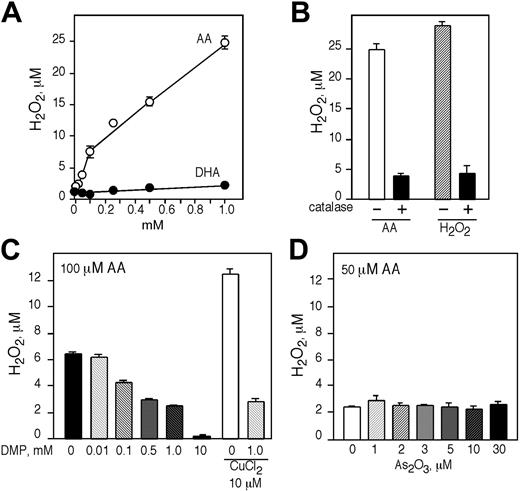
AA generates H2O2 in cell-free culture media and its generation is dependent on the availability of free transition metal ions
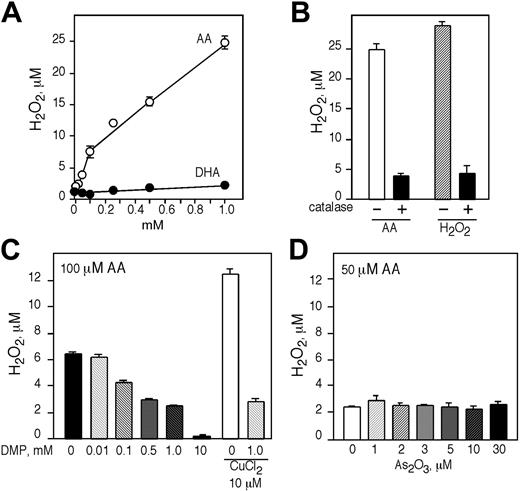
Since direct addition of AA to cells resulted in increased intracellular concentration of ROSs, we tested if AA could generate H2O2 in IMDM in the absence of cells. The generation of H2O2 by AA in cell-free medium was concentration dependent with 0.05, 0.1, 0.25, 0.5, and 1.0 mM of AA generating approximately 4, 7.5, 12, 15, and 25 μMH2O2, respectively (Figure 6A). Comparable concentrations of DHA did not generate any H2O2, identical to the control media alone (Figure 6A). The addition of 500 U/mL catalase resulted in a reduction of H2O2 generated by 1.0 mM AA from about 25 μMto4 μM (Figure 6B). Similar results were obtained when H2O2 was added to IMDM containing 500 U/mL catalase (Figure 6B). We then tested if the generation of H2O2 in cell-free media by AA was dependent on the presence of free transition metal ions in the culture media. IMDM containing 100 μM AA was incubated with increasing concentrations of the transition metal chelator DMP for 1 hour at 37°C and H2O2 generation was quantified. In the absence of DMP, 100 μM AA generated about 7 μM H2O2 and increasing concentrations of DMP reduced the amount of H2O2 produced (Figure 6C). In media containing 0.1, 1.0, and 10.0 mM DMP the concentration of H2O2 generated was approximately 5, 2.5, and 0.5 μM, respectively (Figure 6C). DMP also reduced the amount of H2O2 generated in association with the addition of copper, a transition metal ion that interacts with AA to generate H2O2. Incubation of 10 μM CuCl2 and 100 μM AA generated about 13 μMH2O2, and in the presence of 1.0 mM DMP the concentration of H2O2 was reduced to 3 μM (Figure 6C). Another metal chelator, DTPA, reduced the generation of H2O2 in the presence of AA in a similar manner (data not shown).
AA generates H2O2 in a cell-free system that is dependent on the presence of free transition metal ions. (A) Different concentrations of AA (○) and DHA (•) were incubated in IMDM for 1 hour at 37°C and H2O2 was determined by Amplex red. (B) The generation of H2O2 by 1 mM AA in IMDM in the presence or absence of 500 U/mL catalase. (C) Generation of H2O2 by 100 μM of AA or 10 μM CuCl2 in IMDM and increasing concentrations of DMP. (D) Generation of H2O2 by 50 μM of AA and increasing concentrations of As2O3 or 10 μM CuCl2 in 50 mM sodium phosphate buffer (pH 7.4). (A-D) Experiments were performed in triplicate and are expressed as the mean ± SD of 2 independent experiments.
AA generates H2O2 in a cell-free system that is dependent on the presence of free transition metal ions. (A) Different concentrations of AA (○) and DHA (•) were incubated in IMDM for 1 hour at 37°C and H2O2 was determined by Amplex red. (B) The generation of H2O2 by 1 mM AA in IMDM in the presence or absence of 500 U/mL catalase. (C) Generation of H2O2 by 100 μM of AA or 10 μM CuCl2 in IMDM and increasing concentrations of DMP. (D) Generation of H2O2 by 50 μM of AA and increasing concentrations of As2O3 or 10 μM CuCl2 in 50 mM sodium phosphate buffer (pH 7.4). (A-D) Experiments were performed in triplicate and are expressed as the mean ± SD of 2 independent experiments.
We also examined whether AA directly interacted with As2O3 to generate H2O2 in a phosphate buffer and found that incubation of 50 μM AA with increasing concentrations of As2O3 (1-30 μM) did not result in H2O2 generation (Figure 6D). The addition of catalase partially inhibited the cytotoxic effect of AA and As2O3 in HL60 cells (approximately 20%; data not shown).
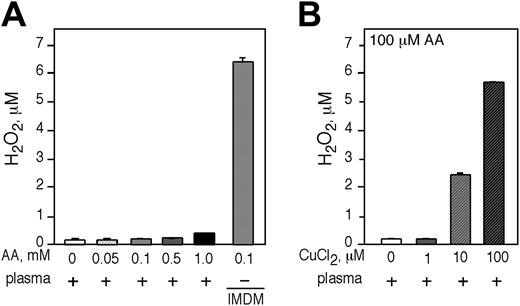
AA does not generate H2O2 in human plasma
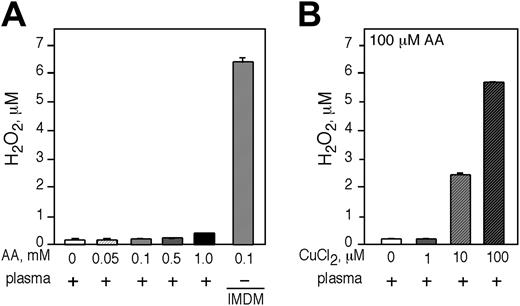
We sought to approximate the in vivo circumstance by testing if adding AA to human plasma led to the generation of H2O2.AAup to 1 mM was incubated in plasma for 1 hour and H2O2 was quantified. We did not detect significant H2O2 production by AA in plasma (Figure 7A). AA generated H2O2 only in tissue culture media (Figure 7A last bar). Addition of 100 μM of CuCl2 to human plasma was required to approximate the AA-dependent generation of H2O2 in IMDM (Figure 7B).
Discussion
The mechanisms by which As2O3 induces cell death are not completely elucidated, however, the generation of ROSs is a significant component of its cytotoxic action. Consistent with this notion is the observation that high levels of GSH are associated with cellular resistance to arsenic16,21,24,54-56 and decreasing intracellular GSH concentrations cause increased sensitivity to As2O3.24 Given the involvement of ROSs in the cytotoxicity of As2O3, it appears paradoxical that vitamin C, a strong antioxidant, enhances the cytotoxicity of As2O3 in vitro.24,28,29 In vivo, vitamin C protects GSH-depleted animals from death, and vitamin C and GSH have interchangeable and redundant antioxidant properties in protection of cells from oxidative stress.25,57 Based on the in vitro findings, clinical studies have been undertaken to ascertain if cotreatment of patients with AA to As2O3 could enhance the therapeutic efficacy of As2O3.32
AA does not generate H2O2 in human plasma and the generation of H2O2 in plasma is dependent on the presence of free transition metal ions. Incubation of freshly isolated human plasma with increasing concentrations of AA. (A) The amount of H2O2 generated was determined by Amplex red. As a positive control, 100 μM AA was incubated in IMDM (last bar). (B) The generation of H2O2 was determined in human plasma incubated with 100 μM AA and increasing concentrations of CuCl2. (A-B) Experiments were performed in triplicate and are expressed as the mean ± SD of 2 independent experiments.
AA does not generate H2O2 in human plasma and the generation of H2O2 in plasma is dependent on the presence of free transition metal ions. Incubation of freshly isolated human plasma with increasing concentrations of AA. (A) The amount of H2O2 generated was determined by Amplex red. As a positive control, 100 μM AA was incubated in IMDM (last bar). (B) The generation of H2O2 was determined in human plasma incubated with 100 μM AA and increasing concentrations of CuCl2. (A-B) Experiments were performed in triplicate and are expressed as the mean ± SD of 2 independent experiments.
The toxic effects of AA in vitro are attributed to the generation of ROSs and the consequent depletion of GSH from cells. The pro-oxidant and cytotoxic activity of AA in vitro is well known58-62 and depends on the reduction of free transition metal ions (Fe3+ to Fe2+ and Cu2+ to Cu+), which then act as catalysts to generate H2O2 and subsequently free radicals (O2·- and OH·) via Fenton chemistry.26,37,38,63 AA is known to generate H2O2 in tissue culture media without cells37,63,64 and catalase partially inhibits the cytotoxic effects of AA in vitro.13,24,37,58,65 Sequestering transition metal ions by metal binding compounds such as DTPA66,67 prevents the generation of H2O2 and the cytotoxic effects of AA. The direct toxic action of AA in vitro is enhanced because many cells cannot directly transport AA, which remains in the culture medium reducing transition metal ions and generating H2O2 and free radicals. Consequently, the pro-oxidant and cytotoxic properties of AA in vitro are thought to be an in vitro artifact.37,63,64
To circumvent the in vitro pro-oxidant effects of AA, we loaded HL60 and U266 cells (which do not transport AA directly) with vitamin C by incubating them with DHA.68-70 Cells loaded with vitamin C were significantly protected from As2O3 toxicity. Increasing concentrations of intracellular vitamin C in HL60, U266, and RPMI-8226 cells provided increasing protection from As2O3 cytotoxicity with maximum protection achieved at 2 to 8 mM intracellular AA. GSH-depleted cells showed the expected increased sensitivity to As2O3, and vitamin C loading protected these cells from As2O3 cytotoxicity even more prominently. In previous studies we showed that GSH-depleted cells were sensitized to the cytotoxic effect of H2O2 and cells loaded with vitamin C were protected.70
The protection of HL60 and U266 cells from the toxicity of As2O3 by DHA loading was likely due to the marked reduction of intracellular ROSs. Treating cells directly with AA prominently increased intracellular ROSs, and high concentrations of AA were quite cytotoxic on themselves with a 50% inhibitory concentration (IC50) of 5 mM. The cytotoxicity of AA was greatly enhanced when GSH was depleted and the IC50 was reduced from 5 to 0.5 mM. H2O2 is a major ROS product generated in vitro by AA and evidence suggested that the enhancement of arsenic's toxicity by AA is partly H2O2 dependent, as killing in cells cotreated with As2O3 and AA is inhibited in the presence of catalase.24 We confirmed that AA causes the generation of H2O2 in cell-free tissue culture media with 25 μMH2O2 generated at 1 mM AA in 1 hour at 37°C. Unlike AA, DHA did not stimulate the production of H2O2 in tissue culture media. Chelators of free transition metal ions blocked the production of H2O2 by AA and we found no effect of AA with As2O3 in terms of H2O2 production in buffers lacking transition metal ions.
To approximate in vivo conditions, we performed experiments to determine if AA generated H2O2 in human plasma. Concentrations of AA as high as 1 mM resulted in no measurable H2O2 generation. These results are consistent with the observation that adding increasing amounts of human serum or plasma to in vitro–cultured cells reduces the cytotoxicity of AA, presumably by sequestering free transition metal ions.60,61,71 Recently it was shown that AA does not act as a pro-oxidant toward lipids and proteins in human plasma exposed to both transition metal ions and H2O2.72 There is little evidence that vitamin C acts as a pro-oxidant in vivo largely because transition metals in plasma and tissues are sequestered by metal binding proteins, such as transferrin and caeruloplasmin, and are not available to generate free radicals. 26,27,63,64,73,74 In vivo, vitamin C has been shown to function as a strong antioxidant even in the presence of an iron overload.75-77
Intracellular signal transduction is often ROS dependent and antioxidants are known to inhibit signaling.78 For example, vitamin C inhibits granulocyte-macrophage colony stimulating factor (GM-CSF) responses53 and nuclear factor κB (NFκB) activation.79 We found that vitamin C inhibits NFκB activation prior to IκB kinase activation.79 Inhibition of NFκB by vitamin C could enhance cellular apoptosis. The notion that AA will increase the therapeutic effect of As2O3 in the treatment of myeloma is currently being tested in clinical trials.32 These studies are based on the hypothesis that AA could enhance the therapeutic efficacy of As2O3 by a mechanism similar to the one shown in vitro in which AA was cytotoxic and decreased the intracellular concentration of GSH. Although, the authors believe that their data show that AA decreases GSH levels in mononuclear cells, the GSH levels were not consistently lowered nor was it clear that the effect was not due to As2O3. Evidence from studies in vivo indicated that vitamin C and GSH have functional interchangeability, sparing each other in antioxidant defense.25,57 More importantly, a direct study in humans indicated that AA increased GSH levels in lymphocytes.80 In vivo animal experiments have not shown that a combination of vitamin C and As2O3 leads to increased tumor responses. Recher et al31 found that AA administration did not enhance the effect of As2O3 in a T-cell prolymphocytic leukemia model in mice. Also, the data in a P338D1 lymphoma model24 do not appear statistically compelling. In those experiments, As2O3 itself did not increase survival.24 Other studies in rats indicate that vitamin C ameliorated sodium arsenite–induced toxicity.33-36 Similarly, vitamin E inhibits arsenic cytotoxicity in vitro and in vivo.33,35,81
The pro-oxidant effects of AA in vitro and the wide belief in potential antitumor effects of vitamin C have lead to confusion regarding antioxidants in cancer therapy. While vitamin C can effect many signaling events in cells,53,79 it undoubtedly functions as a strong antioxidant. We provided evidence that vitamin C can decrease oxidative-induced mutations52 and we and others have shown that vitamin C can protect cells from H2O2 and radiation.68-70 The evidence presented here indicates that increasing intracellular concentrations of vitamin C ameliorates the cytotoxicity of As2O3, likely by quenching ROSs generated intracellularly. These results suggest that AA is not likely to increase the antineoplastic activity of arsenic but could provide protection to both normal and neoplastic cells.
Prepublished online as Blood First Edition Paper, January 27, 2005; DOI 10.1182/blood-2003-03-0772.
Supported by grant no. R01 CA30388 from the National Institutes of Health, the NY State Department of Health grant M020313, and the Schultz and Lebensfeld Foundations.
Our mentor and inspiration behind this work, David W. Golde, died on August 9, 2004.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.