Several studies suggest a poor prognosis for patients with myelofibrosis (MF) with an abnormal karyotype, but the prognostic impact of specific cytogenetic lesions is difficult to define.1 Using conventional cytogenetics, Tefferi et al were able to demonstrate that +8 and 12p- were associated with inferior survival in 165 MF patients.2 Notably, abnormal metaphases were obtained in only 48% of patients at diagnosis, most likely due to the low proliferative capacity of MF tumor cells in vitro. Thus, the prevalence of defined chromosomal aberrations is a matter of uncertainty, and correlations of cytogenetic findings to clinical course are hampered by the low number of informative cases. Interphase cytogenetic analysis (fluorescence in situ hybridization [FISH]) can overcome some of these limitations, as suggested by a small study including 42 MF patients.3
To obtain further information on the prognostic impact of specific chromosomal aberrations, we have retrospectively studied 107 MF patients from 2 institutions. FISH was performed on deparaffinized bone marrow sections as described previously,4 using commercially available probes for chromosomal regions 7q31, 12p, 13q14, 17p13.1, 20q13.2, 21q22, cen7, cen8, cen11, and cen17 (probes used in dual combinations; eg, cen7/7q31). The diagnostic bone marrow paraffin-block was obtained in 96 cases and from a later stage of disease in 11 patients. Cutoff values for FISH were based on 11 patients with lymphoma without bone marrow involvement. The cutoff for loss of signals was mean + 3 × SD of controls (ie, 11.33 + 3 × 5.75 for -cen7 and 11.82 + 3 × 6.17 for 7q31-). The mean number of interphase nuclei analyzed was 89.
There were 53 females and 54 males, with a median age of 68 years (range, 24-87 years). Of the patients, 28 (26%) were 60 years or younger, and 15 (14%) were 50 years or younger. There were 83 patients who had chronic idiopathic MF (CIMF), and 24 had secondary MF (19 postpolycythemic and 5 postthrombocythemic). Median follow-up was 34 months and median survival was 64 months.
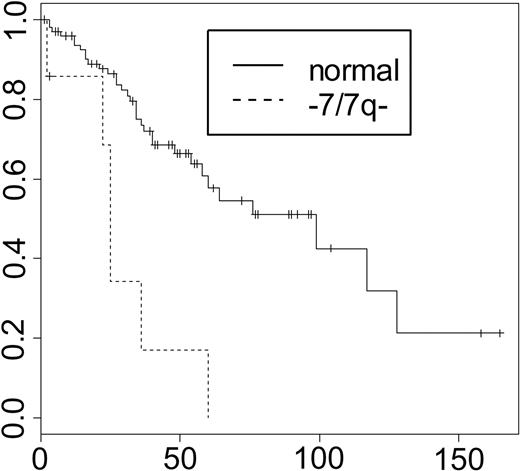
Neither the presence of any cytogenetic abnormality (56% of patients) nor the number of abnormalities (35.5% had more than one abnormality) was associated with prognosis.2 Of all defined cytogenetic abnormalities, only -7 and/or 7q- (6.5% of patients) were significantly associated with adverse prognosis in univariate and multivariate analyses including all cytogenetic parameters (Figure 1). In 6 of 7 patients, -7/7q- was associated with other chromosomal aberrations, but the presence of -7/7q- was not correlated with a specific second abnormality. The parameter “-7/7q-” remained prognostically significant in all patients and the subset of CIMF patients when compared with the LILLE score in multivariate analysis.5
In a recent report focusing on MF patients with leukemic transformation, -7/7q- were present in only 6.3% of patients at diagnosis, but in 19.6% at the time of transformation.6 In our series, the presence of -7/7q- did not correlate with leukemic transformation.
Survival by -7/7q-. Median survival was 99 and 25 months for good- and poor-risk patients, respectively (P < .001 for comparison of survival curves). The x-axis indicates survival in months; the y-axis, percent survival.
Survival by -7/7q-. Median survival was 99 and 25 months for good- and poor-risk patients, respectively (P < .001 for comparison of survival curves). The x-axis indicates survival in months; the y-axis, percent survival.
Chromosome 7 deletions were reported to be associated with adverse prognosis in acute myeloid leukemia and myelodysplastic syndrome.7,8 Our findings show an adverse impact in MF and underline the prognostic significance of chromosome 7 deletions in myeloid disorders.