Comment on May et al, page 4337
A monoclonal antibody against the immediate N-terminal region of VE-cadherin targets endothelial cell junction formation and inhibits nascent tumor vasculature, but spares normal vessels where the epitope is masked.
Monoclonal antibodies took a couple of decades to enter mainstream hematology and oncology, but are currently the leading type of molecule in the pipeline of many (if not most) pharmaceutical companies. After a first generation of antibodies were able to target cancer cells but were unable to distinguish them from their normal counterpart, a second generation of more specific molecules able to distinguish friend from foe is in sight. In this issue of Blood, May and colleagues explain why the E4G10 monoclonal antibody against VE-cadherin is able to target nascent vasculature (such as that of cancer) while sparing existing vessels.
Homology model of VE-cadherin. See the complete figure in the article beginning on page 4337.
Homology model of VE-cadherin. See the complete figure in the article beginning on page 4337.
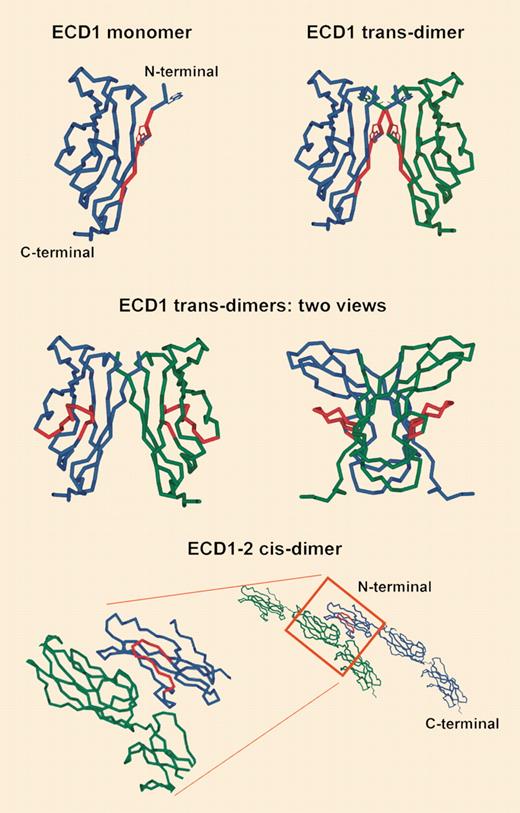
VE-cadherin is localized within endothelial cell–specialized structures called adherens junctions. It is crucial for cell-cell contacts, mediates homophilic adhesion between neighboring endothelial cells, and is constitutively expressed throughout the entire vasculature, representing one of the most endothelial-specific proteins known so far. Its importance is highlighted by the finding of embryonic (E9.5) lethality due to vascular assembly impairment in VE-cadherin–null mice, and it is involved in endothelial cell migration, survival, and growth; in vascular integrity; and in vessel tube formation.
In the present era of discovery and validation of antiangiogenic therapies for cancer and other diseases, VE-cadherin seems to be a very convenient target. However, among the first series of antimurine monoclonal antibodies generated against this protein, some showed broader preclinical effects than desired. BV13 and 10G4 monoclonal antibodies, for instance, were shown to inhibit angiogenesis and tumor growth, but also to disrupt normal vessels and to generate severe and life-threatening vascular leakage in treated mice. In sharp contrast, a third monoclonal antibody, E4G10, inhibited tumor growth in the absence of any disruptive activity against normal vessels.
May and colleagues have mapped the epitope of the nondisruptive E4G10 antibody to the immediate N-terminal region of VE-cadherin, and indicated that its tryptophan residues are needed for VE-cadherin–mediated endothelial cell trans-adhesion. Conversely, the disruptive BV13 and 10G4 antibodies target a different epitope that does not seem to be involved in trans-adhesion. Taken together, these data suggest that the target of the nondisruptive E4G10 antibody is available only on the monomer form of the molecule and in particular in nascent vessels prior to trans-dimerization. On already established vessels, conversely, at last part of the epitope is engaged in interactions upon trans-dimer formation. Thus, the epitope highlighted by the E4G10 antibody seems a very convenient target for any kind of therapy designed against neovessels. It defines where the foe (angiogenesis) is different from the friend (established vessels).
What's next? First, it is still unclear how the 2 disruptive BV13 and 10G4 antibodies destabilize adherens junctions. Secondly, the work of May and colleagues has focused on murine VE-cadherin, and the corresponding human protein should be studied in detail before selecting its most convenient epitope for patients' therapy. If friend and foe are not equal, this might be the case also for humans and mice. ▪