Abstract
Reclassification of Hodgkin disease as Hodgkin lymphoma (HL) represents a milestone in the lymphoma field, awarding recent insights in the molecular biology of Hodgkin and Reed-Sternberg (H-RS) cells and their environment. This review summarizes antiapoptotic and proproliferative pathways involved in the pathogenesis of this disease with the ultimate goal of translating laboratory knowledge into clinical decision making. The focus is on potential targets and novel drugs, which are discussed in the context of the complex biology of HL. Considering that HL patients are more likely to die from acute and late treatment-related toxicities than from HL itself, the introduction of targeted, biologically based therapies for HL patients with palliative and eventually curative intention might be justified. (Blood. 2005;105:4553-4560)
Introduction
In 1994, Küppers et al in a true landmark paper proved definitely that Hodgkin and Reed-Sternberg (H-RS) cells in Hodgkin disease (HD) are clonally related B-cell-derived malignant cells.1 This discovery was the starting shot for a new era of molecular research embarking on what can now be called Hodgkin lymphoma (HL). In fact, the WHO classification awarded this fact and reclassified HD as HL.2 Since that time, numerous reports have shed substantial light on the complex biology of HL, and the recent application of genomic technology may help even more to clarify the molecular changes that underlie malignant transformation and cellular proliferation of this malignancy. However, whereas a large variety of secondary molecular aberrations have been defined for HL, no HL-specific primary transforming event has been proven to explain the H-RS phenotype so far. It is the scope of this article to highlight clinical and biologic characteristics of HL in order to develop novel, biologically based translational concepts for the treatment of HL. Hopefully, this process will lead to a shift of paradigms concerning the treatment of HL, similarly to what happened when “HD” got “HL.”
Treatment of HL: achievements and limitations
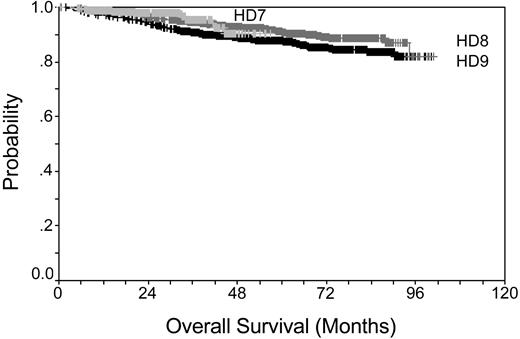
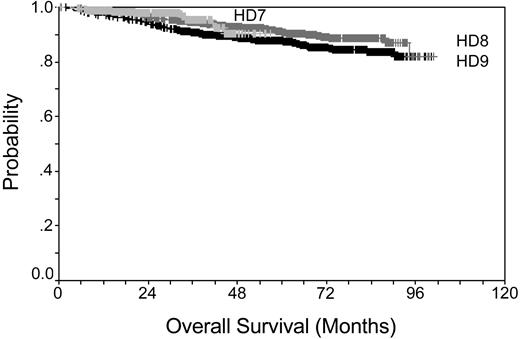
Prior to the middle of the last century, HL disease was fatal for the majority of cases. Introduction of radiotherapy and development of adriamycin, bleomycin, vinblastine, and dacarbazine (ABVD) in the 1970s was a major step forward. A new era was marked by the latest generation of intensified multidrug regimens such as Stanford V,3 ChIVPP/EVA (chlorambucil, vinblastine, procarbazine, and prednisone/etoposide, vincristine, and doxorubicin),4 MEC (meclorethamine, CCNU, vindesine, alkeran, prednisone, epidoxorubicin, vincristine, procarbazine, vinblastine, and bleomycin),5 and BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone)6 that were introduced for the treatment of advanced-stage HL in the 1990s. Overall survival for patients treated within the German Hodgkin Study Group (GHSG) trials is shown in Figure 1 (according to results of the trials: HD7 for early favorable, HD8 for early unfavorable, and HD9 for advanced stage HL), demonstrating that even patients with advanced-stage disease can be cured at a high percentage. Even for the instance of a relapse, HL patients can be salvaged using high-dose chemotherapy and autologous hematopoietic stem cell transplantation.7-9
Although patients and treating doctors could be satisfied by this success accomplished over the last decades, there is one major drawback that puts clinical achievements at risk: late toxicity resulting from HL therapy.10,11 Unfortunately, the increasing incidence of late toxicities is directly associated with increasing cure rates of mostly young patients. Apparently, after 20 or 30 years, patients have a higher risk of dying from toxicities than from HL. The main treatment-associated late toxicities are secondary malignancies (especially solid cancer such as lung cancer12 and breast cancer)13,14 and cardiovascular disease (especially left ventricle insufficiency and coronary artery disease).15
It is therefore the major task of the near future to reduce treatment-associated toxicity while maintaining high cure rates. In the future, this might be achieved by reduction of the amount of radiotherapy,16-18 identification of valid biologic risk factors that allow risk stratification of HL patients similar to the international prognostic score (IPS),19 and identification of patients with an excessive risk of toxicity. Having pointed out some of the major clinical achievements and limitations of HL treatment, it becomes clear that there is a definite need for alternatives for curative treatment strategies for HL that are as effective but less toxic. One promising option relies on the introduction of targeted drugs for the treatment of primary and relapsed HL; these drugs could contribute to a decrease in acute toxicities and especially late toxicities that are currently putting the initial success of cytoreductive combined modality treatment at risk. In the following section, we will describe biologic characteristics of HL that could form the basis for a rational approach for a novel avenue of molecular treatment of HL.
Kaplan-Meier plot showing overall survival rates for early favorable stage, early unfavorable stage, and advanced stage. Data taken from GHSG trials HD7-9.
Kaplan-Meier plot showing overall survival rates for early favorable stage, early unfavorable stage, and advanced stage. Data taken from GHSG trials HD7-9.
Biology of HL: Hodgkin and Reed-Sternberg cells and their environment
Classical and lymphocyte-predominant HL
HL is classified as classical HL (cHL) or nodular lymphocyte predominant HL (LPHL) dependent on the detection of Hodgkin and Reed-Sternberg (H-RS) cells or lymphocytic and histiocytic (L&H) cells embedded in a reactive infiltrate consisting of T cells, histiocytes, eosinophils, and plasma cells. The classical subtype comprises the nodular sclerosis, the mixed cellularity, the lymphocyte-depleted, and the classical lymphocyte-rich variants. Both the cHL and the LPHL subtypes have in common their derivation from germinal center (GC) B cells in most instances, while in few cases cHL is a T-cell-derived lymphoma. cHL and LPHL differ in terms of immunophenotype (absence versus presence of B-cell markers, respectively), mutational status of the heavy chain immunoglobulin (Ig) gene (crippling mutations versus ongoing somatic mutations, respectively), and signaling pathways involved in malignant transformation (eg, absence versus presence of BCL6 expression, respectively). Moreover, Epstein-Barr virus (EBV) is detected in H-RS cells of cHL mostly in the mixed cellularity variant (80%) but rarely in L&H cells of LPHL (< 1%).20
Genetic alterations of H-RS cells
In cHL, subtle DNA sequence changes include mainly IκBα mutations found in at least one third of cases, whereas mutations affecting n-ras, protein 53 (p53), or CD95 were not detected in a significant proportion.20 Similar to most B-cell lymphomas, H-RS cells in cHL do not display a mutator phenotype.21 Instead, chromosomal instability as defined by numeric chromosome abnormality is detected in virtually all H-RS cells as shown by fluorescence immunophenotyping and interphase cytogenetics (FICTION) analyses.22 Although it is known from classical cytogenetic studies that several breakpoints such as 3q27, 6q15, 7q22, 11q23, and 14q32 are detected nonrandomly in H-RS cells,23 known translocation partners such as BCL6, MYC, and MALT1 were identified only in a minority of cases.24 Recent evidence of recurrent genetic alterations came from whole genome studies analyzing genomic imbalances in H-RS cells. Recurrent amplifications were detected in H-RS cells on 2p13-p16, 9p23-p24, and 12q14 affecting c-rel, Janus kinase 2 (Jak2), and murine double minute 2 (MDM2) gene loci, respectively,25-28 the latter binding to the transactivation domain of p53 and thus blocking its ability to activate transcription. As small molecules such as cis-imidazoline analogs inhibit binding of MDM2 to p53,29 these might be useful compounds to activate the p53 pathway in H-RS cells.
According to the concept of gene dosage, the described gene amplifications could explain activation of both the signal transducer and activator of transcription (STAT) and the nuclear factor κB (NFκB) pathway observed in cHL, underscoring the need for further genetic studies of H-RS cells in a nonbiased, genome-wide scale. Taken together, these alterations probably are secondary and may reflect genetic instability of H-RS cells rather than primary transforming events, as none of these changes has been shown to be either necessary or sufficient for the development of an H-RS phenotype.
Transcriptional control in H-RS cells
Since NFκB (p65/RelA) was first described in 1996 to be constitutively up-regulated in HL30 and to be critical for cell survival,31 it gained much interest as it is known to mediate both proproliferative and antiapoptotic gene expression programs.32 Based on this evidence, NFκB might be regarded as a putative transforming master switch for H-RS cells in HL, although it becomes evident that constitutive NFκB activation is not a specific feature of H-RS cells but is also observed in other lymphomas. Besides NFκB, a variety of additional transcription factors are also deregulated in HL. Among these, activator protein 1 (AP-1) has been shown to be aberrantly up-regulated in H-RS cells, mediating some of the proproliferative and antiapoptotic characteristics of H-RS cells partly in concert with NFκB.33
The heterodimeric NFκB is a key transcription factor involved in inflammatory processes. Its shift from the cytoplasm to the nucleus is induced via binding of specific inflammatory cell-surface receptors. Timely correct activation and cessation of its activity is tightly regulated via binding to inhibitory IκBα molecules, whose activity in turn is regulated by ubiquitinating Iκ kinases (IKKs). Mechanistic explanations of NFκB up-regulation in H-RS cells include gene amplification of c-rel27 ; ligand-mediated and ligand-independent activation of cell-surface receptors such as CD30,34 CD40,35 receptor activator of NFκB (RANK),36 or NOTCH137 ; EBV-encoded late membrane protein 1 (LMP1)38 or LMP2a39 expression in EBV-positive cases; or loss-of-function mutations of the tumor suppressor IκBα.20 Importantly, IκBα mutations were not detected in activated B-cell-like diffuse large B-cell lymphoma (ABC-DLBCL), another GC-derived lymphoma with NFκB activation.40 Activated NFκB in H-RS cells is known to induce expression of antiapoptotic genes, such as cFLIP,41-43 XIAP,44 and BclXL,45 that antagonize the extrinsic or intrinsic apoptotic pathway in H-RS cells.
The AP-1 complex is formed by hetero- or homodimers of jun, Fos, and activating transcription factor (ATF) family proteins.46 In HL and also in anaplastic large-cell lymphoma (ALCL), but not in other lymphoma entities, c-jun and junB have been described to be aberrantly expressed in the malignant cell population.33 It appears that c-jun (which seems to be the major transactivating force of the constitutive AP-1 complex) is not under the control of c-Jun N-terminal kinase (JNK) but activated by an autoregulatory mechanism, whereas junB activity is NFκB dependent. Both c-jun and junB support proliferation of H-RS cells, possibly via induction of cyclin D2 and c-MET expression in H-RS cells.
In contrast to the mentioned up-regulated factors, some other factors such as Oct2, Bob1, and PU.147-49 are down-regulated in accordance with the observed loss of B-cell transcriptome50 of H-RS cells. The loss of B-cell-specific factors and the acquisition of lineage-deviant T-cell-specific factors such as GATA-3 and T-bet51 might as well explain some of the characteristic H-RS properties such as insensitivity to CD95-mediated apoptosis. The role of the transcription factors B-cell-specific activator protein (BSAP)52 and multiple myeloma oncogene-1-protein (MUM1)53 for the pathogenesis of HL is not clarified, but their differential expression pattern in cHL, LPHL, ALCL, and ABC-DLBCL indicates biologic relevance for the phenotype of H-RS cells.
Cytokines and cell-surface receptors in cHL
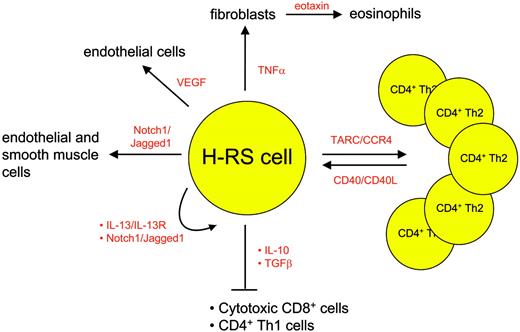
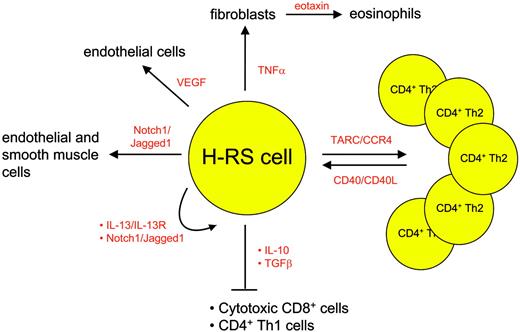
Besides the lack of a B-cell phenotype and transcription program, the scarcity of H-RS cells in the affected tissue is another very characteristic feature of these cells. Up to 99% of the cells in the involved HL tissue is represented by immune cells. These cells are attracted by the H-RS cells via several molecules depicted in detail in Figure 2. H-RS cells secrete mostly T helper 2 (Th2) cytokines and chemokines, leading to a favorable environment for H-RS survival: thymus and activation regulated chemokine (TARC), for instance, causes CD4 cells of Th2 subtype to accumulate and rosette around the malignant cells54 ; eotaxin secreted by fibroblasts that are stimulated by H-RS-derived tumor necrosis factor α (TNFα) attracts eosinophils55 and contributes to the recruitment of Th2 cells; transforming growth factor β (TGFβ) and interleukin-10 (IL-10) secreted by regulatory CD4+CD25+ T cells56 in contrast inhibit cytotoxic function of the T cells, thus creating an environment that protects H-RS cells from apoptosis induction. In addition, cytokines also contribute directly to H-RS cell survival as exemplified by the Th2 cytokine IL-13, which stimulates autocrine IL-13 receptors on H-RS cells57 and finally induces STAT6 up-regulation.
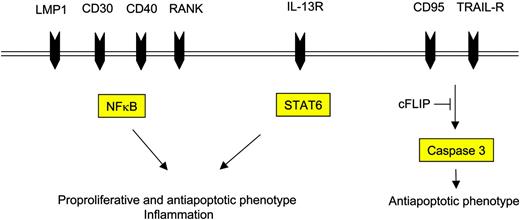
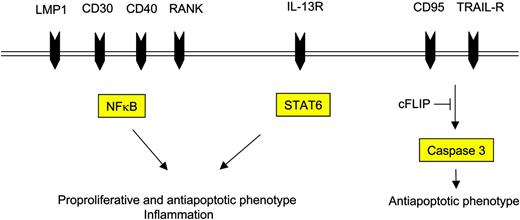
The attracted immune cells in turn influence the H-RS cell phenotype favorably as they provide survival signals (Figure 3). Examples are endothelial smooth muscle cells, eosinophils, and T cells that engage NOTCH1, CD30, and CD40 receptors on H-RS cells, respectively. Binding of members of the TNF receptor (TNFR) family results in NFκB activation by signaling via TRAF molecules, TRAF158 and TRAF259 being expressed and cytoplasmatically aggregated in H-RS cells, and also via the mitogen activated protein kinase (MAPK) pathway.60 The detection of TRAF molecules in H-RS cells might be of special interest, as they recently have been shown to be involved in cancer by exerting antiapoptotic effects.61 Another signaling pathway that is activated upon binding of CD30, CD40, or RANK included the mitogen-activated protein kinase (MEK) kinase, leading to phosphorylation of downstream extracellular signal-related kinase (ERK) and thus promoting cell survival.60 Inhibition of ERK phosphorylation interfered with ligand-induced cell survival in H-RS cell lines and therefore might represent another valid molecular target in HL.
Examples of chemokines and cytokines mediating interaction between H-RS cells and bystanding cells.
Examples of chemokines and cytokines mediating interaction between H-RS cells and bystanding cells.
Cell-surface receptors and their respective intracellular mediators induce the proproliferative and antiapoptotic phenotype of H-RS cells.
Cell-surface receptors and their respective intracellular mediators induce the proproliferative and antiapoptotic phenotype of H-RS cells.
In contrast to TNFR signaling, cytokine signaling is mediated by STAT molecules. It has been shown that STAT3 and STAT6 are expressed in H-RS cells in most instances.62,63 STAT6 expression might be explained by continuous stimulation of H-RS cells via their cytokine receptors, such as IL-13R.57 Another explanation is Jak2 gene amplification,26 which might also account for high STAT levels in H-RS cells making cells independent from physiologic stimuli. STAT3 might be of special interest, as it recently has been shown to mediate immune evasion in cancer.64
Besides exerting antiapoptotic and proproliferative potential, some of the soluble factors secreted by H-RS cells and bystanding stromal cells also induce reorganization of the stromal environment, including neoangiogenesis. Examples include TGFβ, hepatocyte growth factor (HGF),65 or vascular endothelial growth factor (VEGF),66 which probably are positive regulators of angiogenesis in HL.
Proproliferative and antiapoptotic phenotype of H-RS cells
According to Hanahan and Weinberg,67 proproliferative features (ie, self-sufficiency in growth signals; insensitivity to antigrowth signals; and limitless replicative potential) and antiapoptotic features are prerequisites to the development of malignancy. In HL, transcriptional deregulation and aberrant signaling via cell-surface receptors contribute to the proproliferative and antiapoptotic phenotype of H-RS cells.
High-level detection of antiapoptotic mediators might be of special interest for the pathogenesis of HL, as H-RS cells harbor crippled heavy-chain Ig genes in a substantial proportion of cases1 and therefore lack expression of functional B-cell receptors (BCRs) on the cell surface.68 Thus, H-RS cells are supposed to die within the GC reaction of the lymph node by induction of the CD95 pathway similar to nonmalignant B cells expressing a low-affinity BCR.69 Nevertheless, H-RS cells are resistant to CD95-mediated cell death,70 probably due to the constitutive expression of the antiapoptotic protein CFLIP.41 In fact, recruitment of cFLIP to the death-inducing signaling complex (DISC) and inhibition of cleavage of procaspase-8 have recently been demonstrated in 2 elegant studies.42,43 Furthermore, in these studies it could be shown that CD95 resistance could be overcome by silencing cFLIP using RNA interference. These findings have implications for novel therapeutic strategies targeting a centrally important phenotype of cHL: apoptosis resistance.
Besides CD95, other TNFR family members such as tumor necrosis factor receptor 1 (TNF-R1) and TNF-related apoptosis-inducing ligand receptor 1 (TRAIL-R1) and TRAIL-R2 are also expressed in H-RS cells.43 TNF-R1 activation induces the extrinsic pathway, while binding of TRAIL receptors induces both the extrinsic and the intrinsic apoptotic pathway under physiologic circumstances. In H-RS cells, cFLIP expression inhibits proper TRAIL-induced signal transduction similar to the inhibition of the CD95, and TRAIL sensitivity could be restored by down-regulation of cFLIP.43 TRAIL has been shown to act synergistically with other (chemotherapeutic) drugs,71 thus making TRAIL receptors attractive targets for treatment of HL. The use of CD95 ligand (CD95L) for clinical purposes instead has been limited by high, predominantly hepatic, toxicity. Similar to the extrinsic pathway, the intrinsic apoptotic pathway is also inhibited in H-RS cells,44 which can be explained by expression of both the caspase-3- and caspase-7-inhibiting XIAP molecule and the bcl2/BclXL molecules that antagonize the proapoptotic function of bax at the mitochondrial membrane.72 Whether these alterations are necessary for the development of the antiapoptotic phenotype of H-RS cells or secondary epiphenomena remains to be established. The finding that defects of upstream events of both the extrinsic and the intrinsic pathway contribute to apoptosis escape of H-RS cells is, however, even more interesting in the light of proper caspase-3 expression of H-RS cells,73 indicating that the convergent downstream apoptotic effector machinery might be functional in these cells.
Targeted therapy of HL: targets and drugs
Based on biologic characteristics of HL, potential target molecules and drugs will be discussed. Approaches that target extracellular domains consist mainly of antibodies directed against specific cell-surface receptors. In contrast, intracellular approaches include the application of small molecules, which modulate protein function of targets via directing interaction, and technology that prevents expression of the respective protein, such as antisense technology or RNA interference (RNAi) (Table 1). It has been recognized recently that development of new clinical candidate drugs requires a multidimensional optimization process from the very beginning instead of following a sequential approach.74 This would prevent the development of lead compounds with attractive in vitro profiles but suboptimal distribution, metabolism, and excretion properties. We therefore focus within this review article primarily on compounds that are in advanced preclinical or already in clinical phase of development.
Several studies aimed at targeting cell-surface molecules such as CD30 or CD20 in cHL and LPHL, respectively, thus attacking directly the malignant cell population via Fc-receptor-dependent mechanisms including antibody-dependent cellular cytotoxicity (ADCC). Among anti-CD30 antibodies currently tested in clinical phase 1 trials for treatment of cHL, the fully humanized native antibody MDX-060 (Medarex)75 and the chimeric antibody SGN-30 (Seattle Genetics)76 showed only modest clinical efficacy when given as single agents (response rates approximately 10%). There is recent in vitro evidence that treatment of H-RS cells with MDX-060 might further sensitize these cells to bortezomib (PS-341).77 Future developments in the field of HL might also include the anti-IL-13 antibody CAT-354 (Cambridge Antibody Technology); the anti-CD40 antibody CHIR-12.12 (Chiron Oncology); or the anti-RANKL antibody AMG162 (Amgen). That antibody treatment is highly efficient in HL is also demonstrated by the use of the anti-CD20 antibody rituximab (Roche) as a single agent for the treatment of LPHL78,79 and rare forms of CD20+ cHL79 for first-line and second-line treatment. Although in these 2 studies response rates of 100% and 86% have been observed, freedom-from-treatment rates might be improved by incorporating rituximab or other anti-CD20 antibodies such as the radioactively labeled monoclonal ibritumomab tiuxetan (Biogen Idec, Cambridge, MA) in future polychemotherapy regimens.
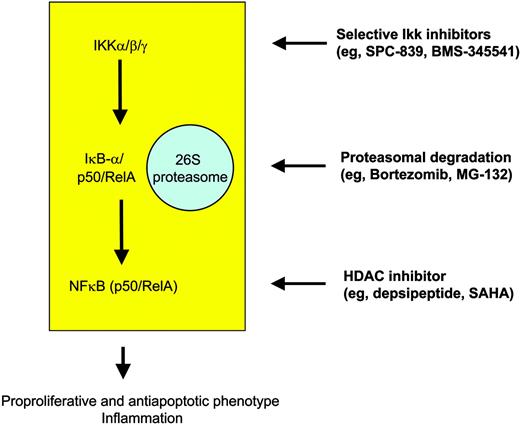
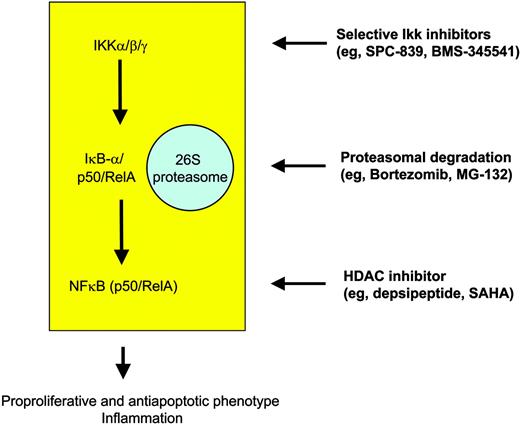
At the intracellular level, many novel compounds have been designed that target the IKK-IκBα-NFκB cascade, particularly IKK inhibitors, proteasome inhibitors, and direct NFκB inhibitors (Figure 4). Most of the selective IKK inhibitors target IKKβ and still are in the preclinical phase of testing, but extensive studies have been performed showing a median inhibitory concentration (IC50) in the nanomolar range. Well-studied molecules include the reversible adenosine triphosphate (ATP)-competitive inhibitor SPC-839 (Celgene), which is a quinazoline analog, and the imidazoquinoxaline BMS-345541 (BMS), which in contrast acts as an ATP-noncompetitive inhibitor.80 It has to be considered that effectiveness of IKK blockage in HL might be counteracted by mutations rendering IκBα molecules nonfunctional in about one third of cases. This is suggested not only by the mechanism of action itself but also by an in vitro study showing that the IKKβ inhibitor arsenic trioxide (As2O3) requires wild-type IκBα to induce apoptosis in H-RS cell models.81 Thus, although arsenic trioxide has been shown to induce remission rates in diseases such as promyelocytic leukemia, the feasibility of this compound in cHL is questionable.
Targeted strategies for modulation of NFκB in order to overcome the proproliferative and antiapoptotic phenotype of H-RS cells in HL.
Targeted strategies for modulation of NFκB in order to overcome the proproliferative and antiapoptotic phenotype of H-RS cells in HL.
Bortezomib (PS-341; Millennium Pharmaceuticals) recently gained FDA approval for the treatment of relapsed and refractory multiple myeloma. It is a reversible inhibitor of the 26S proteasome and thus interferes with degradation of a variety of proteins including IκBα. Similarly to IKK inhibitors, inhibition of the proteasome complex in tumor cells is thought to depend on functional IκBα molecules retaining NFκB in the cytoplasm. Nevertheless, it has been shown that PS-341 has strong apoptosis-inducing activity even in IKBA-mutated H-RS cell lines,82 which might in part be explained by IKK-independent effects of bortezomib, such as an increase in p21 and bax levels or downregulation of bcl2. Although functional data are missing, it might be speculated that a profound disturbance of cell-cycle checkpoints and the tumor-suppressor pathways involving p21 and p27 might be important for H-RS cell pathogenesis.45 This presumed mechanism of action might also work in primary cases of HL, being the basis for clinical trials with PS-341 in relapsed HL independently of the IKBA mutational status of H-RS cells. Other proteasome inhibitors such as MG-132 (Tokyo Metropolitan Institute of Medical Science, Tokyo, Japan), shown to be efficient in H-RS cell lines via inhibition of the Jak/STAT pathway,83 are in preclinical evaluation. Taken together, clinically active inhibitors of the IKK complex are already on the shelf that might prove beneficial in cHL, which critically relies on the constitutive transcriptional activity of NFκB. However, an unanswered issue in the field of molecular targeting in cHL is whether those cases harboring IκBα gene mutations do benefit from IKK-targeted therapy. Data from our own group in fact suggest that IKK inhibition is active in lymphomas with constitutively active NFκB, regardless of the mutational status of IKBA (R.K.T., unpublished results, 2004).
Direct inhibition of NFκB activity at a transcriptional level might be achieved using RelA antisense oligonucleotides, but there is little clinical evidence in support of this strategy. More recently, regulation of intranuclear events regulating the duration and strength of NFκB binding to the respective DNA element gained much interest.84 Expression of NFκB target genes is under the influence of coactivators (eg, p300/creb-binding protein [CBP]) and corepressors (eg, HDAC) that posttranslationally acetylate and deacetylate the RelA molecule. Compounds such as depsipeptide (FK228; Gloucester Pharmaceuticals) or suberoylanilide hydroxamic acid (SAHA; Aton Pharma) inhibit HDAC enzymes, interfere with NFκB transactivating potential, and thus mediate apoptosis induction via p21 up-regulation,85 inhibition of cFLIP,86 or generation of reactive oxygen species (ROSs).87 Moreover, these compounds also favor the assembly of NFκB with its repressor IκBα and the nuclear export of NFκB toward the cytoplasm finally leading to down-regulation of NFκB activity. Recent data suggest that combining SAHA with bortezomib (PS-341) might act synergistically at least in leukemia.88 Clinical trials using depsipeptide89 and SAHA90 are under way for lymphoma and even for HL: so far, 2 of 5 SAHA-treated HL patients experienced antitumor activity. We therefore strongly believe that this class of molecules should be further tested in HL.
Alternatively to the inhibition of NFκB, interfering with TRAF, MEK/ERK, or phosphatidylinositol-3 kinase/AKT signaling might represent a useful strategy for HL. Although inhibitors of MEK/ERK60 or phosphatidylinositol-3 kinase/AKT91 have been shown to induce apoptosis in H-RS cells, to our knowledge there are no compounds available that meet clinical necessities. Similarly, induction of apoptosis using chemical inhibitors of STAT molecules, such as the tyrosine kinase inhibitor AG490, works efficiently in H-RS cells in vitro62 ; these or similar compounds are not clinically available. More recently, the STAT3 antisense molecule ISIS 345794 (ISIS Pharmaceuticals) has been selected for further clinical development, and it might be speculated that targeting STAT3 with that molecule is a promising strategy for HL.
As mentioned earlier, the antiapoptotic phenotype is a hallmark of H-RS cells. As more preclinical evidence becomes available, implementing antiapoptotic compounds into early clinical studies is thus a challenging task for the future. Besides acting as a caspase inhibitor, XIAP seems to be also an attractive target for HL, as it influences cell-cycle progression and strengthens NFκB activity (positive feedback).92 XIAP antisense oligonucleotide AEG35156 is currently tested in clinical phase 1 trials (Aegera Therapeutics). Chemical inhibition of XIAP can be achieved by compounds mimicking the endogenous IAP inhibitor second mitochondria-derived activator of caspase (SMAC). To date, screening strategies focus on the identification of novel small molecule peptidyl and nonpeptidyl inhibitors of XIAP, such as SMAC peptides and polyphenylurea pharmacophores, respectively. For example, SMAC-mimicking peptides have already been shown to be effective in xenograft tumor models in combination with TRAIL.71 TRAIL has strong antitumor potency by itself,93 and phase 1 studies using anti-TRAIL-R1 antibodies (Human Genome Sciences) have been initiated for malignant disease.
Similar to CD95 signaling, TRAIL signaling is also blocked in HL intracellulary due to the expression of caspase-8 inhibitory cFLIP, thus preventing receptor-mediated apoptosis. Silencing of cFLIP using siRNA restores both CD95 and TRAIL sensitivity. This could also be accomplished by synthetic triterpenoids such as CDDO (RTA401; Reata Pharmaceuticals), which is known to trigger ubiquitination and degradation of cFLIP.94,95 Other possibilities of cFLIP silencing include NFκB inhibition and histone deacetylase (HDAC) inhibition.
Besides XIAP and cFLIP, the bcl2 family member BclXL is another NFκB controlled gene that confers apoptosis resistance in H-RS cells. Nevertheless, in vitro data indicate that deregulation of bcl2 family members is more likely to represent an acquired secondary event during tumorigenesis and anticancer treatment. Thus, targeting bcl2 family members in HL should be performed within multidrug regimens, if at all. Good candidates might be the antisense oligonucleotide oblimersen (Genta/Aventis, Bridgewater, NJ), which is currently tested in a variety of phase 3 trials, or small molecules antagonizing bcl2 and/or BclXL (IDUN/Abbott, San Diego, CA).
Having discussed novel treatment strategies for HL using antibodies and chemical compounds, we now briefly comment on immunologic approaches in the field. Due to the transforming potential of EBV and the expression of EBV nuclear antigen 1 (EBNA1), LMP1, and LMP2 (type II latency gene expression pattern) in H-RS cells in approximately 40% of all cases, cellular strategies directed against EBV-encoded proteins are being tested in clinical trials. After in vitro generation of mostly LMP2a-specific autologous CD8+ cytotoxic T cells,96,97 clinical remissions have been seen in some instances. Of note is that this approach is limited to EBV-positive HL cases and requires establishment and preclinical testing of autologous cells for each individual to be treated. Usually, the engineering of EBV-specific cytotoxic cells in sufficient quantity and quality takes months, which is a major obstacle to the clinical application. Considering immunologic approaches, it is noteworthy to mention that vaccination strategies with keyhole limpet hemocyanin (KLH) administered together with autologous anti-idiotype vaccines—similar to current phase 3 trials in follicular lymphoma98 —are not applicable to HL due to the lack of Ig gene expression of H-RS cells. The role of tumor antigens99 for therapeutic applications has not been addressed in HL so far.
Conclusion: how to move from bench to bed
The discovery that H-RS cells derive from preapoptotic B cells in most instances was a breakthrough for the understanding of HL biology. Much has been learned since then about transcriptional programs that govern the antiapoptotic and proproliferative phenotype of H-RS cells; genetic characteristics and cellular networks have been elucidated; and finally, this knowledge opened the door for the development of novel, biologically based therapies. The experiences with both the small-molecule inhibitor STI571 for chronic myelogenous leukemia and the bcl2 antisense oligonucleotide for melanoma, exemplifying both successes and limitations of targeted strategies, suggest that one should aim at identifying and silencing primary transforming events in malignant disease rather than targeting secondary events. In HL, these underlying primary events have not yet been identified unambiguously. However, given their clear and decisive role in establishment and maintenance of the malignant phenotype, targeting mediators of the antiapoptotic and proproliferative phenotype of H-RS cells might be a promising strategy to follow.
Similarly, novel therapeutic concepts based on well-defined molecular targets rather than on empiric testing are needed for future clinical trials. This is particularly true, as obviously mostly young HL patients can be cured at a high rate only at the price of eventually suffering from late therapy-associated toxicities. As HL patients can be salvaged at relatively high percentage after the first relapse, trials focusing on targeted therapies will be initiated for patients with a second relapse or multiple recurrences or primary progressive disease, as these patients are to receive palliative treatment in any instance. Examples of such efforts are therapies with anti-CD20 or anti-CD30 antibodies and recently also bortezomib-based approaches. It is most likely that similar to STI571 for the treatment of chronic myelogenous leukemia (CML), these and novel drugs will be included in established chemotherapy protocols substituting well-known cytotoxic drugs. Eventually, these strategies then might even be considered for first-line treatment for poor-risk subgroups that still have to be determined.
The role of tumor antigens for therapeutic applications99 has been addressed recently for EBV-positive HL patients,100 but clinical data are not yet available.
Prepublished online as Blood First Edition Paper, February 22, 2005; DOI 10.1182/blood-2004-12-4750.
Supported by a grant from the Friedrich and Maria Sophie Moritz′sche Foundation, the Center for Molecular Medicine Cologne (CMMC), the Frauke-Weiskam Foundation, and the Deutsche Krebshilfe.