Abstract
We tested the hypothesis that interaction between autoantigens and chemoattractant receptors may be an important step in the development of autoimmunity. The retinal autoantigens S-antigen (S-Ag) and interphotoreceptor retinoid binding protein (IRBP) can induce autoimmune uveitis in rodent models. We evaluated the chemotactic activity of S-Ag and IRBP and found that both induced migration of human and mouse immature dendritic cells (iDCs) and lymphocytes, but not neutrophils, monocytes, or mature DCs. Cross-desensitization studies and single-receptor transfected cells revealed that subfamily of alpha chemokine receptors CXCR5 and CXCR3 mediated the chemotactic effect of IRBP, while only CXCR3 was required for the chemotactic response to S-Ag. Examination of the relationships between chemoattraction and the ability to elicit pathology at the protein or peptide levels in the mouse uveitis model revealed dissociation of the capacity to induce uveitis, lymphocyte proliferation, and chemoattraction. These studies suggest that IRBP and S-Ag can initiate innate and, in sensitive individuals, adaptive immune response by attracting iDCs and T and B cells expressing CXCR3 and CXCR5.
Introduction
We showed, in an earlier study, that histidyl- (HisRS) and asparaginyl-(AsnRS) transfer ribonucleic acid (tRNA) synthetases, which are cytoplasmic proteins involved in protein synthesis and also autoantigens in myositis, are chemotactic for a subset of lymphocytes and immature dendritic cells (iDCs).1 HisRS does not share amino acid sequence similarities with chemokines; nevertheless, it utilizes a chemokine receptor, subfamily of beta chemokine receptor CCR5, to induce migration. The autoantigenic epitope and chemoattractant domains appear to overlap. The capacity of autoantigens associated with myositis to recruit lymphocytes and iDCs by triggering select chemokine receptors led us to propose that this may be a general property of autoantigens in other autoimmune diseases. To test this hypothesis, we chose to evaluate the chemotactic activities and receptor interaction of 2 autoantigens involved in autoimmune uveitis.
Human autoimmune uveitis encompasses a spectrum of potentially blinding inflammatory eye diseases exemplified by sympathetic ophthalmia, Vogt-Koyanagi-Harada disease, Behçet disease, sarcoid uveitis, and others.2 Several self antigens such as the retinal S-antigen (S-Ag), tyrosinase-related proteins, and interphotoreceptor retinoid binding protein (IRBP) have been implicated in the pathogenesis of various forms of uveitis. Uveitis patients present with detectable antibodies and lymphocyte proliferation to these antigens.3-7 Autoimmune uveitis is thought to be primarily mediated by CD4+ T cells with a T-helper 1 (Th1)–like lymphokine profile in human disease and in animal models.2,8,9
IRBP and S-Ag can induce experimental autoimmune uveitis (EAU) in rodent models. IRBP is an extracellular 135 to 140 kDa glycoprotein in the photoreceptor matrix10 that is essential for normal retinal morphology and function due to its participation in the transport of all-trans retinol to the retinal pigment epithelium and regeneration of rhodopsin. Transcription of IRBP is limited to photoreceptors and pinealocytes.11 S-Ag (visual arrestin) is a 48-kDa intracellular photoreceptor protein, which is also found in the pineal gland, that quenches photoactivated rhodopsin.12 These 2 immunologically non-crossreactive proteins induce similar autoimmune pathologies in different rodent models.13 IRBP is uveitogenic in mice, while S-Ag is uveitogenic in guinea pigs; both elicit EAU in rats. Strains within each species vary in susceptibility to these antigens.
In the present study we investigated the ability of S-Ag and IRBP to act as chemoattractants for human and murine leukocytes, and sought to identify the corresponding chemoattractant receptors. The results indicated that both proteins are chemotactic for selected mononuclear leukocyte populations and use Gαi-protein–coupled subfamily of alpha chemokine receptors, CXCR3 and/or CXCR5. Unlike our observations with HisRS, the lymphoproliferative and chemotactic fragments of IRBP were at times distinct. Taken together, our data suggest that the ability of self antigens to be chemoattractants may be a general phenomenon resulting in the attraction of selected leukocyte populations to sites of tissue damage.
Materials and methods
Reagents
All chemokines and cytokines were obtained from the National Institutes of Health (NIH) cytokine repository or Peprotech (Rocky Hill, NJ). IRBP was isolated from bovine retinas, as previously described.14 Native bovine and recombinant human S-Ag were kindly provided by Drs Paul Hargrave and Hugh McDowell, and by Dr Clay Smith, respectively, of the University of Florida (Gainsville, FL). They were made and purified as described.15 Synthetic 20-residue peptides overlapping by 10 residues and spanning the first homologous repeat of human IRBP16 were generously provided by Dr L. A. Donoso from the Wills Eye Institute (Philadelphia, PA). Synthetic 20-residue peptides overlapping by 10 residues and representing the entire human S-Ag were synthesized by AnaSpec (San Jose, CA) using conventional solid-phase techniques as described.17 S-Ag peptide 1-20 (MAASGKTSKSEPNHVIFKKI) was resynthesized by Invitrogen (Carlsbad, CA) for the extracellular signal–related kinase (ERK) phosphorylation studies. Polyclonal rat antibodies against IRBP and polyclonal rabbit antibodies against S-Ag were generated in-house using the native bovine proteins. All other reagents were purchased from Sigma (St Louis, MO) unless otherwise noted. All monoclonal antibodies used to phenotype DCs were purchased from BD Biosciences (San Diego, CA), unless otherwise indicated.
Cells
Primary human leukocytes were isolated from fresh normal donor leukapheresis packs under an approved human subjects protocol (99-CC-0168). Approval for these studies was obtained from the National Cancer Institute Institutional Review Board (Frederick, MD). Informed consent was provided according to the Declaration of Helsinki. Subsets of cells were purified as previously reported.18 In some studies, percoll-purified lymphocytes or monocytes were cultured at 1 × 106 cells/mL in RPMI 1640 (BioWhittaker, Walkersville, MD) containing 10% fetal bovine serum (HyClone, Logan, UT) and 2 mM glutamine, and 100 U/mL each penicillin and streptomycin (Quality Biologicals, Gaithersburg, MD), with 100 U/mL of recombinant human interleukin-2 (rhuIL-2) for 16 hours or 7 days in a 5% CO2 humidified tissue-culture incubator. B cells were purified using positive-selection magnetic activated cell-sorting (MACS) beads as recommend by the manufacture (Miltenyi Biotech, Auburn, CA). The purity for all primary cell types was 90% or more. Human embryonic kidney-293 cell line (HEK-293) cells were cultured in Dulbecco modified Eagle medium (DMEM; BioWhittaker) containing 10% fetal bovine serum (HyClone) and 2 mM glutamine, 100 U/mL each penicillin and streptomycin (Quality Biologicals). Parental HEK-293 cells were transfected with linearized wild-type CXCR5 by electroporation.19 After selection in media containing 800 μg/mL antibiotic G418 (Geneticin; Life Technologies, Rockville, MD) for 2 weeks, cells were used for analysis.
Human immature DCs were generated from purified human peripheral blood monocytes (> 95%) as previously described20 and confirmed by flow cytometry analysis. Immature DCs were CD1a+ (clone H1149), CD14- (clone M5E2), CD40low (clone 5C3), CD83- (clone HB15ϵ), CD86low (clone 2331 [FUN-1]), human leukocyte antigen–DR (HLA-DR) medium (G46-6 [L243]). Mature DCs were generated by culturing iDCs with 1 μg/mL of lipopolysaccharide (LPS; Sigma L-9764) for 48 hours. Flow cytometry analysis showed the mature DCs (mDCs) to be CD83high, CD86high (clone 2331 [FUN-1]), HLA-DRhigh (G46-6 [L243]).
Murine immature DCs were generated from bone marrow (BM). BM was obtained by aspiration from the femurs and tibias of 8-to 10-week-old female B10.RIII or C57BL/6 mice as previously described.21,22 The 6-day-old cultures were harvested and stained with anti-CD11c antibody-conjugated microbeads (Miltenyi Biotech) to magnetically sort CD11c+ immature DCs. The purity of the sorted immature DCs was consistently more than 98% as analyzed by immunofluorescence CD11c staining. As a positive control, immature DCs generated from the bone marrow of C57Bl/6 or B10.RIII donor mice migrated to 10 ng/mL of the beta chemokine CCL5 with a chemotactic index (CI) of 7.0 or greater, but not to CCL21 (secondary lymphoid organ chemokine [SLC]; data not shown).
Chemotaxis
Cells were resuspended in chemotaxis media (RPMI 1640 media containing 1% bovine serum albumin, 25 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH 8.0) at 1-5 × 106 cells/mL. Chemokines diluted in chemotaxis medium were placed in the lower wells of a microBoyden chemotaxis chamber (NeuroProbe, Gaithersburg, MD). When primary leukocytes were analyzed, 5-μm polycarbonate membranes were placed over the chemokines. Detection of chemotaxis by lymphocytes required that the membranes be precoated with 50 μg/mL of fibronectin. When HEK transfectants were analyzed, 10-μm polycarbonate membranes precoated with 50 μg/mL rat tail collagen type 1 (Collaborative Biomedical Products, Bedford, MA) at 37°C for 2 hours were placed over the chemoattractants. After the microchemotaxis chamber was assembled, 50 μL of cells were placed in the upper wells. The filled chemotaxis chambers were incubated in a humidified CO2 incubator for 60 minutes (neutrophils, immature murine DCs), 90 minutes (monocytes, immature human DCs), 3 hours (lymphocytes), or 5 hours (HEK transfectants). After incubation, the membranes were removed from the chemotaxis chamber assembly followed by gently removing cells from the upper side of the membrane. The cells on the lower side of the membrane were stained using Rapid Stain (Richard Allen, Kalamazoo, MI). The number of migrated cells in 3 high-powered fields (× 200) was counted by light microscopy after coding the samples. Quantitation of human cell chemotaxis was computer assisted using the BIOQUANT program (R & M Biometrics, Nashville, TN). Results are expressed as the mean value of the migration of triplicate sample with the standard deviation shown by bars. CIs were calculated as follows: [(mean number of migrating cells in sample)/(mean number of cells in media control)].
Calcium mobilization
HEK-293 cells transfected to stably express CXCR5 or murine pre-B cell line 300-19 cells transfected to stably express CXCR3 were loaded with Fura-2 in the following manner; 2 × 107 cells/mL in loading medium (DMEM, 10% fetal calf serum [FCS]) were incubated with 5 μM Fura-2 am (Molecular Probes, Eugene, OR) for 30 minutes at room temperature in the dark. The dye-loaded cells were washed 3 times and resuspended in saline buffer (138 mM NaCl, 6mM KCl, 1 mM CaCl2, 19 mM HEPES pH 7.4, 5 mM glucose, and 0.1% bovine serum albumin [BSA]). The cells were then transferred into quartz cuvettes (2 × 106 cells in 2 mL) that were placed in a luminescence spectrometer (LS-50B; Perkin-Elmer, Beaconsfield, England). Stimulants at different concentrations were added in 20-μL volume to each cuvette at the indicated time points. The ratio of fluorescence at 340- and 380-nm wavelengths was calculated using the FL WinLab program (Perkin-Elmer).
Fluorescence-activated cytometry analysis
The cells were washed in ice-cold phospate-buffered saline (PBS) containing 1% fetal bovine serum followed by blocking nonspecific sites with anti-CD32, 500 ng/106 cells (StemCell Technologies, Vancouver, BC, Canada). CXCR3 (FAB160F) and CXCR5 (FAB190P) were purchased from R&D Systems (Minneapolis, MN). Fluorescence-activated cell-sorting (FACS) analysis was performed as previously described.23
ERK phosphorylation assay
CXCR3/300-19–transfected cells were cultured in media containing only 0.5% serum overnight. Cells (2 × 106) in 0.5 mL per sample were incubated at 37°C in the absence or presence of indicated concentrations of CXCL11 and S-Ag peptide 1 (0-5 μg/mL) for a specified period of time ranging from 0 to 30 minutes. Ice-cold PBS (1 mL) was added to stop the stimulation, and the cells were spun down at 400g for 5 minutes at 4°C. After complete removal of the supernatant, the cells were lysed by adding 50 μL of sodium dodecyl sulfate (SDS) sample buffer (62.5 mM Tris [tris(hydroxymethyl)aminomethane]–HCl, pH 6.8, at 25°C, 2% wt/vol SDS, 10% glycerol, 50 mM dithiothreitol [DTT], 0.01% bromphenol blue). The lysate was sonicated for 10 seconds on ice to shear DNA and boiled for 10 minutes afterward. Protein (60 μg) was loaded and separated on a 4% to 12% NuPAGE Bis-Tris Gel (Invitrogen) using NuPAGE MES SDS Running buffer (Invitrogen). After the electrophoresis, proteins in the gel were electrotransferred (30 V constant for 1 hour) onto a piece of Immobilon membrane (Millipore, Bedford, MA) using NuPAGE transfer buffer (12 mM Tris, 96 mM glycine, pH 8.3, 0.1% vol/vol antioxidant, 10% vol/vol methanol). The membrane was sequentially washed (1 × Tris-buffered saline [TBS] plus 0.1% Tween-20–0.025 M Tris, 0.137 M NaCl, 0.003 M KCl) blocked for 1 hour (TBS Tween-20 + 5% nonfat milk) at room temperature, washed, and incubated at 4°C overnight in the presence of 1/1000 dilution of rabbit anti–phospho-p44/42 (catalog no. 9101; Cell Signaling Technology, Beverly, MA). On the next day, the membrane was washed and incubated with 1/2000 dilution of horseradish peroxide (HRP)–conjugated anti–rabbit immunoglobulin G (IgG) (catalog no. 7074; Cell Signaling Technology) for 1 hour. The membrane was washed and incubated with 4 mL working solution of ECL Plus Western Blotting Detection System (Amersham Biosciences, Piscataway, NJ) for 5 minutes at room temperature, and exposed to a piece of BioMax x-ray film (Kodak, Rochester, NY) for 5 seconds. The x-ray film was developed using an automatic processor (Kodak X-OMAT 200A). The same membrane was stripped and probed for p44/42 protein essentially in the same manner except using rabbit anti-p44/42 (catalog no. 9102; Cell Signaling Technology) as the primary antibody.
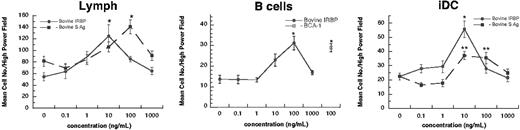
Human lymphocytes and iDCs migrate to IRBP and S-Ag. Freshly isolated human B cells (95% purity), 16-hour IL-2–cultured lymphocytes (Lymph; 90%), or monocyte-derived immature dendritic cells (iDC; 95%) were placed in the upper chamber of a micro-Boyden chemotaxis chamber. The number of migrated cells in 3 high-powered fields (× 200) was determined by light microscopy. The mean number of migrating cells and corresponding standard deviation are graphed on the y-axis while ligand concentrations are shown on the x-axis. • indicates bovine IRBP, while ▪ represents bovine S-Ag. Cells tested include lymph nodes cells (left), B cells (middle), and iDCs (right). For B cells, a control chemoattractant BCA-1 (CXCL13; □) is shown at 100 ng/mL. StatView Student t test (Abacus Concepts, Berkeley, CA) with *P ≤ .0001 for maximum chemotactic responses vs “0” control; **P ≤ .005. N ≥ 2. A representative experiment is shown. Error bars indicate standard deviation (s).
Human lymphocytes and iDCs migrate to IRBP and S-Ag. Freshly isolated human B cells (95% purity), 16-hour IL-2–cultured lymphocytes (Lymph; 90%), or monocyte-derived immature dendritic cells (iDC; 95%) were placed in the upper chamber of a micro-Boyden chemotaxis chamber. The number of migrated cells in 3 high-powered fields (× 200) was determined by light microscopy. The mean number of migrating cells and corresponding standard deviation are graphed on the y-axis while ligand concentrations are shown on the x-axis. • indicates bovine IRBP, while ▪ represents bovine S-Ag. Cells tested include lymph nodes cells (left), B cells (middle), and iDCs (right). For B cells, a control chemoattractant BCA-1 (CXCL13; □) is shown at 100 ng/mL. StatView Student t test (Abacus Concepts, Berkeley, CA) with *P ≤ .0001 for maximum chemotactic responses vs “0” control; **P ≤ .005. N ≥ 2. A representative experiment is shown. Error bars indicate standard deviation (s).
Results
IRBP and S-Ag are chemoattractants
First we investigated if any human leukocytes migrated in response to the EAU antigens bovine IRBP and S-Ag. The rodent, bovine, and human proteins are evolutionarily conserved and show extensive homology between species. Bovine and human proteins induce autoimmune uveitis in rodents, indicating that they are interchangeable as autoantigens.4,8,9,24,25 Lymphocytes (90% pure) and monocyte-derived iDCs (95% pure) migrated in response to bovine IRBP and S-Ag, while B cells (95% pure) migrated only to IRBP (Figure 1). Peripheral blood–derived neutrophils and monocytes did not migrate in response to these antigens (data not shown).
To demonstrate that IRBP and S-Ag induce chemotaxis rather than chemokinesis, we performed a checkerboard assay, which calls for increasing amounts of stimulant to be mixed with the migrating cells. When the concentration of the IRBP or S-Ag mixed with the cells was equal to or greater than the concentration eliciting an optimal response, the number of migrating cells was decreased to the level of spontaneous migration, indicating that these antigens induce directional chemotaxis (n = 3, Tables 1, 2). The maximal response (chemotactic index [CI] = 2.3) to bovine IRBP was observed at 10 ng/mL (0.07 nM); the maximal response (CI = 1.7) to bovine S-Ag was at 100 ng/mL (2.3 nM). The chemotaxis induced by 100 ng/mL of S-Ag was significantly greater than spontaneous migration (Student t test, P ≤ .005).
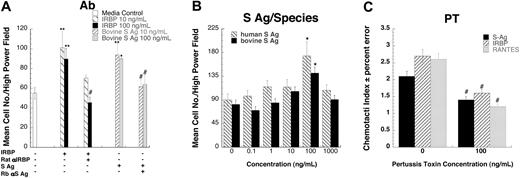
To provide further evidence that these autoantigens are indeed chemotactic, we tested the effects of neutralizing polyclonal antibodies to IRBP or S-Ag on chemotactic responses. Rat polyclonal anti-IRBP completely inhibited lymphocyte migration induced by bovine IRBP, as did rabbit polyclonal anti–S-Ag with bovine S-Ag (Figure 2A.) Although the amino acid sequence of S-Ag is generally conserved across species, there are species-specific regions. Therefore, the chemotactic effects of recombinant human S-Ag were compared with bovine S-Ag. Both bovine (CI = 1.7) and human S-Ag (CI = 1.9) were chemotactic for human lymphocytes (Figure 2B), confirming the validity of results obtained with heterologous antigens in these assays. Next we investigated the possibility that IRBP and S-Ag utilize chemokine receptors, which are G-protein–coupled receptors of the Gαi subfamily (GαiPCR). Preincubating cells with pertussis toxin, which blocks the G-protein–mediated signal, inhibited both IRBP and S-Ag induced chemotaxis, implicating GαiPCR (Figure 2C).
IRBP- and S-Ag–induced leukocyte migration is specific and G-protein–coupled receptor mediated. (A) Antibodies against either bovine IRBP or S-Ag block lymphocyte migration. 10 (▧) or 100 (▪) ng/mL of IRBP were mixed with 20 μg/mL of rat polyclonal anti-IRBP prior to being placed in the lower wells of a micro-Boyden chemotaxis chamber. S-Ag (10, ▨, or 100, ▦, ng/mL) was mixed with 20 μg/mL of rabbit polyclonal anti–S-Ag prior to being placed in the lower wells of a micro-Boyden chemotaxis chamber. Lymphocytes cultured for 16 hours with IL-2 were placed in the upper wells. The mean number of migrating cells and corresponding standard deviation are graphed on the y-axis while chemoattractant and antibody conditions are shown on the x-axis. □ indicates media control. n = 3. A representative experiment is shown. (B) Both human and bovine S-Ag induce lymphocyte migration. Various concentrations of S-Ag were placed in the lower well of a micro-Boyden chemotaxis chamber. Human lymphocytes cultured for 16 hours with IL-2 were placed in the upper wells. The mean number of migrating cells and corresponding standard deviation are graphed on the y-axis while ligand concentrations are shown on the x-axis. ▧ represents human S-Ag; ▪, bovine S-Ag. n = 2. A representative experiment is shown. (C) Both S-Ag–and IRBP-induced lymphocyte migration is pertussis toxin sensitive. Human lymphocytes cultured for 16 hours with IL-2 were pretreated for 30 minutes at 37°C with increasing amounts of pertussis toxin. Various concentrations of chemoattractants were placed in the lower wells of a micro-Boyden chamber. Pertussis toxin–pretreated lymphocytes were placed in the upper wells. ▪ indicates S-Ag; ▨, IRBP; and ▦, regulated on activation, normal T-cell–expressed and secreted (RANTES). *StatView Student t test with P ≤ .0001 for maximum chemotactic responses vs “0” control; **P ≤ .005. Inhibited chemotaxis that was not significantly different from media control was noted with #. n = 3. Representative experiment is shown. Error bars indicate standard deviation.
IRBP- and S-Ag–induced leukocyte migration is specific and G-protein–coupled receptor mediated. (A) Antibodies against either bovine IRBP or S-Ag block lymphocyte migration. 10 (▧) or 100 (▪) ng/mL of IRBP were mixed with 20 μg/mL of rat polyclonal anti-IRBP prior to being placed in the lower wells of a micro-Boyden chemotaxis chamber. S-Ag (10, ▨, or 100, ▦, ng/mL) was mixed with 20 μg/mL of rabbit polyclonal anti–S-Ag prior to being placed in the lower wells of a micro-Boyden chemotaxis chamber. Lymphocytes cultured for 16 hours with IL-2 were placed in the upper wells. The mean number of migrating cells and corresponding standard deviation are graphed on the y-axis while chemoattractant and antibody conditions are shown on the x-axis. □ indicates media control. n = 3. A representative experiment is shown. (B) Both human and bovine S-Ag induce lymphocyte migration. Various concentrations of S-Ag were placed in the lower well of a micro-Boyden chemotaxis chamber. Human lymphocytes cultured for 16 hours with IL-2 were placed in the upper wells. The mean number of migrating cells and corresponding standard deviation are graphed on the y-axis while ligand concentrations are shown on the x-axis. ▧ represents human S-Ag; ▪, bovine S-Ag. n = 2. A representative experiment is shown. (C) Both S-Ag–and IRBP-induced lymphocyte migration is pertussis toxin sensitive. Human lymphocytes cultured for 16 hours with IL-2 were pretreated for 30 minutes at 37°C with increasing amounts of pertussis toxin. Various concentrations of chemoattractants were placed in the lower wells of a micro-Boyden chamber. Pertussis toxin–pretreated lymphocytes were placed in the upper wells. ▪ indicates S-Ag; ▨, IRBP; and ▦, regulated on activation, normal T-cell–expressed and secreted (RANTES). *StatView Student t test with P ≤ .0001 for maximum chemotactic responses vs “0” control; **P ≤ .005. Inhibited chemotaxis that was not significantly different from media control was noted with #. n = 3. Representative experiment is shown. Error bars indicate standard deviation.
S-Ag and IRBP use CXCR3 and CXCR5
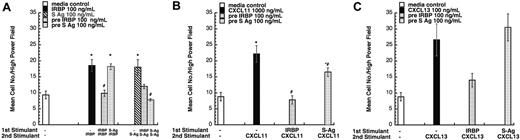
We performed desensitization assays to identify the receptors employed by these autoantigens. Pretreatment with IRBP desensitized the chemotactic response of iDCs to both S-Ag and IRBP, suggesting that IRBP and S-Ag share a receptor (Figure 3A). Pretreatment with S-Ag, however, only desensitized cells to itself but not to IRBP. Similar data were obtained for lymphocytes (data not shown). The failure of IRBP and S-Ag to induce neutrophils, monocytes, and mDCs to migrate excluded receptors expressed by those cells, such as CXCR1, CXCR2, CXCR4, CCR1, CCR2, CCR7, and CX3CR1. Therefore, we evaluated a number of chemokines that use other receptors as possible desensitizing ligands, including CXCL16 (a CXCR6 ligand), CCL1 (I309, a CCR8 ligand), CCL11 (eotaxin, a CCR3 ligand), CCL17 (TARC, a CCR4 ligand), CCL27 (CTAC, a CCR10 ligand), CCL25 (TECK, a CCR9 ligand), and XCL1 (lymphotactin, a XCR1 ligand). None of these ligands desensitized lymphocyte migration to IRBP or S-Ag. Furthermore, a number of single receptor–expressing human embryonic kidney (HEK-293) cells, including CCR5 and CCR6, did not migrate to IRBP or S-Ag.
IRBP and S-Ag receptor crosstalk. (A) IRBP and S-Ag share a common chemoattractant receptor. Monocyte-derived iDCs were subjected to cross-desensitization analysis. Pretreating iDCs with 100 ng/mL IRBP (▨) inhibited iDC migration to both IRBP and S-Ag. In contrast, pretreating iDCs with 100 ng/mL of S-Ag (▦) only inhibited iDC migration to S-Ag. (B) IRBP and S-Ag desensitize iDC migration to CXCL11 (ITAC). Pretreating iDCs with 100 ng/mL IRBP (▨) or 100 ng/mL of S-Ag (▦) inhibited iDC migration to CXCL11. (C) IRBP desensitizes iDC migration to CXCL13 (BCA-1). Pretreating iDCs with 100 ng/mL IRBP (▨) inhibited iDC migration to CXCL13. *StatView Student t with P ≤ .0001 for maximum chemotactic responses vs “0” control; **P ≤ .005. Inhibited chemotaxis that was not significantly different from media control (□) was noted with #. n = 3. Representative experiments are shown. Error bars indicate standard deviation.
IRBP and S-Ag receptor crosstalk. (A) IRBP and S-Ag share a common chemoattractant receptor. Monocyte-derived iDCs were subjected to cross-desensitization analysis. Pretreating iDCs with 100 ng/mL IRBP (▨) inhibited iDC migration to both IRBP and S-Ag. In contrast, pretreating iDCs with 100 ng/mL of S-Ag (▦) only inhibited iDC migration to S-Ag. (B) IRBP and S-Ag desensitize iDC migration to CXCL11 (ITAC). Pretreating iDCs with 100 ng/mL IRBP (▨) or 100 ng/mL of S-Ag (▦) inhibited iDC migration to CXCL11. (C) IRBP desensitizes iDC migration to CXCL13 (BCA-1). Pretreating iDCs with 100 ng/mL IRBP (▨) inhibited iDC migration to CXCL13. *StatView Student t with P ≤ .0001 for maximum chemotactic responses vs “0” control; **P ≤ .005. Inhibited chemotaxis that was not significantly different from media control (□) was noted with #. n = 3. Representative experiments are shown. Error bars indicate standard deviation.
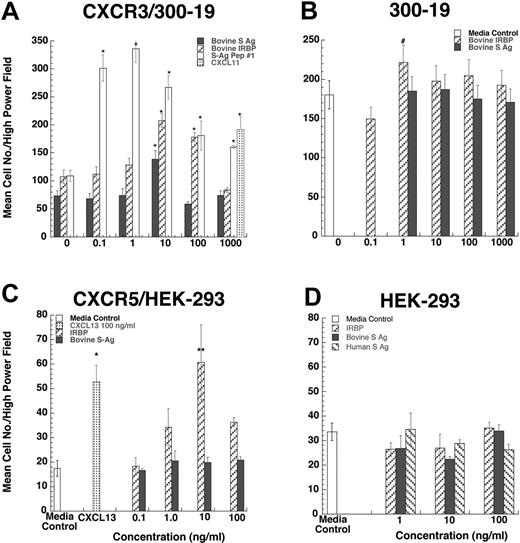
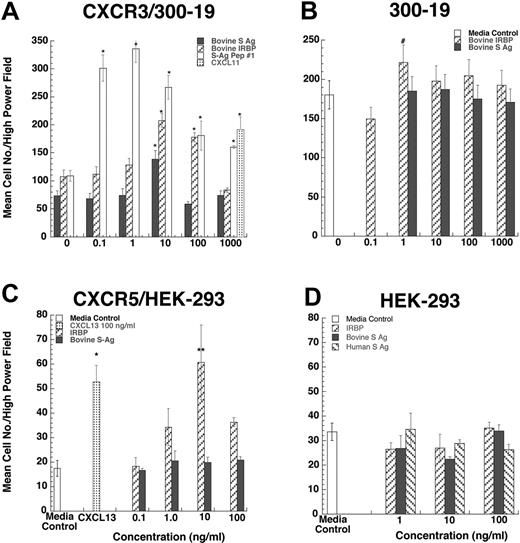
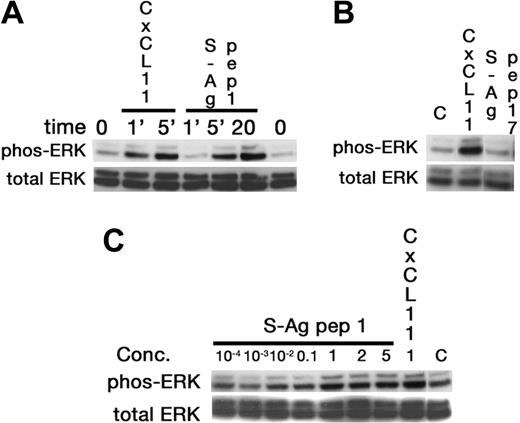
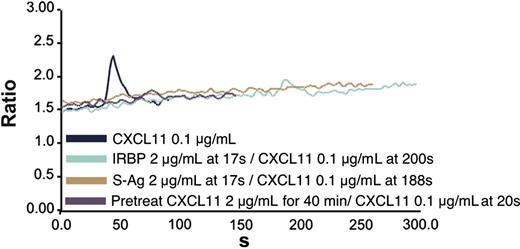
Since lymphocytes and iDCs express CXCR3 and CXCR5, we determined whether pretreatment with IRBP and S-Ag could desensitize migration of iDCs to either CXCL11 (ITAC, a CXCR3 ligand) or CXCL13 (BCA, a CXCR5 ligand). Pretreatment with IRBP desensitized iDCs to both CXCL11 and CXCL13, whereas pretreatment of iDCs with S-Ag partially decreased their response only to CXCL11 (Figure 3B-C). Furthermore, IRBP, S-Ag and S-Ag peptide 1-20 (labeled S-Ag no. 1) induced cells that express CXCR3 to migrate (Figure 4A-B) and IRBP also induced CXCR5–transfected cells to migrate (Figure 4C), while neither ligand induced the parental untransfected cells to migrate (Figure 4B,D). Using a chemotactic synthetic peptide corresponding to the amino terminal 1-20 amino acid (aa) of S-Ag, we show that in a time-(Figure 5A) and dose-dependent (Figure 5C) fashion, S-Ag peptide 1 induces activation of the mitogen-activated protein (MAP) kinase pathway, specifically p42 and p44 ERK2. An S-Ag peptide that was not chemotactic, S-Ag peptide 171-190 called S-Ag pep17 (TDAEEDKIPKKSSVRYLIRS), did not induce ERK2 kinase phosphorylation (Figure 5B). In addition, prior exposure of CXCR3-expressing cells to either IRBP or S-Ag blocked calcium mobilization by CXCL11 (Figure 6). Thus, IRBP appears to interact with both CXCR5 and CXCR3, while S-Ag only attracts CXCR3-expressing cells. Neither autoantigen induced calcium flux in CXCR3-expressing 300-19 cells or in CXCR5-expressing HEK cells. Furthermore, neither autoantigen competed with radiolabeled CXCL11 binding to CXCR3/300-19 cells (data not shown), suggesting that the autoantigen binding site(s) may be distinct from those of the cognate chemokine ligands.
CXCR3-transfected 300-19 cells migrate to both IRBP and S-Ag. Various concentrations of S-Ag or IRBP placed in the lower well of a micro-Boyden chemotaxis chamber. (A-B) Parental 300-19 or CXCR3-expressing 300-19 cells were placed in the upper wells of a micro-Boyden chamber. The mean number of migrating cells and corresponding standard deviation are graphed on the y-axis while ligand concentrations are shown on the x-axis. ▪ represents bovine S-Ag; ▨, IRBP; ▦, CXCL11; and □, 1-20 aa S-Ag peptide (A) or media control (B). *StatView Student t with P ≤ .0001 for maximum chemotactic responses vs “0” control; #P not significantly different from control. n = 3. (C-D) CXCR5-transfected HEK-293 cells migrate to IRBP. Various concentration of S-Ag or IRBP placed in the lower well of a micro-Boyden chemotaxis chamber. Parental human embryonic kidney cells (HEK-293) or CXCR5-expressing HEK cells were placed in the upper wells of a micro-Boyden chamber. The mean number of migrating cells and corresponding standard deviation are graphed on the y-axis while ligand concentrations are shown on the x-axis. ▪ represents bovine S-Ag; ▧, human S-Ag; ▨, IRBP; ▦, CXCL13; and □, media control. *StatView Student t with P ≤ .0001 for maximum chemotactic responses vs. “0” control; **P ≤ .005. n = 3.
CXCR3-transfected 300-19 cells migrate to both IRBP and S-Ag. Various concentrations of S-Ag or IRBP placed in the lower well of a micro-Boyden chemotaxis chamber. (A-B) Parental 300-19 or CXCR3-expressing 300-19 cells were placed in the upper wells of a micro-Boyden chamber. The mean number of migrating cells and corresponding standard deviation are graphed on the y-axis while ligand concentrations are shown on the x-axis. ▪ represents bovine S-Ag; ▨, IRBP; ▦, CXCL11; and □, 1-20 aa S-Ag peptide (A) or media control (B). *StatView Student t with P ≤ .0001 for maximum chemotactic responses vs “0” control; #P not significantly different from control. n = 3. (C-D) CXCR5-transfected HEK-293 cells migrate to IRBP. Various concentration of S-Ag or IRBP placed in the lower well of a micro-Boyden chemotaxis chamber. Parental human embryonic kidney cells (HEK-293) or CXCR5-expressing HEK cells were placed in the upper wells of a micro-Boyden chamber. The mean number of migrating cells and corresponding standard deviation are graphed on the y-axis while ligand concentrations are shown on the x-axis. ▪ represents bovine S-Ag; ▧, human S-Ag; ▨, IRBP; ▦, CXCL13; and □, media control. *StatView Student t with P ≤ .0001 for maximum chemotactic responses vs. “0” control; **P ≤ .005. n = 3.
Activation of ERK-2 by S-Ag peptide 1. Western blotting was used to show that in a time-dependent manner the chemoattractant 1-20 aa S-Ag peptide, labeled S-Ag pep1, induced increased ERK phosphorylation, with a maximum response observed at 20 minutes (A). Further, a non-chemoattractant S-Ag peptide corresponding to aa 171-190 did not induce ERK phosphorylation (B), even when 5μg/mL of peptide were used for a 20-minute stimulation. Finally, ERK activation by S-Ag pep 1 was dose dependent, with increased phosphorylation observed from 100 ng/mL and to 5 μg/mL (C).
Activation of ERK-2 by S-Ag peptide 1. Western blotting was used to show that in a time-dependent manner the chemoattractant 1-20 aa S-Ag peptide, labeled S-Ag pep1, induced increased ERK phosphorylation, with a maximum response observed at 20 minutes (A). Further, a non-chemoattractant S-Ag peptide corresponding to aa 171-190 did not induce ERK phosphorylation (B), even when 5μg/mL of peptide were used for a 20-minute stimulation. Finally, ERK activation by S-Ag pep 1 was dose dependent, with increased phosphorylation observed from 100 ng/mL and to 5 μg/mL (C).
CXCR3 and CXCR5 expression by DCs
Previous reports indicate that monocyte-derived iDCs express little or no CXCR5 or CXCR3 on their cell surface that can be detected by flow cytometry,26 and our studies confirm this (data not shown). Nevertheless, iDCs exhibited significant chemotaxis to CXCL11 and CXCL13, indicating the presence of accessible and functional CXCR3 and CXCR5 (Figure 1). FACS analysis showed that the majority of permeabilized cells were highly stained for CXCR3 and CXCR5, indicating that most of these receptors are intracellular (data not shown). In contrast, DCs that had undergone maturation with LPS expressed moderate levels of both CXCR3 and CXCR5 on their surface. Unexpectedly, although LPS-stimulated DCs expressed surface CXCR3 and CXCR5, these receptors were nonfunctional and no longer migrated to S-Ag, IRBP, CXCL11, or CXCL13 (data not shown).
Lymphocyte proliferation, EAU pathology, and chemoattraction
In an effort to identify the chemotactic fragments of human IRBP and S-Ag, synthetic 20–amino acid peptides overlapping by 10 residues were evaluated. There was no detectable migration of human leukocytes to peptides 1-20 or 161-180 of human IRBP, (which are uveitogenic in C57BL/6 mice and B10.RIII mice, respectively), in a 1 to 10 000-ng/mL range. Chemotactic responses were observed with lymphocytes isolated from healthy controls to S-Ag peptides corresponding to amino acids 1-20, 11-30, 41-60, 121-140, and 131-150 (Table 3),3,17 but peptides corresponding to human S-Ag amino acids 141-160, 151-170, 161-180, and 171-190 did not induce migration when tested in the 1 to 1000-ng/mL range. Combined with data from published literature, our observations suggest that, unlike HisRS, S-Ag has multiple and distinct epitopes responsible for chemotaxis, leukocyte proliferation, and elicitation of autoantibodies (Table 3).
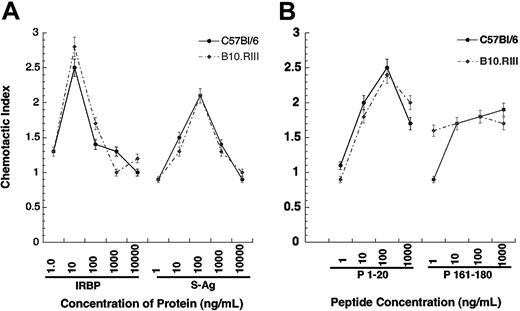
To dissect this question more rigorously, we examined the relationship between chemoattraction, antigenicity (ability to elicit a lymphoproliferative response), and pathogenicity (ability to elicit disease) using the well-characterized mouse EAU model. Our previous studies indicated that B10.RIII mice develop high EAU scores and C57BL/6 mice develop moderate scores in response to immunization with IRBP. Neither strain develops EAU when immunized with S-Ag, but both exhibit proliferative responses to this molecule. In the present experiments, iDCs from both strains consistently exhibited chemotaxis to rhIRBP (CI ≥ 2.0) that is recognized as pathogenic by both strains. However, iDCs from both strains also migrated to S-Ag, which is not pathogenic for either strain, with a CI of 2.0 or more (Figure 7). This leads to the conclusion that, at the protein level, the chemoattraction that we have observed here is dissociated from the pathogenic potential of a self antigen.
Blockade of CXCR3-expressing 300-19 cell calcium mobilization by prior treatment with IRBP or S-Ag. CXCR3-expressing cells were stimulated with CXCL11 at 0.1 μg/mL and showed calcium mobilization (navy blue line). However, neither S-Ag (brown line) nor IRBP (green line) alone induced calcium mobilization of CXCR3-expressing cells and blocked CXCL11-induced calcium flux. Pretreatment of cells with CXCL11 blocked subsequent CXCL11-induced calcium mobilization (purple line).
Blockade of CXCR3-expressing 300-19 cell calcium mobilization by prior treatment with IRBP or S-Ag. CXCR3-expressing cells were stimulated with CXCL11 at 0.1 μg/mL and showed calcium mobilization (navy blue line). However, neither S-Ag (brown line) nor IRBP (green line) alone induced calcium mobilization of CXCR3-expressing cells and blocked CXCL11-induced calcium flux. Pretreatment of cells with CXCL11 blocked subsequent CXCL11-induced calcium mobilization (purple line).
We next addressed the relationship between chemoattraction, antigenicity, and pathogenicity at the epitope level, based on the previously characterized responses of B10.RIII and C57BL/6 mice to peptide epitopes derived from human IRBP. As shown in Table 4,27,28 peptide 161-180 is a dominant IRBP epitope recognized by the B10.RIII mouse (H-2r) that is highly uveitogenic and antigenic. It is also antigenic for C57BL/6 mice (H-2b) but is not uveitogenic in that strain. Conversely, peptide 1-20 of human IRBP is a cryptic epitope recognized by C57BL/6 mice as both uveitogenic and antigenic, but is neither antigenic nor uveitogenic in B10.RIII mice. The 1-20 peptide induced a maximal chemotactic response at 100 ng/mL by iDCs from C57Bl/6 (CI = 2.5) and B10.RIII (CI = 2.4) mice. However, the 161-180 peptide did not induce the characteristic chemokine bell-shaped response curve, but instead the response appeared to plateau at approximately 1.7-fold greater than media control. Thus, the chemotactic activity of an autoantigen is unrelated to its ability to stimulate lymphocyte proliferation or to elicit EAU.
Murine myeloid DCs migrate to IRBP, S-Ag, and IRBP-related peptides. Murine immature DCs were generated from the bone marrow of EAU-resistant C57Bl/6 (• and solid line) or sensitive B10.RIII (• and broken line) mice. Chemotactic indexes are graphed on the y-axis while the chemoattractant and concentration are shown of the x-axis. *P ≤ .05. Error bars indicate standard deviation.
Murine myeloid DCs migrate to IRBP, S-Ag, and IRBP-related peptides. Murine immature DCs were generated from the bone marrow of EAU-resistant C57Bl/6 (• and solid line) or sensitive B10.RIII (• and broken line) mice. Chemotactic indexes are graphed on the y-axis while the chemoattractant and concentration are shown of the x-axis. *P ≤ .05. Error bars indicate standard deviation.
Discussion
We demonstrate that S-Ag and IRBP, which are tissue-specific self antigens, can be chemoattractants for normal human immunocytes, specifically, lymphocytes and immature DCs. These autoantigens, which have no primary or secondary structural homology to chemokines, induce cell migration by interacting with specific chemokine receptors. Both IRBP and S-Ag induce CXCR3-expressing cells to migrate, and IRBP also induces CXCR5 expressing cells to migrate. IRBP and S-Ag are potent chemoattractants for CXCR3-expressing cells, with a maximum chemotactic response observed at 0.07 nM or 2.3 nm, respectively, compared with 120 nM for CXCL11. IRBP is also a more potent chemoattractant than the CXCR5 ligand, CXCL13 (12.5 nM). While not used in this study, others have compared the other ligands for CXCR3, CXCL9 (MIG), and CXCL10 (IP10) to CXCL11. Those studies showed that at 100 ng/mL (8-12 nM) the ranking of chemotactic efficacy was CXCL11 > CXCL10 > CXCL9.29 CXCL9 and the autoantigens appear to be equally weak chemoattractants, consistently inducing only 2-fold increases in the number of migrating cells over control. Nevertheless, the autoantigen-induced migration was inhibited by pertussis toxin and was not due to chemokinesis. Furthermore, prior exposure to these autoantigens inhibited subsequent calcium flux and chemotactic responses to the cognate chemokine ligands. Further, the 1-20 aa S-antigen induced phosphorylation of ERK2, which is known to be in the chemotaxis pathway, providing further support for autoantigen stimulation of target cells. These observations, together with our ongoing studies, which show that HisRS is internalized only by CCR5-expressing cells (O.M.Z.H., H.F.D., and J.J.O., unpublished observations, October 2003), support our view that autoantigens interact with and activate chemokine receptors. There are several distinctions between chemokine and autoantigen-receptor interactions. Although both classes of proteins are chemoattractant, autoantigens do not induce calcium flux and they do not compete with chemokines for receptor binding. Based on our previous observations that HisRS did not compete with CCL5 for receptor binding due to interaction with the third and fourth extracellular domains of CCR5,1 it is likely that IRBP and S-Ag will use domains on CXCR3 distinct from its cognate chemokine ligands.
Although uveitis antigens stimulated cells to migrate only 2 to 4-fold over background and were therefore not as efficacious as chemokines, they were potent, requiring nanomolar (S-Ag) and picomolar (IRBP) concentrations to induce migration in in vitro micro-Boyden chamber assays. While in vivo concentrations of human IRBP and S-Ag protein have not been determined, levels of IRBP in the interphotoreceptor matrix of Xenopus were revealed to be in excess of 100 pmol.30 Previous studies have shown that antigens placed in the anterior chamber leave the eye by several pathways within a few hours, draining to the cervical and submandibular lymph nodes via the lymphatics and reaching the spleen via the blood stream.31-33 Further, clonal expansion of antigen-specific T cells in submandibular lymph nodes can occur when antigen is introduced into the eye.34 Thus, it is reasonable that the chemoattraction of iDCs and lymphocytes observed in our in vitro studies could occur in vivo because there is sufficient IRBP in the eye at any one time to establish a gradient, and in pathologic conditions the exposed DCs could relocate to the draining lymph nodes, leading to the expansion of autoimmune T cells.
The distribution of CXCR3 is differentially regulated in normal and inflamed tissue.35 Early studies showed that CXCR3 was expressed on a subset of Th1 lymphocytes and natural killer (NK) cells.36,37 More recent studies have shown that CXCR3 is also expressed by plasmacytoid DCs (pDCs)38 and CD11c+ myeloid DCs (myDCs).35 Our results confirmed that monocyte-derived iDCs express CXCR3, although most of the expression was intracellular, possibly due to persistent exposure in culture to ligands that resulted in its internalization. Furthermore, activation of naive CD4+ T cells by either pDCs or myDCs results in a rapid, transient increase in CXCR3- and CXCR5-expressing cells.38 Cell-surface expression of CXCR3 reappears on activated CD4+ T cells after several cell divisions,39 suggesting that DCs and T cells can be recruited to areas where S-Ag and IRBP are abundant, such as to a traumatized or ultraviolet (UV)–damaged eye. There are 2 forms of CXCR3: A and B.40 In our studies, we have shown that CXCR3A-expressing cells are chemoattracted to IRBP and S-Ag and may be among the first cells recruited to damaged ocular tissues.
CXCR5 is considered to be a principal regulator of DCs and T- and B-cell localization in secondary lymphoid organs,41 and CXCR5 appears to participate in the formation of autoimmune disease–associated follicles outside of normal lymphoid tissue.42,43 In our studies, human monocyte–derived iDCs express little CXCR5 on their surface, but contain a large intracellular reservoir. The small amount of CXCR5 on the cell surface of iDCs is sufficient to support migration induced by IRBP, but LPS-matured DCs, which express higher levels of CXCR5, do not migrate to either IRBP or CXCL13. This observation suggests a deficiency in an essential CXCR5 signaling component as the DCs mature; however, more extensive studies are needed to address this intriguing incongruity.
Because the ability to chemoattract leukocytes could contribute to the induction of autoimmunity, it was important to examine the relationship between the ability of an autoantigen to chemoattract leukocytes and its capacity to induce autoimmune pathology. While S-Ag and IRBP are implicated in human disease, the causal role of these autoantigens can be examined more directly in murine EAU models. Our data indicate that, in murine models, an autoantigen does not have to be autopathogenic or even antigenic in order to be a chemoattractant, since IRBP, which is uveitogenic in mice, is as good a chemoattractant for murine iDCs as S-Ag, which is not uveitogenic. At the epitope level, iDCs migrate equally well to IRBP epitopes regardless whether they are pathogenic or even antigenic in a particular mouse strain. Thus, within a pathogenic autoantigen, fragments that chemoattract iDCs can be distinct from the fragments that elicit lymphoproliferative and pathogenic responses, indicating that the ability to chemoattract is not predicated on the ability to bind to major histocompatibility complex (MHC) or be recognized by the T-cell receptor.
Recently a number of studies pointed to a role for “beneficial autoimmunity” in tissue repair processes, and suggested that a failure of adequate immunoregulatory mechanisms underlie conversion of this physiologic autoreactivity to autoimmune disease.44-46 It is tempting to speculate that the ability to chemoattract, which appears to be independent of the ability to induce lymphoproliferative responses and disease, might be involved in attracting immunocytes to sites of tissue damage as part of the tissue repair process. In fact, Monsonego et al47 observed that prior administration of the autoantigen myelin basic protein facilitated repair of spinal and optic nerve injuries. Thus, autoantigens may serve as “warning signals” that chemoattract iDCs to participate in the recruitment of innate and adaptive immune system cells. Other intrinsic and extrinsic factors, such as the presence of DC maturational signals, persistence of lymphocytes with high-affinity receptors for the antigen, and lack of regulatory cell populations, would then determine whether the process will develop into autoaggressive tissue pathology.48
The physiologic relevance of these findings would clearly depend on whether these tissue-specific antigens can produce an in vivo gradient sufficient to chemoattract leukocytes. Although technology to directly measure such a gradient in vivo is currently unavailable, the human retina contains close to a milligram of S-Ag and similar quantities of IRBP.49 It is therefore conceivable that under conditions of tissue damage, or during inflammatory responses in which proteolytic enzymes are present, a sufficient gradient of antigen and/or its fragments in the vicinity of the lesion could be established to chemoattract migrating leukocytes.
In conclusion, 2 self antigens, IRBP and S-Ag, which are associated with uveitis, induce iDC and lymphocyte migration. IRBP-induced migration is mediated through CXCR3 and CXCR5; however, S-Ag only induces migration of CXCR3-expressing cells. Our observations suggest that self-antigens use GαiPCR receptors to facilitate inflammatory and immune response to tissue damage, promoting normal repair process, and potentially contribute to the development of autoimmune diseases.
Prepublished online as Blood First Edition Paper, February 15, 2005; DOI 10.1182/blood-2004-07-2697.
Supported in whole or in part with federal funds from the National Cancer Institute, under contract no. N01-CO-012400.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organization imply endorsement by the U.S. Government.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.