Abstract
It is widely believed that self-tolerance of natural killer (NK) cells occurs because each NK cell expresses at least one inhibitory receptor specific for a host major histocompatibility complex (MHC) class I molecule. Here we report that some NK cells lack all known self-MHC–specific inhibitory receptors, yet are nevertheless self-tolerant. These NK cells exhibit a normal cell surface phenotype and some functional activity. However, they respond poorly to class I–deficient normal cells, tumor cells, or cross-linking of stimulatory receptors, suggesting that self-tolerance is established by dampening stimulatory signaling. Thus, self-tolerance of NK cells in normal animals can occur independently of MHC-mediated inhibition, and hyporesponsiveness plays a role in self-tolerance of NK cells, as also proposed for B and T cells.
Introduction
Natural killer (NK) cells attack transformed, infected, and allogeneic cells. Target cell recognition depends on stimulatory receptors with various specificities and inhibitory receptors specific for major histocompatibility complex (MHC) class I molecules.1-3 The stimulatory receptors associate with signaling adapter molecules including DNAX activating protein 12 (DAP12), CD3ζ, or Fc receptor γ (FcRγ), which contain immunoreceptor tyrosine-based activation motifs (ITAMs), whereas the inhibitory receptors contain cytoplasmic immunoreceptor tyrosine-based inhibitory motifs (ITIMs). The balance of stimulatory and inhibitory signaling determines whether target cells are lysed and stimulate cytokine production.
NK stimulatory receptors3 recognize pathogen-encoded molecules (eg, Ly49H ligand4,5 ) or host molecules that are up-regulated in transformed or infected cells (eg, NKG2D ligands6 ). Because NK cells attack certain uninfected and untransformed cell types, such as bone marrow cells or lymphoblasts from MHC-deficient or allogeneic animals, it is believed that even these normal cells express a ligand that is recognized by an NK stimulatory receptor (reviewed in Raulet et al7 ).
There are 3 different families of MHC-specific inhibitory receptors that have been defined: Ly49, a family of approximately 10 lectinlike receptors expressed by murine NK cells8 ; killer immunoglobulin (Ig)–like receptors (KIRs), a family of approximately 10 Ig-like receptors expressed by human NK cells2,9 ; and CD94/NKG2A, a lectinlike receptor heterodimer expressed by both human and murine NK cells.10,11 Ly49 receptors and KIRs bind to classical class Ia MHC molecules. In contrast, CD94/NKG2A interacts with a nonclassical class Ib molecule called Qa-1 in mice and HLA-E in humans. The Qa-1/HLA-E molecules are β2-microglobulin (β2m)–dependent and present a conserved peptide from the cleaved signal sequences of certain class Ia MHC molecules, which is recognized by CD94/NKG2A. Therefore, the CD94/NKG2A receptor indirectly recognizes class Ia molecules. All 3 types of inhibitory NK receptors distinguish subgroups of MHC class I molecules and all are expressed in a variegated fashion such that each NK cell expresses a more or less random set of receptors, with an average number per cell of 2 to 3.12,13 Whether a target cell inhibits a given NK cell depends on whether the NK cell expresses an inhibitory receptor specific for one or more of the target cell's class Ia molecules.
NK cells from normal mice attack bone marrow cells or lymphoblasts from mice lacking class I MHC molecules due to mutations in β2m,14 transporter associated with antigen processing (TAP),15 or the Kb and Db class I molecules.16,17 Comparable studies have also been performed with human NK cells.18 Furthermore, NK cells from normal animals attack bone marrow cells or lymphoblasts from MHC allogeneic animals lacking one or more MHC molecules of the host.9,19,20 The capacity of NK cells to attack normal cell types that lack some or all host MHC molecules raises the important question as to how NK reactivity against normal cells is prevented.
Significantly, NK cells arise in near normal numbers in MHC-deficient β2m-/-,14,21,22 TAP-/-,15,18 or Kb-/-Db-/-16,17 animals, but these NK cells do not attack class I–deficient normal cells. NK cells from class I–deficient mice exhibit a somewhat generalized reduction in functional activity against various types of target cells, including tumor target cells, antibody-coated target cells (ADCCs), and target cells presenting FcR–bound antibodies specific for NK-cell stimulatory receptors such as NKR-P1C (reverse ADCC).21-23 This form of self-tolerance has been demonstrated only in MHC-deficient mice and humans, however.
In humans, it has been reported that tolerance of NK cells arises by a mechanism that provides to each NK cell at least one inhibitory receptor specific for one or more of the host's MHC class Ia molecules.24 The notion that each NK cell in a normal animal expresses at least one self-MHC–specific inhibitory receptor is widely accepted in the literature, and is proffered as the explanation for NK-cell self-tolerance in a major textbook in the field.25 Given that initial inhibitory receptor expression appears to be largely random, the “at least one” hypothesis implies that an education process acts during NK-cell development to ensure that only NK cells with self-MHC–specific inhibitory receptors are allowed to mature (reviewed in Raulet et al7 ). Contrary to this expectation, we report here the identification of an appreciable population of NK cells in normal mice that lack inhibitory receptors specific for self-MHC class I molecules, yet attain self-tolerance by a mechanism of hyporesponsiveness.
Materials and methods
Mice
All mice were bred at the University of California, Berkeley in compliance with institutional guidelines. Some mice to be bred were purchased from Jackson Laboratories (Bar Harbor, ME) (C57BL/6J [B6, H-2b] and B10.M [H-2f]) or Charles River Laboratories (Frederick, MD) (B6-Ly5.1 mice [catalog name, B6-Ly5.2/Cr]). B6-β2m-/- mice have been described.23,26 B6-β2m-/--Ly5.1 mice were derived in our facilities.
Staining protocols
The monoclonal antibodies (mAbs) 16a11 (anti-NKG2A27 ), 20d5 (anti-NKG2A/C/E28 ), SW5E6 (anti-Ly49C and Ly49I29 ), 4D11 (anti-Ly49G230 ), JR9-318 (anti-Ly49A31 ), HBF-719 (anti-Ly49F32 ), YLI-90 (anti-Ly49I32 ), and PK136 (anti-NK1.133 ) were purified from INTEGRA CELLine CL1000 (Integra Biosciences, Chur, Switzerland) supernatants. MI-6 was purified as described.34 The remaining mAbs were purified by precipitation with caprilic acid followed by precipitation with 45% saturated ammonium sulfate as described.35 Purified mAbs were conjugated to biotin or fluorescein isothiocyanate (FITC) according to standard methods. Biotinylated anti-Ly49C (4LO3311) has been described previously.36 Anti–NK1.1–phycoerythrin (PE) or peridinin chlorophyll-alpha protein (PerCP)–cyanin 5.5 (Cy5.5), anti–CD3-CyChrome, anti–interferon-γ (IFN-γ)–FITC, anti–Ly49I-biotin (YLI-90), anti–CD11b-FITC, anti–CD16-FITC, 2B4-FITC, DX5-FITC, 2F1-FITC,37 anti–Ly5.1-PE, and streptavidin-PE were purchased from Pharmingen (San Diego, CA). Streptavidin-PE-Texas red (TR) was purchased from Caltag Laboratories (Burlingame, CA). For IFN-γ staining of NK subsets, the cells were preincubated for 20 minutes with 2.4G2 (HB-197; American Type Culture Collection [ATCC], Manassas, VA) hybridoma supernatant to block FcγRII/III receptors, incubated with SW5E6-biotin and 20d5-biotin, followed after washing with PK136-PE or DX5-PE (as indicated), CD3-CyChrome, and streptavidin-PE-TR. Cells were permeabilized and stained for intracellular IFN-γ using the Cytoperm/Cytofix kit (Pharmingen). Flow cytometry was performed on EPICS XL-MCL machines (Coulter, Hialeah, FL), using FlowJo software for analysis (Tree Star, Ashland, OR).
In some staining experiments, T cells (including NK1.1+ T cells) were depleted from splenocyte preparations by incubation with anti–CD5-biotin mAb (eBiosciences, San Diego, CA) and antibiotin microbeads followed by separation with the AutoMACS magnetic cell separator (Miltenyi Biotec, Auburn, CA). The depletion efficiency was always at least 98%.
NK-cell stimulation and functional assays
Freshly isolated NK cells were studied because culturing the cells in interleukin-2 (IL-2) for several days usually led to a partial reversal of the hyporesponsive phenotype of CI/NKG2- NK cells (data not shown). Because fully naive NK cells do not respond well to target cells in vitro, donor mice were injected with 70 μg poly(I:C) 1.5 days before harvesting the cells. Spleen cell suspensions were depleted of dead cells and erythrocytes by centrifugation in Lympholyte-Mammalian M (Cedarlane Laboratories, Hornby, ON, Canada). For intracellular IFN-γ assays, 1 × 106 cells/well were incubated in round-bottom plates coated with antibody or containing stimulator cells, in RPMI 1640 containing 5% fetal calf serum, 2-mercaptoethanol, 0.5 μg/mL recombinant human IL-2 (Chiron, Emeryville, CA), and 1 μg/mL brefeldin A (GolgiPlug; Pharmingen). Where indicated, 1 × 106 lymphoblasts or tumor cells were added as stimulator cells. After 5 hours, the cells were stained as described in “Staining protocols.”
Stimulator tumor cells included YAC-1 cells, RMA cells, or RMA cells transduced with the NKG2D ligands Rae1γ38 or H60.39 Lymphoblast stimulator cells were prepared from splenocytes from which NK cells had been depleted using anti-DX5–coated microbeads and negative selection with the AutoMACS (Miltenyi Biotec). NK depletion ensured that only responder-type NK cells were analyzed at the end of the experiment. Depleted cells were stimulated for 24 hours with concanavalin A (Con A, 2.5 μg/mL; Sigma-Aldrich, St Louis, MO) and treated with α-methyl mannopyranoside before use.
For cytolysis assays, groups of 2 to 3 mice were injected intraperitoneally on day -2 with 100 μg poly(I:C). On day -1, they were treated with antibodies to deplete NK subsets (1 mg 16a11 plus 0.75 mg SW5E6, or 0.3 mg A1 plus 0.3 mg 4D11 per mouse) or with phosphate-buffered saline (PBS) as a control. On day 0, splenocytes from each group were pooled. Triplicate samples, titrated according to the percentage of CD3- NK1.1+DX5+ cells as determined by flow cytometry, were incubated for 3 hours (YAC-1) or 4 hours (lymphoblasts) with 51Cr-labeled target cells.21 Data represent mean specific lysis ± SD.
For stimulation with mAbs, round-bottom high-protein binding plates were coated overnight at 4°C with the indicated concentration of mAbs in 100 μL PBS. The wells were washed 4 times with PBS before adding the cells. Cells were transferred to fresh round-bottom plates for the staining procedure. For stimulation with phorbol 12-myristate 13-acetate (PMA)/ionomycin, 250 ng/mL PMA and 2.5 μg/mL ionomycin were used as the starting concentrations, and the mixture was diluted as depicted in Figure 4. The stimulants were added directly to the wells for the 5-hour incubation period.
Statistical comparisons were performed using 2-tailed Student t tests.
Infections with Listeria
B6 mice (groups of 3) were inoculated intravenously with 1 × 104 colony-forming units (CFUs) Listeria monocytogenes 10403S. At the indicated time points, spleen cells were harvested and stained (without brefeldin A addition) with mAbs for self-MHC–specific inhibitory receptors (Ly49C, Ly49I, NKG2A), NK1.1, CD3, and intracellular IFN-γ as described in “Staining protocols.”
Bone marrow engraftment assay
Bone marrow cells of donor mice were adjusted to 5 × 106 cells/mL in PBS, and incubated with 5 μM carboxyfluorescein diacetate succinimidyl ester (CFSE) for 10 minutes at 37°C. The labeled cells were washed 3 times in PBS–10% FCS. Labeled β2m-/-Ly5.1+ bone marrow cells were mixed with an equal number of labeled B6 (Ly5.2) bone marrow cells, which should not be rejected by any of the recipients and therefore served as an internal reference population. A total of 1 × 107 of each donor cell type (or fewer cells for the supplemental figures; at the Blood website, see the Supplemental Figures link at the top of the online article) was inoculated intravenously into each recipient in 0.5 mL on day 0. Before marrow cell inoculation, the recipients had in some cases been treated on day -3 and day 0 with 300 μg each of the indicated mAbs to deplete NK cells or NK subsets. All the mice were irradiated with 9.5 Gy from a 137Cs source shortly before marrow cell injection. On day 3, recipient spleen cells were harvested, stained with anti–Ly5.1-PE, and analyzed on a flow cytometer after gating on CFSE+ (donor) cells. The data are depicted as the ratio of Ly5.1-positive to Ly5.1-negative cells among CFSE+ cells. Due to cell counting errors, the starting ratio of donor cells varied from experiment to experiment. Pilot experiments demonstrated that none of the donor cells had proliferated sufficiently to completely lose the CFSE dye during the 3-day span of the experiment.
Results
Expression of self-MHC–specific inhibitory receptors by NK cells
To gauge whether NK cells generally express at least one inhibitory receptor specific for self-MHC class I molecules we studied H-2b mice. Of the Ly49 receptors in C57BL/6 (B6) mice, only Ly49C and Ly49I bind to the Kb class Ia molecule and none of the Ly49 receptors show appreciable binding to the Db class Ia molecule.13,40,41 The alternative inhibitory class I–specific receptor in mice, CD94/NKG2A, recognizes a Db-derived signal peptide, presented by Qa-1.11 Direct analysis using knock-out mice demonstrated that Db is the only source of this peptide in lymphoblasts from B6 mice.42 Hence, Ly49C, Ly49I, and CD94/NKG2A represent the only self-specific inhibitory receptors in B6 mice.
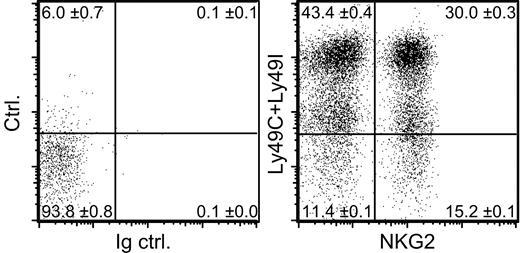
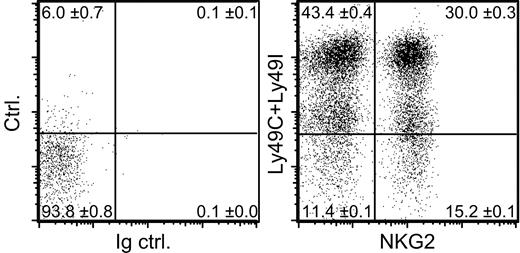
Of the NK cells in B6 mice (H-2b), 10% to 13% lacked expression of all 3 of these self-MHC–specific receptors (Ly49C, Ly49I, and NKG2A) (Figure 1). The 20d5 mAb used here to detect NKG2A also detects NKG2C and NKG2E, but essentially all NK cells that stain with 20d5 also stain with an NKG2A-specific mAb,27 and we find no evidence that NKG2C and E are expressed at significant levels on normal B6 NK cells.28 Although Ly49C is known to be expressed at only low levels on the surface of NK cells in H-2b mice,36 our protocol was sufficiently sensitive to reveal these cells as an intermediate staining population (Figure 1). Recent data suggest that Ly49A may also react weakly with Db,43 but this interaction is insufficient to cause detectable functional inhibition.41,44,45 Furthermore, staining experiments showed that including Ly49A in the category of an H-2b–specific receptor did not substantially change the conclusion: approximately 10% of NK cells in B6 mice lack expression of Ly49A, Ly49C, Ly49I, and NKG2A (data not shown).
NK cells lacking self-MHC–specific inhibitory receptors respond poorly to class I–deficient lymphoblasts
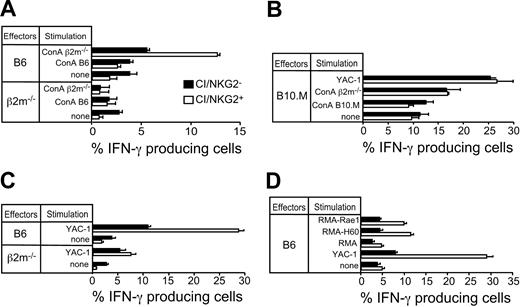
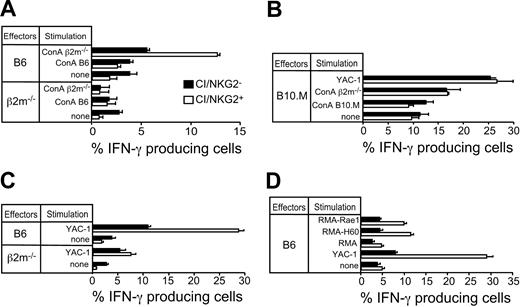
It was possible that the 10% to 13% of Ly49C/Ly49I/NKG2-negative NK cells (hereafter abbreviated CI/NKG2- NK cells) expressed unidentified inhibitory receptors specific for H-2b, which prevented the NK cells from attacking autologous H-2b cells. If so, these NK cells should be inhibited by normal H-2b–expressing cells, but not by comparable class I–deficient cells. To address this possibility, the in vitro response of CI/NKG2- NK cells was compared with the response of NK cells expressing one or more of these receptors (abbreviated CI/NKG2+ NK cells). Freshly isolated NK cells from mice treated with the NK-cell–inducing agent poly(I:C) were stimulated in cell culture with class I–deficient (β2m-/-) or H-2b lymphoblasts (Con A blasts) for 5 hours, and the percentage of NK cells in each subset producing IFN-γ was determined. A small percentage of NK cells produced IFN-γ in the absence of a stimulus in vitro (Figure 2A). Strikingly, CI/NKG2- NK cells from B6 mice did not respond appreciably to β2m-/- lymphoblasts over the background level (Figure 2A), nor did they respond to Kb-/-Db-/- lymphoblasts (Supplemental Figure S1), whereas CI/NKG2+ NK cells mounted a substantial response (Figure 2A). CI/NKG2- NK cells from B6 mice always responded poorly to β2m-/- lymphoblasts, although the response was in some cases marginally higher than the background level (data not shown). Because these NK cells fail to attack normal cell types lacking MHC class I molecules, it cannot be argued that they are self-tolerant because they are inhibited by self-MHC class I molecules.
A subset of NK cells lacking known inhibitory receptors specific for self-MHC molecules. Freshly isolated B6 splenocytes were depleted of T cells, including NK1.1+ T cells, and stained with mAbs specific for NK1.1, Ly49C, Ly49I, and NKG2. Control staining was performed with control rat IgG (Ig ctrl.) instead of anti-NKG2 mAb and streptavidin-PE alone instead of anti-Ly49C and anti-Ly49I mAbs (ctrl.). Dot plots are gated on NK1.1+ cells. Numbers in the quadrants represent mean percentages ± SEM (n = 3 mice). An average of 11.4% of the NK cells lacked Ly49C, Ly49I, and CD94/NKG2 (CI/NKG2- NK cells).
A subset of NK cells lacking known inhibitory receptors specific for self-MHC molecules. Freshly isolated B6 splenocytes were depleted of T cells, including NK1.1+ T cells, and stained with mAbs specific for NK1.1, Ly49C, Ly49I, and NKG2. Control staining was performed with control rat IgG (Ig ctrl.) instead of anti-NKG2 mAb and streptavidin-PE alone instead of anti-Ly49C and anti-Ly49I mAbs (ctrl.). Dot plots are gated on NK1.1+ cells. Numbers in the quadrants represent mean percentages ± SEM (n = 3 mice). An average of 11.4% of the NK cells lacked Ly49C, Ly49I, and CD94/NKG2 (CI/NKG2- NK cells).
CI/NKG2- NK cells in B6 mice exhibit reduced responsiveness to NK-sensitive target cells. Splenocytes from poly(I:C)-treated β2m-/-, B6, or B10.M mice were stimulated for 5 hours in the presence of brefeldin A with Con A–activated lymphoblasts from the designated mice or with tumor cell lines before staining the cells with mAbs specific for cell-surface markers and intracellular IFN-γ. Results are depicted as the percentage ± SEM (n = 3 mice) of IFN-γ+ cells in each subset (CI/NKG2+ or CI/NKG2-). (A) CI/NKG2- NK cells in B6 mice respond poorly to β2m-/- lymphoblasts. Similar results were obtained in 5 independent experiments (P = .006 for the % IFN-γ+ cells in CI/NKG2+ versus CI/NKG2-). (B) CI/NKG2- NK cells in B10.M mice respond as well as CI/NKG2+ NK cells to β2m-/- lymphoblasts or YAC-1 tumor cells. A repetition of the experiment yielded similar results. (C) CI/NKG2- NK cells in B6 mice respond poorly to YAC-1 tumor cells. Results are representative of 3 experiments (P = .003 for CI/NKG2+ versus CI/NKG2-). (D) CI/NKG2- NK cells in B6 mice respond poorly to RMA tumor cell transductants expressing NKG2D ligands Rae1 or H60. Results are representative of 3 experiments (P < .001 for CI/NKG2+ versus CI/NKG2-).
CI/NKG2- NK cells in B6 mice exhibit reduced responsiveness to NK-sensitive target cells. Splenocytes from poly(I:C)-treated β2m-/-, B6, or B10.M mice were stimulated for 5 hours in the presence of brefeldin A with Con A–activated lymphoblasts from the designated mice or with tumor cell lines before staining the cells with mAbs specific for cell-surface markers and intracellular IFN-γ. Results are depicted as the percentage ± SEM (n = 3 mice) of IFN-γ+ cells in each subset (CI/NKG2+ or CI/NKG2-). (A) CI/NKG2- NK cells in B6 mice respond poorly to β2m-/- lymphoblasts. Similar results were obtained in 5 independent experiments (P = .006 for the % IFN-γ+ cells in CI/NKG2+ versus CI/NKG2-). (B) CI/NKG2- NK cells in B10.M mice respond as well as CI/NKG2+ NK cells to β2m-/- lymphoblasts or YAC-1 tumor cells. A repetition of the experiment yielded similar results. (C) CI/NKG2- NK cells in B6 mice respond poorly to YAC-1 tumor cells. Results are representative of 3 experiments (P = .003 for CI/NKG2+ versus CI/NKG2-). (D) CI/NKG2- NK cells in B6 mice respond poorly to RMA tumor cell transductants expressing NKG2D ligands Rae1 or H60. Results are representative of 3 experiments (P < .001 for CI/NKG2+ versus CI/NKG2-).
Neither CI/NKG2+ nor CI/NKG2- NK cells responded to self (B6) lymphoblasts, demonstrating that both subsets were selftolerant (Figure 2A). As expected, the CI/NKG2+ NK cells from class I–deficient β2m-/- mice, unlike those from B6 mice, failed to respond to β2m-/- lymphoblasts, demonstrating their tolerance to self (β2m-/-) cells (Figure 2A). Therefore, these cells, like the CI/NKG2- NK cells from B6 mice, exhibit attenuated responsiveness to target cells that lack MHC class I molecules. The results indicate that a subpopulation of NK cells in normal mice responds poorly to lymphoblasts independently of any inhibition by MHC class I molecules on target cells, mimicking the functional phenotype of all NK cells in class I–deficient mice.
To test whether the hyporesponsiveness of CI/NKG2- NK cells is caused by the absence of inhibitory receptors reactive with self-MHC or is an invariant property of cells with this phenotype, we examined the same NK subset in B10.M (H-2f) mice. H-2f class I molecules, unlike H-2b class I molecules, fail to detectably bind Ly49I, bind Ly49C poorly, and bind Ly49A relatively well.41 Because the class I molecules in H-2f mice bind a different set of inhibitory receptors, the CI/NKG2- subset is not predicted to be devoid of cells expressing self-MHC–specific receptors. CI/NKG2- and CI/NKG2+ NK cells from B10.M mice responded similarly to YAC-1 tumor cells or to β2m-/- Con A blasts (Figure 2B), indicating that the low responsiveness of CI/NKG2- NK cells is dependent on the host's MHC and is not an intrinsic property of cells with this phenotype.
Responses of NK subsets to tumor cells and in cytotoxicity assays
The CI/NKG2-B6 NK cells were also hyporesponsive in other respects. They consistently responded poorly to stimulation with YAC-1 tumor cells (Figure 2C-D) or to RMA tumor cell transfectants expressing Rae1 or H60, both of which are ligands of the stimulatory NKG2D receptor (Figure 2D).1 Again, NK cells from β2m-/- mice (either CI/NKG2+ or CI/NKG2-) also responded poorly to these tumor cells (Figure 2C and data not shown).
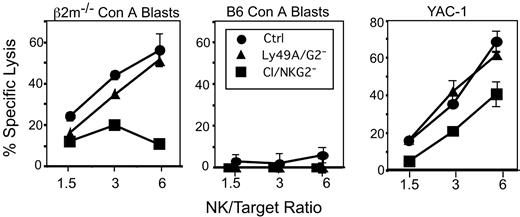
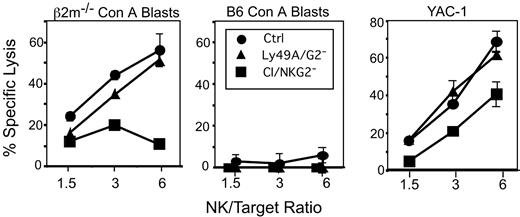
The low responsiveness of CI/NKG2- NK cells was also evident in cytotoxicity experiments. To obtain sufficient numbers of CI/NKG2- NK cells for cytolytic assays, CI/NKG2+ NK cells were depleted in vivo by treating B6 mice with mAb SW5E6, specific for Ly49C and I, and mAb 16a11, specific for NKG2A. Since the treatments deplete most NK cells, as a control we treated mice with depleting antibodies specific for the irrelevant Ly49A and Ly49G2 receptors, which together also deplete a large percentage (70%-75%) of all NK cells. None of the NK-cell populations lysed B6 Con A blasts, demonstrating that they are all self-tolerant. β2m-/- lymphoblasts and YAC-1 tumor targets were lysed comparably by Ly49A/Ly49G2- NK cells and unfractionated NK cells. In contrast, CI/NKG2- NK cells were much less efficient than CI/NKG2+ NK cells in lysing β2m-/- Con A blasts, and consistently less efficient in lysing YAC-1 target cells (Figure 3). Thus, the hyporesponsiveness of CI/NKG2- NK cells extends to cytolytic activity.
Responses of NK subsets to cross-linking of stimulatory NK receptors
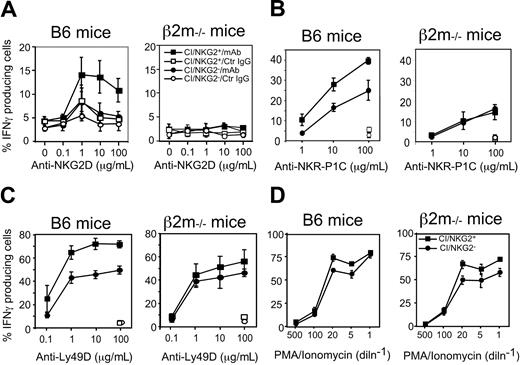
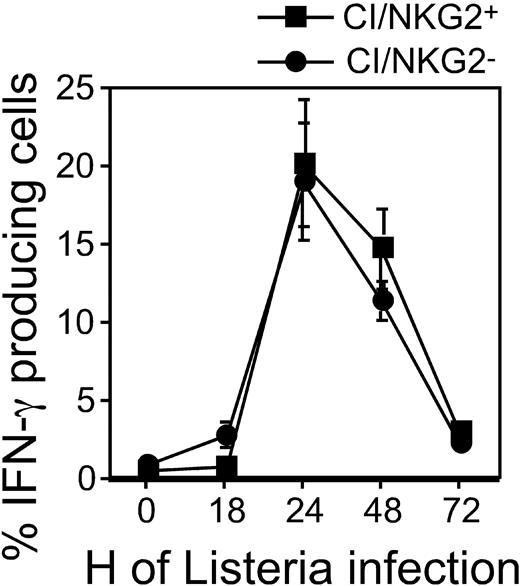
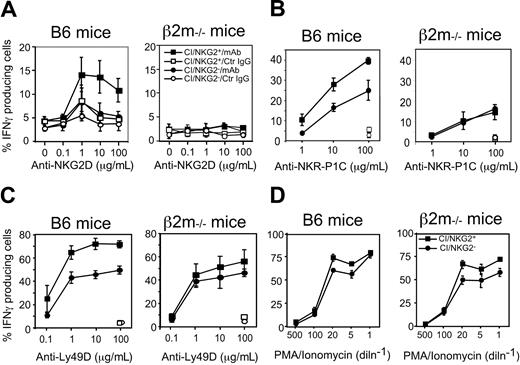
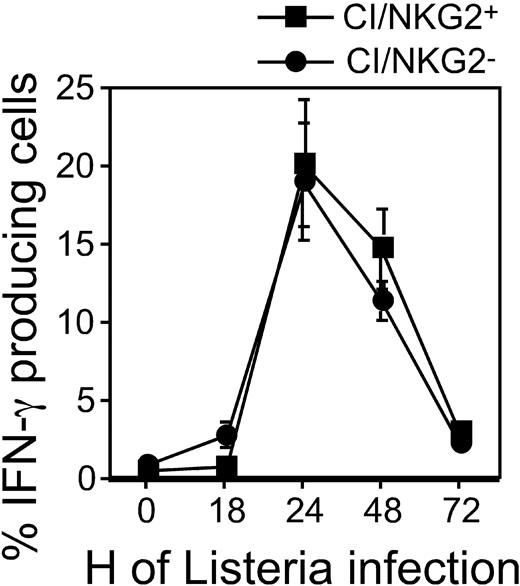
Hyporesponsiveness might reflect attenuated stimulatory signaling or enhanced inhibitory signaling. To address the first possibility, we examined the responsiveness of CI/NKG2- NK cells to direct cross-linking of stimulatory receptors in the absence of target cells. The stimulatory receptor NKG2D is expressed by essentially all NK cells, and cross-linking NKG2D activates NK cells directly.34 Cross-linking NKG2D with plate-bound MI-6 mAb resulted in significant responses of CI/NKG2+ NK cells but substantially lower responses by CI/NKG2- NK cells (Figure 4A). CI/NKG2- NK cells also responded less well than CI/NKG2+ NK cells to cross-linking of 2 other stimulatory receptors, NKR-P1C (NK1.1 antigen) and Ly49D (Figure 4B-C). These results suggest that the poor response of CI/NKG2- NK cells to self cells is due to attenuated stimulatory signaling. Importantly, the CI/NKG2- NK cells from B6 mice responded nearly as well as CI/NKG2+ NK cells to PMA plus ionomycin, even at limiting doses of these pharmacologic agents (Figure 4D). Furthermore, in terms of IFN-γ production, the CI/NKG2- NK cells responded as well as CI/NKG2+ NK cells to infections with Listeria monocytogenes bacteria in vivo (Figure 5). Therefore, the diminished responsiveness of CI/NKG2- NK cells to cross-linking of stimulatory receptors does not reflect a general inability of the cells to execute an activation program.
Cytotoxicity of CI/NKG2- NK cells. B6 mice were depleted of CI/NKG2+ NK cells by treatment with a mixture of the SW5E6 and 16a11 mAbs. The SW5E6 and 16a11 mAbs routinely deplete 95% or 80%, respectively, of the corresponding NK-cell subsets (data not shown). Control NK-cell populations were depleted of irrelevant Ly49A+ and Ly49G2+ NK cells by treatment with A1 and 4D11 mAbs, or were from untreated mice. The NK-cell populations were tested for lysis of β2m-/- Con A blasts (left) or B6 Con A blasts (middle), or, in a separate experiment, YAC-1 tumor cells (right). The effector-target (E/T) ratio was calculated according to the number of NK1.1+CD3- cells in the population. Each experiment was repeated with comparable results. Splenocytes from CI/NKG2--depleted mice were significantly less cytotoxic than both Ly49A/Ly49G2-depleted or control mice against β2m-/- Con A blasts (P ≤ .008 at 6:1 or 3:1 E/T ratios) or YAC-1 targets (P ≤ .03 for all E/T).
Cytotoxicity of CI/NKG2- NK cells. B6 mice were depleted of CI/NKG2+ NK cells by treatment with a mixture of the SW5E6 and 16a11 mAbs. The SW5E6 and 16a11 mAbs routinely deplete 95% or 80%, respectively, of the corresponding NK-cell subsets (data not shown). Control NK-cell populations were depleted of irrelevant Ly49A+ and Ly49G2+ NK cells by treatment with A1 and 4D11 mAbs, or were from untreated mice. The NK-cell populations were tested for lysis of β2m-/- Con A blasts (left) or B6 Con A blasts (middle), or, in a separate experiment, YAC-1 tumor cells (right). The effector-target (E/T) ratio was calculated according to the number of NK1.1+CD3- cells in the population. Each experiment was repeated with comparable results. Splenocytes from CI/NKG2--depleted mice were significantly less cytotoxic than both Ly49A/Ly49G2-depleted or control mice against β2m-/- Con A blasts (P ≤ .008 at 6:1 or 3:1 E/T ratios) or YAC-1 targets (P ≤ .03 for all E/T).
CI/NKG2- NK cells in B6 mice respond poorly to cross-linking of stimulatory receptors. (A-C) Splenocytes from poly(I:C)-treated β2m-/- or B6 mice were cultured for 5 hours on plates coated with increasing concentrations of the anti-NKG2D mAb MI-6 (A), the anti–NKR-P1C mAb PK136 (B), the anti-Ly49D mAb SED85 (C), or control rat IgG (A-C) in the presence of brefeldin A before staining and analysis. NK cells were gated as NK1.1+CD3- cells except in panel B, where they were gated as DX5+CD3- cells. Results are depicted as percentage ± SEM (n = 3 mice) of IFN-γ+ cells in each subset. In panels B and C, data were normalized based on percentage of NKR-P1C+ or Ly49D+ NK cells, respectively, in each subset, as determined before stimulation. CI/NKG2- NK cells produced significantly less IFN-γ than their CI/NKG2+ counterparts after anti-NKG2D (P ≤ .03 for all doses), anti–NKR-P1C (P ≤ .04 for all but the lowest dose), or anti-Ly49D stimulation (P ≤ .03 for all but the lowest dose). (D) NK cells were stimulated with the indicated dilutions of a mixture of ionomycin and PMA for 5 hours in the presence of brefeldin A before analysis. The highest concentrations (dilution factor = 1) were 2.5 μg/mL ionomycin and 250 ng/mL PMA.
CI/NKG2- NK cells in B6 mice respond poorly to cross-linking of stimulatory receptors. (A-C) Splenocytes from poly(I:C)-treated β2m-/- or B6 mice were cultured for 5 hours on plates coated with increasing concentrations of the anti-NKG2D mAb MI-6 (A), the anti–NKR-P1C mAb PK136 (B), the anti-Ly49D mAb SED85 (C), or control rat IgG (A-C) in the presence of brefeldin A before staining and analysis. NK cells were gated as NK1.1+CD3- cells except in panel B, where they were gated as DX5+CD3- cells. Results are depicted as percentage ± SEM (n = 3 mice) of IFN-γ+ cells in each subset. In panels B and C, data were normalized based on percentage of NKR-P1C+ or Ly49D+ NK cells, respectively, in each subset, as determined before stimulation. CI/NKG2- NK cells produced significantly less IFN-γ than their CI/NKG2+ counterparts after anti-NKG2D (P ≤ .03 for all doses), anti–NKR-P1C (P ≤ .04 for all but the lowest dose), or anti-Ly49D stimulation (P ≤ .03 for all but the lowest dose). (D) NK cells were stimulated with the indicated dilutions of a mixture of ionomycin and PMA for 5 hours in the presence of brefeldin A before analysis. The highest concentrations (dilution factor = 1) were 2.5 μg/mL ionomycin and 250 ng/mL PMA.
Phenotype of NK subsets
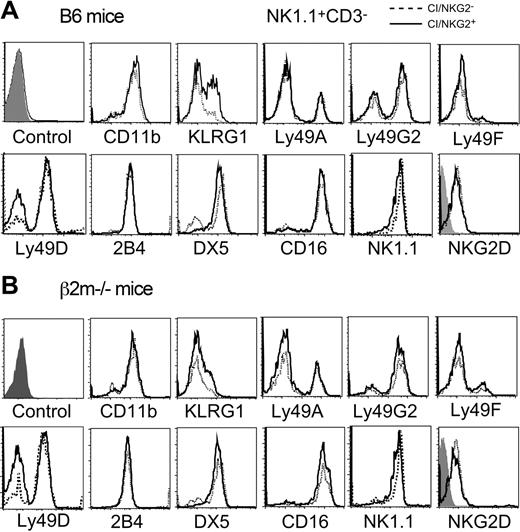
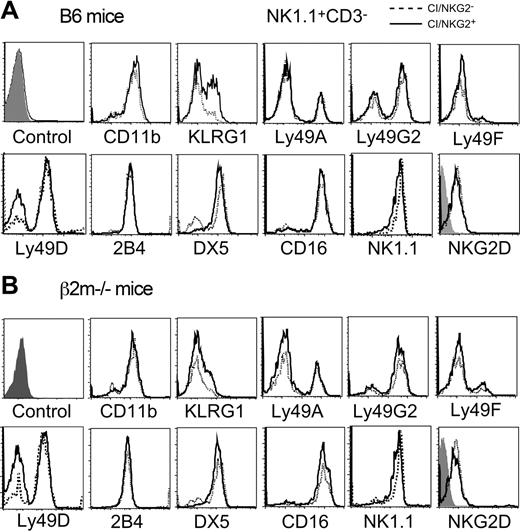
A variety of markers and receptors were compared in CI/NKG2+ and CI/NKG2- NK cells. No significant differences were seen in the cell-surface levels for most of these molecules, including Ly49A, Ly49G2, Ly49D, Ly49F, CD16, 2B4, NKG2D, and NKR-P1C (NK1.1) (Figure 6). Therefore, the poor response of NK cells to NKG2D, NKR-P1C, or Ly49D cross-linking cannot be explained by reduced cell surface levels of these receptors. The percentage of Ly49D+ NK cells was higher among CI/NKG2- NK cells (77.3% ± 3.3 versus 52.9% ± 5.4), reflecting the previously reported nonstochastic expression of this receptor.27,46 This difference occurred even in mice lacking MHC expression (Figure 6B),27 and is therefore not caused by a difference in MHC reactivity of the 2 subsets. Interestingly, a human NK subset has been described that is impaired in lysing MHC-deficient leukemic cells, and expresses low levels of certain other stimulatory receptors, such as NKp46, NKp44, and NKp30.47 Antibodies to test these receptors are not available in mice.
NK-subset activation in B6 mice infected with Listeria monocytogenes. At the indicated time points, splenocytes from Listeria-infected B6 mice were harvested and immediately stained with mAbs. The results are depicted as the percentage ± SEM of cells in each subset (gated also for NK1.1+CD3- phenotype) with intracellular IFN-γ (n = 3 mice). A repetition of the experiment yielded similar results.
NK-subset activation in B6 mice infected with Listeria monocytogenes. At the indicated time points, splenocytes from Listeria-infected B6 mice were harvested and immediately stained with mAbs. The results are depicted as the percentage ± SEM of cells in each subset (gated also for NK1.1+CD3- phenotype) with intracellular IFN-γ (n = 3 mice). A repetition of the experiment yielded similar results.
Cell-surface phenotype of CI/NKG2+ and CI/NKG2- NK subsets. Freshly isolated splenocytes from B6 mice (A) or β2m-/- mice (B) were stained with mAbs for the 3 self-specific receptors (Ly49C, Ly49I, and NKG2), NK1.1, CD3, and the other markers indicated. Expression of various markers was compared on gated CI/NKG2+NK1.1+CD3- cells (solid line) and CI/NKG2-NK1.1+CD3- cells (dashed line), except the NK1.1 staining, which was gated on CD3-NKG2D+ cells. The first histogram represents the negative control for all the samples with the exception of NKG2D; the negative control for NKG2D staining is the shaded histogram within that panel.
Cell-surface phenotype of CI/NKG2+ and CI/NKG2- NK subsets. Freshly isolated splenocytes from B6 mice (A) or β2m-/- mice (B) were stained with mAbs for the 3 self-specific receptors (Ly49C, Ly49I, and NKG2), NK1.1, CD3, and the other markers indicated. Expression of various markers was compared on gated CI/NKG2+NK1.1+CD3- cells (solid line) and CI/NKG2-NK1.1+CD3- cells (dashed line), except the NK1.1 staining, which was gated on CD3-NKG2D+ cells. The first histogram represents the negative control for all the samples with the exception of NKG2D; the negative control for NKG2D staining is the shaded histogram within that panel.
Notably, a similar percentage (approximately 85%) of cells in each subset expressed CD11b, a marker of mature NK cells,48 indicating that the poor response of CI/NKG2- NK cells is not due to immaturity. Although potentially immature NK1.1+DX5- NK cells represented a somewhat higher proportion of CI/NKG2A- NK cells (27%) than of CI/NKG2A+ NK cells (11%), this small difference does not account for the large differences in responsiveness that we observed. The only other marker showing significant differential expression was killer cell lectinlike receptor G1 (KLRG1,49 also known as mast cell function-associated antigen [MAFA]50 ), which was expressed by 46% ± 2% CI/NKG2+ NK cells but only 19% ± 3% CI/NKG2- NK cells (Figure 6A). KLRG1 is expressed poorly by NK cells from class I–deficient mice,37 including both CI/NKG2- and CI/NKG2+ subsets of NK cells (Figure 6B), indicating a more general correlation with hyporesponsiveness. However, the correlation is unlikely to reflect a direct role for KLRG1 in hyporesponsiveness, because the receptor is inhibitory yet is expressed poorly by the hyporesponsive CI/NKG2 subset. Furthermore, hyporesponsiveness correlated better with absence of CI/NKG2 expression than with absence of KLRG1 expression (data not shown). As suggested previously, it is possible that KLRG1 expression is stimulated, albeit sporadically, by engagement of class I MHC–specific inhibitory receptors,37 without being involved in self-tolerance.
Role of NK subsets in rejection of bone marrow grafts in vivo
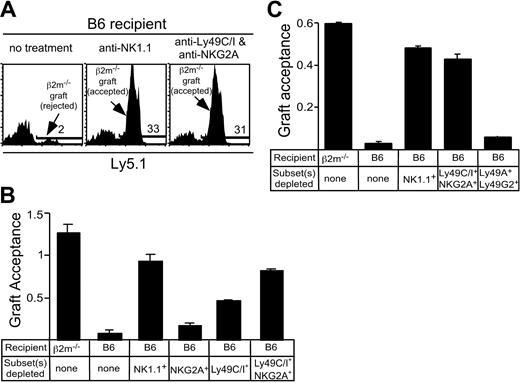
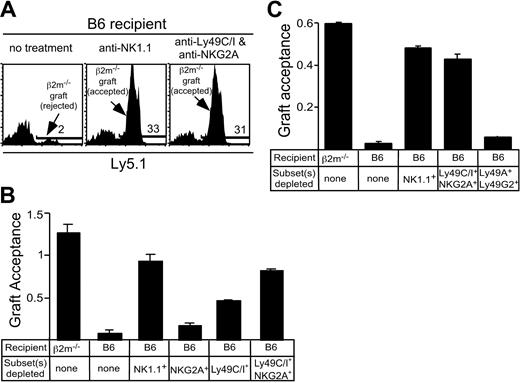
To address whether CI/NKG2- NK cells participate in vivo in the rejection of class I–deficient bone marrow grafts, a stringent competitive assay for bone marrow rejection was used. This assay tested the rejection or acceptance of CFSE-labeled B6-β2m-/- bone marrow cells in comparison with CFSE-labeled B6 bone marrow cells mixed in the same inoculum (1 × 107 cells each). The new assay confirmed that class I–deficient bone marrow grafts are rejected by normal B6 mice, but accepted by β2m-/- mice, and that depletion of NK1.1+ cells with PK136 mAb prevented rejection by B6 mice14 (Figure 7A-B). Depletion of Ly49C/I+ NK cells had a significant impact on graft acceptance, while the effect of depleting NKG2A+ cells was very modest (Figure 7B). However, depletion of both Ly49 C/I+ and NKG2A+ NK cells with a mixture of SW5E6 and 16A11 mAbs nearly abrogated rejection of β2m-/- grafts, suggesting that CI/NKG2+ NK cells play by far the major role in rejecting β2m-/- bone marrow grafts, while CI/NKG2- NK cells play little if any role (Figure 7B-C). As a control, no effect on rejection was observed when mice were depleted of a large fraction (70%-75%) of NK cells that expressed the irrelevant Ly49G2 and Ly49A receptors (Figure 7C). Similar results were obtained with substantially lower and highly limiting doses of bone marrow (as low as 1.25 × 106 of each donor cell type; see Supplemental Figure S2). These data suggest that relevant NK cells are in excess in this assay and that the effects of depleting CI/NKG2+ cells are specific. Thus, CI/NKG2- NK cells appear to play little or no role in rejecting β2m-/- bone marrow grafts, consistent with their hyporesponsive phenotype as revealed by in vitro studies.
Depletion of CI/NKG2+ NK cells prevents rejection of bone marrow grafts from class I–deficient mice. Recipient mice (β2m-/- or B6) were treated with mAbs to deplete the indicated NK-cell subset, followed by lethal irradiation. At day 0, a mixture of CFSE-labeled bone marrow cells from β2m-/- Ly5.1+ and B6 (Ly5.2) mice was injected intravenously. After 3 days, splenocytes were stained with PE-conjugated anti-Ly5.1 Ab. The results are represented as graft acceptance, calculated as the ratio of β2m-/- (Ly5.1+) bone marrow cells to B6 (Ly5.1-) bone marrow cells among gated CFSE-positive cells. The B6 cells serve as a nonrejected internal reference population. (A) Representative histograms depicting results in which the test graft was rejected (in untreated B6 mice, left) or accepted (B6 mice from which NK1.1+ cells were depleted, middle). The effect of depleting Ly49C/I+ and NKG2+ NK cells is shown in the right panel. The percentage of positive cells (marked by a bar) are indicated in each panel. Note that many of the nonstaining cells in all the panels are in the lowest fluorescence channel along the left axis due to the compensation settings of the flow cytometer. (B) Acceptance of β2m-/- bone marrow grafts by control mice or B6 mice depleted of NKG2A+ NK cells, Ly49C/I+ NK cells, or both subsets. Data represent the mean ± SEM (n = 3 mice). Results were reproduced in a replicate experiment. (C) Acceptance of β2m-/- bone marrow grafts by B6 mice treated with mAbs to deplete the indicated cell populations, or by control β2m-/- mice. Data represent the mean ± SEM (n = 2 mice). Results were reproduced in 2 replicate experiments. The Ly49C/I- and NKG2A-depleted group differed significantly from the Ly49A/G2-depleted group (P ≤ .04) but not from the NK1.1-depleted group (P = .5).
Depletion of CI/NKG2+ NK cells prevents rejection of bone marrow grafts from class I–deficient mice. Recipient mice (β2m-/- or B6) were treated with mAbs to deplete the indicated NK-cell subset, followed by lethal irradiation. At day 0, a mixture of CFSE-labeled bone marrow cells from β2m-/- Ly5.1+ and B6 (Ly5.2) mice was injected intravenously. After 3 days, splenocytes were stained with PE-conjugated anti-Ly5.1 Ab. The results are represented as graft acceptance, calculated as the ratio of β2m-/- (Ly5.1+) bone marrow cells to B6 (Ly5.1-) bone marrow cells among gated CFSE-positive cells. The B6 cells serve as a nonrejected internal reference population. (A) Representative histograms depicting results in which the test graft was rejected (in untreated B6 mice, left) or accepted (B6 mice from which NK1.1+ cells were depleted, middle). The effect of depleting Ly49C/I+ and NKG2+ NK cells is shown in the right panel. The percentage of positive cells (marked by a bar) are indicated in each panel. Note that many of the nonstaining cells in all the panels are in the lowest fluorescence channel along the left axis due to the compensation settings of the flow cytometer. (B) Acceptance of β2m-/- bone marrow grafts by control mice or B6 mice depleted of NKG2A+ NK cells, Ly49C/I+ NK cells, or both subsets. Data represent the mean ± SEM (n = 3 mice). Results were reproduced in a replicate experiment. (C) Acceptance of β2m-/- bone marrow grafts by B6 mice treated with mAbs to deplete the indicated cell populations, or by control β2m-/- mice. Data represent the mean ± SEM (n = 2 mice). Results were reproduced in 2 replicate experiments. The Ly49C/I- and NKG2A-depleted group differed significantly from the Ly49A/G2-depleted group (P ≤ .04) but not from the NK1.1-depleted group (P = .5).
Discussion
Our results demonstrate that self-tolerance of a fraction of NK cells is accompanied by acquisition of a hyporesponsive functional phenotype, much as has been proposed for B and T cells.51,52 The self-tolerant NK cells exhibit a mature CD11b+DX5+ phenotype, and express Ly49 receptors, characteristic of the latest stage in NK-cell development.48 Furthermore, these cells are capable of cytokine production when stimulated with pharmacologic agents in vitro or with Listeria monocytogenes in vivo. In addition, the CI/NKG2- subset was not hyporesponsive in MHC-different B10.M mice, indicating that hyporesponsiveness of this subset occurs only when its receptors fail to bind host MHC class molecules. Presumably, a distinct hyporesponsive NK subset exists in B10.M mice, but its definition would require a different set of antireceptor antibodies. Thus, the hyporesponsive phenotype is not correlated with immaturity and is determined by the MHC environment. The existence of NK cells lacking self-MHC–specific inhibitory receptors is a definitive refutation of the hypothesis that self-tolerance of NK cells necessarily involves the expression by each NK cell of at least one inhibitory receptor specific for self-MHC molecules. While additional inhibitory receptors specific for H-2b may exist, ligand engagement by such receptors cannot account for the self-tolerance of CI/NKG2- NK cells, which fail to attack even cells that lack all class I expression. If such receptors exist, they may be only weakly reactive with H-2b class I molecules, or be expressed by only a small subset of CI/NKG2- NK cells.
The present results along with earlier data lead us to propose the following model of NK-cell self-tolerance, in which developing NK cells are actively rendered hyporesponsive if they encounter self cells lacking a cognate MHC ligand. Developmentally, NK cells acquire inhibitory receptors in a largely stochastic and cumulative fashion, leading to a repertoire in which cells expressing all combinations of receptors are represented.7 Receptor accumulation is to some extent limited by a feedback process dependent on MHC engagement,7 but even in the absence of MHC expression the process ultimately stops long before each NK cell expresses all possible receptors.53,54 Consequently, NK cells arise that lack inhibitory receptors specific for self-MHC. These developing NK cells will encounter surrounding potential target cells, such as self bone marrow cells. Self bone marrow cells presumably express unidentified stimulatory ligands for NK cells, because they are lysed by mature NK cells when MHC inhibition is prevented. We propose that the balance of stimulatory and inhibitory signaling that occurs in the encounters of developing NK cells and surrounding self cells determines the subsequent responsiveness of the mature NK cells. When MHC-dependent inhibition is absent, a greater degree of hyporesponsiveness is induced. When MHC-dependent inhibition is intense, less hyporesponsiveness occurs. This model can account for the hyporesponsiveness of all NK cells in class I–deficient mice, as well as the hyporesponsiveness of the CI/NKG2- NK cells studied here. It may also account for the long-puzzling observations that although many NK cells in neonatal mice lack expression of MHC-specific inhibitory receptors,27,55-57 these NK cells are nevertheless self-tolerant.
An alternative interpretation of the tolerance data is that engagement of self-MHC molecules with Ly49 receptors actually stimulates (rather than inhibits) the developing NK cell, endowing it with a high level of responsiveness. However, the latter model, unlike the tolerance induction model, does not fit well with the finding that wild-type NK cells maturing in a chimeric MHC+/MHC- host acquire the hyporesponsive phenotype,58,59 indicating that MHC- cells dominantly induce tolerance.
A previous comprehensive study of panels of human NK-cell clones from 2 donors provided valuable information concerning genetic control of the NK repertoire,24 yet found that each NK clone expressed at least one inhibitory receptor (KIR and/or CD94/NKG2A) for the donor's MHC class I molecules. It is possible that the NK repertoire is regulated differently in mice and humans. However, the “at least one” hypothesis predicts significant variations in the frequencies of NK receptor–defined populations in MHC-different animals, whereas only modest differences were observed in both mouse60 and human.53 These differences are arguably insufficient to equip each NK cell with a self-specific inhibitory receptor when MHC alleles differ. Alternatively, it is possible that hyporesponsive NK cells are specifically under-represented among NK clones, perhaps because they exhibit a disadvantage in cell growth or maintenance under cloning conditions.
The absence of reactivity against self cells could potentially be due to inhibitory receptors specific for non-MHC ligands expressed by normal cells. The action of recently described inhibitory NKR-P1D receptor and its Clr-b ligands61,62 cannot account for the low responses of CI/NKG2- NK cells, because anti–Clr-b mAb failed to restore responses to β2m-/- lymphoblasts or YAC-1 target cells (data not shown). Furthermore, we found that the CI/NKG2- NK cells responded poorly when stimulated with antibodies specific for the NKG2D, NKR-P1, and Ly49D stimulatory receptors. The latter observations suggest that some component(s) of the stimulation pathway is attenuated in the CI/NKG2- NK cells. Dampened stimulatory signaling could account for self-tolerance by diminishing the signals provided by receptors that engage naturally expressed stimulatory self ligands. If such signals were diminished below a threshold level necessary for cell triggering, the autoreactivity of the cells would be curtailed.
Cell-surface levels of the stimulatory receptors we studied were normal but the cells responded poorly to receptor ligation, suggesting that hyporesponsiveness is due to alterations in intracellular signaling components. We did not observe down-regulation of DAP12 or DAP10 intracellular protein levels in CI/NKG2- NK cells, or a decreased level of mRNA encoding the short isoform of NKG2D that was previously shown to be associated with DAP1263 (data not shown). Therefore, alterations in signaling molecules that are more distal in the signaling pathways or alterations in the spatial organization of membrane proteins may contribute to the hyporesponsive phenotype. While hyporesponsiveness is evident when stimulating NK cells through several known NK receptors, it remains possible that self-tolerance of this subset is partly due to down-regulation of an unidentified stimulatory receptor necessary for recognition of normal cells.
Whereas the hyporesponsive NK cells exhibited impaired stimulatory receptor signaling, it does not follow that they are generically hyporesponsive or that they do not play any role in biologically important responses. Although such cells are less responsive to stimulation, they are also not subject to inhibition by self-MHC molecules, and this could compensate for diminished stimulatory signaling in some responses. Furthermore, these NK cells may respond well to cytokines or to engagement of other types of stimulatory receptors. In either case, this NK-cell subset could be an active participant in immunity to pathogens or tumor cells. As one example, the cells exhibited significant (though reduced) cytotoxicity against YAC-1 cells (though the IFN-γ response was more impaired, Figures 3 and 2C-D). The finding that CI/NKG2- NK cells produce IFN-γ normally in Listeria-infected mice is also interesting in this regard, but does not prove that these NK cells are protective. Testing this possibility is currently difficult because the CI/NKG2- NK cells represent only 10% to 13% of NK cells. Interestingly, however, studies have shown that NK cells in class I–deficient mice can provide normal early protection from murine cytomegalovirus infections, indicating that NK cells exhibiting a similar hyporesponsiveness can function in antiviral immunity.64 One response where these hyporesponsive NK cells clearly have little or no role is in “missing self” reactivity, in which NK cells attack self cells that down-regulate MHC class I molecules.
Prepublished online as Blood First Edition Paper, February 22, 2005; DOI 10.1182/blood-2004-08-3156.
Supported by National Institutes of Health (NIH) grant RO1-AI35021 to D.H.R. N.C.F. and E.T. were recipients of postdoctoral fellowships from the Cancer Research Institute.
N.C.F. and E.T. contributed equally to this study.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Jen Hsia and Lily Zhang for excellent technical assistance, Thomas Hanke for discussions, Philippe Bousso for reviewing the manuscript, Hector Nolla for expert assistance with flow cytometry, and Laurel Lenz and Daniel Portnoy (UC Berkeley) for advice concerning Listeria.