Abstract
ABO histo-blood group antigens have been postulated to modify pathogen spread through the action of natural antibodies and complement. The antigens are generated by a polymorphic glycosyl-transferase encoded by 2 dominant active and a recessive inactive allele. In this study we investigated whether ABO sugars are incorporated into the envelope of HIV-1 virions. HIV vectors derived from cells expressing ABO antigens displayed sensitivity to fresh human serum analogous to ABO incompatibility, and ABO histo-blood group sugars were detected on the viral envelope protein, glycoprotein 120 (gp120). Moreover, lymphocyte-derived virus also displayed serum sensitivity, reflecting the ABO phenotype of the host when cultured in autologous serum due to adsorption of antigens to cell surfaces. Serum sensitivity required both active complement and specific anti-ABO antibodies. Thus, incorporation of ABO antigens by HIV-1 may affect transmission of virus between individuals of discordant blood groups by interaction with host natural antibody and complement. (Blood. 2005;105:4693-4699)
Introduction
The ABO blood group system, discovered by Landsteiner1 more than a century ago, is one of the best-studied polymorphic genetic loci in the human genome.2 The blood group, a carbohydrate antigen, is synthesized by allelic forms of a single glycosyltransferase that catalyses the addition of N-acetylgalactosamine (A) or d-galactose (B) to a core fucosylated oligosaccharide generated by the H transferase (fucosyltransferase 1; FUT1) product of the fut1 gene; the O group is found in individuals homozygous for a prematurely truncated form of the ABO transferase and displays the H phenotype without A or B. ABO antigens are incorporated into the surface glycoproteins and glycolipids of red blood cells. Also free glycolipids in the serum bearing ABO sugars can be incorporated into membranes of other cells and tissues, including lymphocytes that do not normally express the glycosyltransferases.3-5 In more than 80% of individuals in which the blood group is present in secretions, ABO antigens are expressed on epithelial mucosae, including those of the male and female genital tract. The secretor phenotype is defined by the activity of a separate H synthesizing enzyme, FUT2.2 Due to molecular mimicry by gut flora, individuals acquire “natural” antibodies (mainly immunoglobulin M [IgM]) to the antigen structure that they do not synthesize, which gives rise to transfusion incompatibility mediated by complement fixation by these antibodies. This process is analogous to the hyperacute rejection of xenotransplants seen in old world primates and humans due to their lack of a functional α(1-3)galactosyltransferase gene.6,7 Indeed ABO compatibility is crucial for successful organ transplantation in humans; hence, ABO antigens are now referred to as histo-blood group antigens.
The ABO transferase is evolutionarily old but acquired its polymorphic nature during mammalian evolution.6 The selective pressures that retain a balanced polymorphism are undefined, but their role in modulating the spread and pathogenesis of infectious disease has been postulated6,8 (and references therein). Bacteria and viruses often coat themselves in carbohydrate structures to mask their antigenic domains and ward off adaptive immune responses, as well as using oligosaccharide moieties as attachment sites. Notable examples are the enteric pathogens Norwalk-like caliciviruses and Helicobacter pylori that use carbohydrate blood group antigens as cellular receptors on the gastric mucosa.9-12
In this study we address the question of whether ABO antigens can be incorporated into the envelope of HIV-1. The surface envelope protein of HIV-1, glycoprotein 120 (gp120), is highly glycosylated, especially over the side of the molecule that faces outward from the virion after trimerization during assembly, protecting this area from neutralizing antibodies.13 Interestingly, analyses of viruses that escape from neutralizing antibodies often do so through repositioning of glycosylation sites in gp120.14 Although many of the N-linked carbohydrate groups of gp120 are of the high mannose type, others have a mature structure,15,16 which can act as substrates for ABO transferases. The presence of O-linked sugars on gp120 has been suggested to be dependent on the host cell (reviewed by Feizi17 ). The lipid envelope of HIV derived from the host lymphocyte may also contain glycolipids as well as host cell glycoproteins such as major histocompatibility complex (MHC) class II and complement regulatory proteins.18
Modification of viral glycosylation is known to render enveloped virions sensitive to natural antibody-mediated complement lysis. Retroviruses, lentiviruses, and rhabdoviruses raised in nonprimate mammalian cells incorporate α(1-3)galactoside moieties that sensitize them to human serum.7,19,20 Arendrup et al21 reported that HIV-1 IIIB derived from lymphocytes from an A donor could be neutralized by mouse monoclonal antibodies to the A antigen. Additionally, a recent study showed that measles virus grown in cells overexpressing ABO transferases is sensitive to human sera in an ABO-dependent manner.22 Incorporation of these antigens into HIV-1 particles may determine the relative risk of transmission between individuals of discordant blood groups. Recent studies have shown that the presence of natural antibodies can have a profound effect on the generation of cytotoxic T-cell responses against pathogens.23 Such innate immune responses against sugar structures on incoming viruses may also determine the speed at which the adaptive immune response against HIV is generated and thus help limit primary viral replication. We, therefore, sought to analyze the incorporation of ABO antigens into HIV-1 virions and their sensitivity to complement-mediated antibody-dependent inactivation.
Materials and methods
Cell lines, lectins, and plasmids
293T cells were obtained from the American Tissue Culture Collection (ATCC, Manassas, VA). The glioma cell line NP2 expressing CD4 and CXC chemokine receptor 4 (CXCR4) or CC chemokine receptor 5 (CCR5) have been described elsewhere24 and were kindly provided by H. Hoshino. All cell lines were maintained in Dulbecco-modified Eagle Medium (Invitrogen, Paisley, United Kingdom) supplemented with 10% fetal calf serum (FCS) and 1000 U/mL penicillin and streptomycin. Fluorescein isothiocyanate (FITC)-conjugated and biotinylated lectins selective for blood group A (Bandeiraea simplicifolia BSI-A4, Helix pomatia), blood group B (Bandeiraea simplicifolia BSI-B4), the H antigen (Ulex europeanus) and α(1-3)-linked galactoside (Bandeiraea simplicifolia BSI) were obtained from Sigma, Poole, United Kingdom. Plasmid pSG encoding A and B transferases25 were kindly provided by F. Yamamoto, La Jolla, CA, and pcDNA encoding porcine α(1-3)αgalactosyltransferase26 was from C. Porter (Institute of Cancer Research, London, United Kingdom). The H transferase, FUT-1, was isolated from HeLa cells by reverse transcription-polymerase chain reaction and cloned into pBabe-Puro as a BamH1-EcoR1 fragment using the primers GCGCGGATCCCGCCACCATGTGGCTCCGGAGCCAGTCG and GCGCGAATTCTCAAGGCTTAGCCAATGTCC. Lentiviral vector plasmids pCSGW (vector genome expressing enhanced green fluorescent protein [eGFP]) and p8.2 (HIV-1 packaging construct) were kindly provided by M. Collins, United Kingdom, and the HXB2 gp160 envelope expression vector pSVIII-HXB2 was obtained from P. Clapham, Worcester, MA.
Serum preparation
Fresh venous blood was drawn from healthy HIV-negative volunteers with informed consent (in accordance with the Declaration of Helsinki) and allowed to clot. We confirm that approval for the use of human serum from healthy volunteers was granted to Professor R. Weiss by the University College London Committee on Ethics (project ID 0335/001) in accordance with the Helsinki Protocol. Sera from ABO-defined donors were pooled and stored frozen until required. Total IgM was purified from serum using a HiTrap IgM purification column (Nycomed Amersham, Buckinghamshire, United Kingdom) as per the manufacturer's instructions.
Virus propagation
HIV-1 IIIB and SF2 are T-cell line-adapted X4 strains, SF162 is an R5 using primary isolate, 89.6 is a molecular clone derived from an R5 × 4 isolate, and 2028 and 204427,28 are low-passage primary isolates that use R5 × 4 and X4, respectively. Peripheral blood mononuclear cells (PBMCs) were prepared from ABO-defined donated buffy coats (National Blood Transfusion Service, Brentwood, United Kingdom) by Ficoll gradient centrifugation. Purified PBMCs were stimulated with 50 ng/mL phytohemagglutinin and cultured at 106 cells/mL in RPMI 1640/10% FCS or autologous human serum for 2 days. The cells were then split and cultured for a further 3 days in medium supplemented with 10 U/mL recombinant human interleukin 2 (Roche, Hertfordshire, United Kingdom). Cells (5 × 105) were then exposed to 1 × 105 focus-forming units (FFU) of HIV-1 stocks for 2 hours and cultured for 2 days. Infected cells were then cocultivated with fresh PBMCs and grown for a further 3 to 5 days. For cells cultured in autologous serum, fresh serum was added every 2 days. The cell supernatants were cleared by centrifugation and snap-frozen in liquid nitrogen. Titers were determined by serial dilution on NP2 cells expressing CD4 and the appropriate coreceptor and processed as described under “Serum sensitivity assays.”
Production of HIV-1 vector pseudotypes
Subconfluent monolayers of 293T cells on 10-cm plates were transfected with a total of 8 μg plasmid (pCSGW, p8.2, and pSVIII-HXB2 in weight ratio of 3:2:1) using Fugene-6 (Roche). For blood group incorporation, the cells were also cotransfected with pBabe-H, pSG-A, pSG-B, or pcDNA-α(1-3)α galactosyltransferase (GT). Viral supernatants were harvested 48 hours after transfection, filtered, and snap-frozen in liquid nitrogen. Viral titers were determined on NP2/CD4/CXCR4 by fluorescence-activated cell sorting (FACS) as described.29
Serum sensitivity assays
Dilutions of fresh human sera were mixed with 200 FFU of each virus, and the serum concentration was adjusted to 10% with matched heat-inactivated serum, in OptiMEM (Invitrogen). The virus was then incubated at 37°C for 1 hour and plated on NP2 cells expressing CD4 and the appropriate coreceptor. After 2 hours of adsorption, the target cells were washed and cultured for a further 72 hours in fresh medium. The monolayers were then fixed in cold acetone/methanol (1:1 vol/vol) for 10 minutes and immunostained for in situ p24 expression as described previously.30 Titer was determined by counting infected foci by microscopy. Assays using guinea pig complement (Sigma) were performed by using reconstituted complement at 1:5. For assays using vectors, a multiplicity of infection (MOI) of 0.3 as determined on NP2 cells was used as input in the above assay. Virus infection was determined by eGFP expression by FACS 72 hours later.
Envelope Western blot
293T-produced vectors (10 mL) were pelleted by ultracentrifugation at 25 000g for 1 hour and resuspended in phosphate-buffered saline. A volume of 10 μL of each sample was separated on a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel, transferred to an enhanced chemiluminescence (ECL) membrane (Amersham, Buckinghamshire, England), and blocked in tris-buffered saline (TBS) containing 5% bovine serum albumin (BSA) and 0.1% Tween-20. Glycosylated viral proteins were detected using ABO selective biotinylated lectins (10 μg/mL) and streptavidin-linked horseradish peroxidase (HRP). HIV-1 proteins were detected using a 1:2000 dilution of 3 sera from HIV-1-positive individuals and an HRP-linked rabbit anti-human polyclonal antibody. Visualization of the blots was by ECL reagent (Nycomed-Amersham).
Capture assay for gp120
A modified version of a gp120 capture enzyme-linked immunosorbent assay (ELISA) was used to detect ABO sugars on gp120.31 Virus was lysed in 0.5% empigen, and dilutions incubated on Maxisorb plates coated with a gp120 monoclonal antibody D7146 (National Institute for Biological Standards and Control [NIBSC], Hertfordshire, United Kingdom). Immobilized gp120 was then detected with either biotinylated ABO selective lectins or anti-HIV-1 human sera, followed by streptavidin-linked alkaline phosphate (AP; Nycomed Amersham) or AP linked to a goat anti-human polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA), and developed using the LumiPhos reagent. The relative luminescence was the read on a Lucy Luminometer (Rosys Anthos, Hombrechtikon, Austria).
Flow cytometry
ABO antigens were detected on cell surfaces using FITC-conjugated BSI-B4 or U europeanus (Sigma) at 5 μg/mL in phosphate-buffered saline (PBS) plus 5% BSA and 0.05% sodium azide. For blood group A, a mouse anti-A monoclonal IgG (Dako, Glostrup, Denmark) was used followed by a FITC-labeled goat anti-mouse polyclonal antibody. The cells were then analyzed on a FACScan (Becton Dickinson, San Jose, CA) using the Cellquest software.
Results
ABO transferase expression in HIV-producing cells leads to histo-blood group antigens associated with gp120
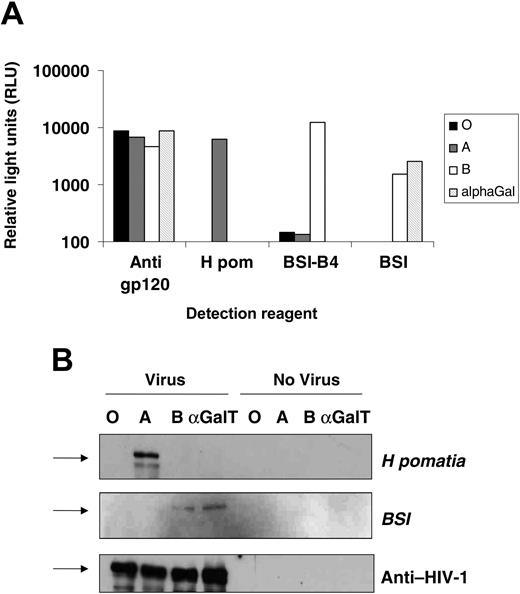
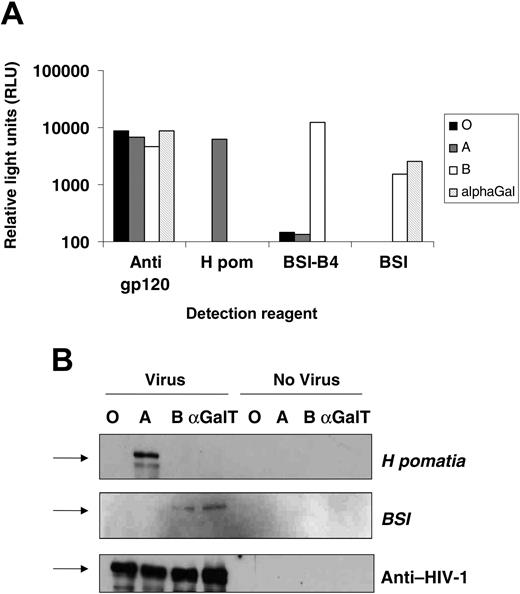
The HIV-1 envelope proteins gp120 and gp41 are highly glycosylated during synthesis. Gp120 itself is both N- and O-glycosylated, especially on its outer face, an area known to exploit glycosylation for immune evasion.13,32 293T cells were transfected with expression vectors encoding either A or B transferases in combination with the H transferase, or the mammalian α(1-3)galactosyltransferase, known to modify gp120 glycosylation,20 as a control. HIV-based vectors pseudotyped with the CXCR4-using gp120 HXB2 and packaging an eGFP reporter genome were raised in these cells in serum-free medium, and titers were established on the indicator cells, NP2/CD4/CXCR4, by FACS detection of GFP-positive cells. Cotransfection of ABO and H transferases had no effect on output viral infectivity with all titers between 4 and 5 × 105 IU/mL (not shown). A gp120 capture ELISA31 was modified to detect ABO sugars using A- or B-selective lectins. The HIV vector stocks were equalized by titer, detergent disrupted, and soluble gp120 was captured by immobilized antibody. Pooled human sera from HIV-1+ individuals was used to detect the capture of gp120 and demonstrated that all stocks contained similar levels of the envelope protein (Figure 1A). The biotinylated A-selective lectin from Helix pomatia (H pom) was used to detect gp120-associated A antigen, with binding only detectable on envelope from A-transfected 293T cells. The B-selective lectin, BSI-B4, reacted strongly with envelope from B transfectants but not with A and O transfectants. The α(1-3)Gal (galactosyl) lectin BSI reacted both against virus from α(1-3)αGalT (galactosyltransferase) and B-transfected cells. While these lectins can bind to other carbohydrate antigens (eg, the Ty antigen for H pom), the reactivity for a particular lectin for gp120 was dependent on the transfection of the specific ABO glycosyltransferase.
These results indicated that ABO transferases add blood group antigens to gp120. Pelleted virions from transfected cells were lysed, and viral proteins were separated by SDS-PAGE and detected by Western blot with lectins or serum from an HIV-1-infected individual. Virus produced from A-expressing cells yielded a band of 120 kDa with the H pomatia, whereas BSI gave 120-kDa bands in the B and α(1-3)αGalT transfectants. The total gp120 loading was equal in all lanes, and the lectin bands were dependent on specific transfection with viral components (Figure 1B). Thus, in cells expressing ABO transferases, HIV-1 gp120 can be modified to carry blood group moieties.
ABO-dependent serum sensitivity of HIV vectors
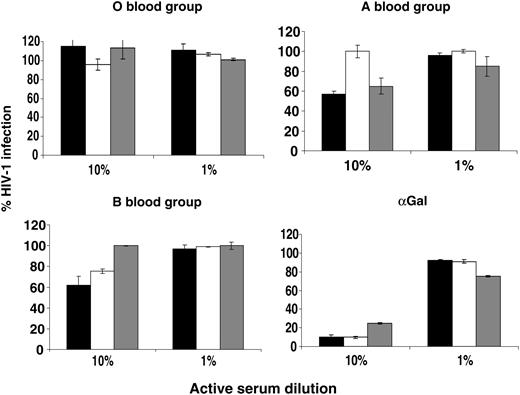
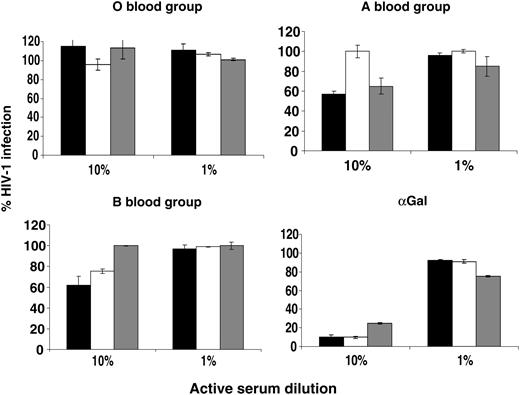
The mammalian α(1-3)αGalT when expressed in human cells modifies the glycosylation of lentiviral envelope proteins, and virions become sensitive to complement fixation by natural antibodies in human serum.20 To see whether the same was true of human ABO transferases, the HIV vector stocks were assessed for sensitivity to human sera from ABO-defined donors. A volume of 2 × 104 IU of vector (MOI of 0.3) was incubated with 10% or 1% active human serum diluted in matched heat-inactivated serum. Serum sensitivity was expressed as the percentage of GFP-positive NP2 cells compared with virus incubated in 10% heat-inactivated serum (Figure 2). O vectors (those produced only in the presence of the H transferase) were not sensitive to inactivation in any sera. Vector raised in cells cotransfected with the A transferase was partially sensitive to inactivation by sera from O and B donors but not in A serum. Conversely, vector raised in B transfectants was partially sensitive in O and A sera but not in B serum. To control for variation in complement in donor sera, vector raised in α(1-3)αGalT-transfected cells was tested for human serum sensitivity, which, as expected, was equally sensitive in all sera. These results indicate that expression of ABO transferases in HIV-1-producing cells sensitizes virus to human complement-mediated inactivation in a manner consistent with the incorporation of ABO antigens into the virion.
Expression of ABO transferases in cells producing HIV-1 particles leads to incorporation of ABO antigens into viral envelopes. (A) HIV-1-based vectors pseudotyped with the HXB2 envelope were produced in 293T cells cotransfected with the H (giving the O blood group) and A or B transferases, or the porcine α(1-3)galactosyltransferase. The envelope protein, gp120, from vectors was captured by immobilized anti-gp120 antibody. Captured ABO sugar was then detected with biotinylated ABO selective lectins H pomatia (A), BSI-B4 (B), or BSI (B and α(1-3)galactoside), or anti-HIV-1 human sera as a loading control. Background lectin binding was controlled by capturing media from ABO-expressing virus-negative 293T cells. Binding was detected by streptavidin-conjugated alkaline phosphates and expressed as relative light units. (B) Viral lysate was separated on a 10% SDS-PAGE gel and Western blotted using the same reagents as in panel A, this time detecting antigen with streptavidin-linked horseradish peroxidase. ABO-expressing 293T cell supernatant was used as a negative control. Arrows represent the 113-kDa molecular weight marker.
Expression of ABO transferases in cells producing HIV-1 particles leads to incorporation of ABO antigens into viral envelopes. (A) HIV-1-based vectors pseudotyped with the HXB2 envelope were produced in 293T cells cotransfected with the H (giving the O blood group) and A or B transferases, or the porcine α(1-3)galactosyltransferase. The envelope protein, gp120, from vectors was captured by immobilized anti-gp120 antibody. Captured ABO sugar was then detected with biotinylated ABO selective lectins H pomatia (A), BSI-B4 (B), or BSI (B and α(1-3)galactoside), or anti-HIV-1 human sera as a loading control. Background lectin binding was controlled by capturing media from ABO-expressing virus-negative 293T cells. Binding was detected by streptavidin-conjugated alkaline phosphates and expressed as relative light units. (B) Viral lysate was separated on a 10% SDS-PAGE gel and Western blotted using the same reagents as in panel A, this time detecting antigen with streptavidin-linked horseradish peroxidase. ABO-expressing 293T cell supernatant was used as a negative control. Arrows represent the 113-kDa molecular weight marker.
HIV-1 isolates propagated in PBMCs from ABO-defined donors are sensitive to anti-ABO sera
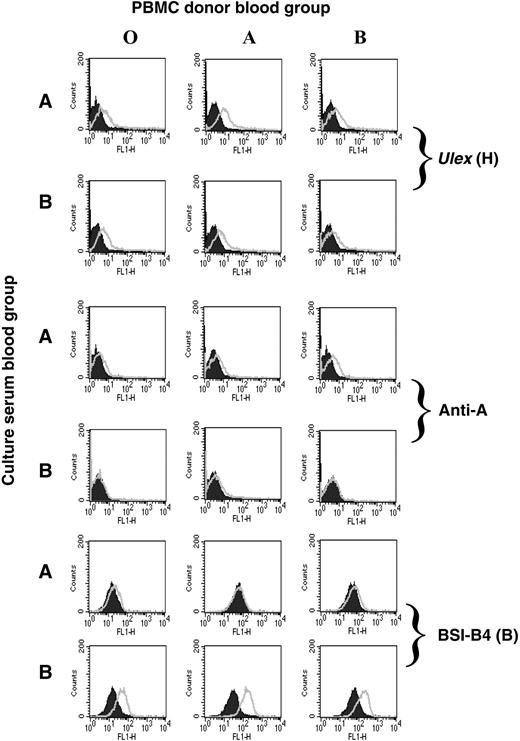
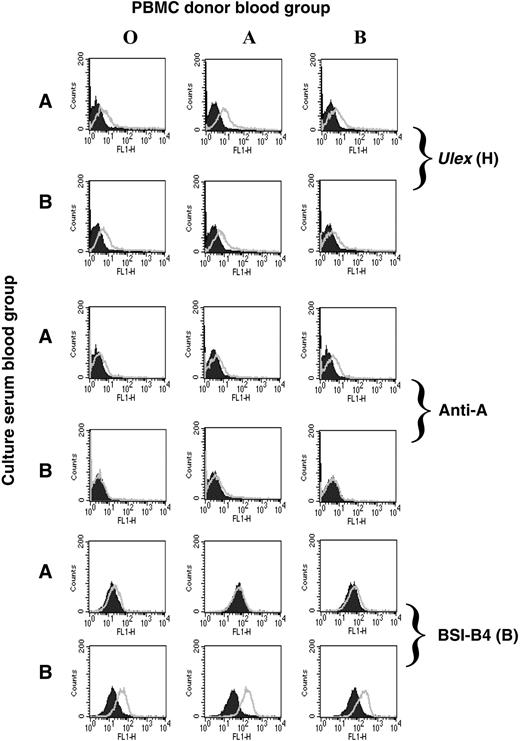
Lymphocytes do not normally express ABO and H transferases, but ABO-glycosylated lipids in the serum can be incorporated into cell membranes. Therefore, lymphocytes can passively acquire these antigens from sera.2 We postulated that HIV-1 grown in PBMCs cultured in autologous serum might acquire the blood group of that individual. PBMCs were isolated from O, A, or B donors and cultured in human serum from A or B donors. The presence of free antigen in the sera was confirmed by their ability to block BSI-A4- or BSI-B4-mediated agglutination of A+ or B+ red blood cells (not shown). All PBMCs were positive for the H antigen as determined by Ulex lectin staining (Figure 3). In addition, PBMCs cultured in A serum, but not B serum, displayed immunoreactivity using a monoclonal anti-A antibody. Conversely, culture in B serum, but not A serum, conferred staining using the B-selective lectin BSI-B4 compared with the background on PBMCs cultured in O serum. This confirmed that ABO antigens could be passively acquired by PBMCs from serum3-5 and, thus, possibly by virions derived under these culture conditions.
Serum sensitivity of HIV-1 vectors encoding eGFP and pseudotyped with the HIV-1 envelope HXB2 produced from cells cotransfected with ABO expression vectors. Vectors were harvested in serum-free medium and titer was determined. An MOI of 0.3 was incubated with 10% or 1% active sera from blood-typed donors for 1 hour at 37°C and plated onto NP2/CD4/X4 target cells. The number of GFP-expressing cells was determined by FACS 48 hours later and is shown as the percentage of infected cells compared with a matched heat-inactivated control. ▪ indicates type O serum; □, type A serum, and ▦, type B serum. Error bars indicate ± SEM.
Serum sensitivity of HIV-1 vectors encoding eGFP and pseudotyped with the HIV-1 envelope HXB2 produced from cells cotransfected with ABO expression vectors. Vectors were harvested in serum-free medium and titer was determined. An MOI of 0.3 was incubated with 10% or 1% active sera from blood-typed donors for 1 hour at 37°C and plated onto NP2/CD4/X4 target cells. The number of GFP-expressing cells was determined by FACS 48 hours later and is shown as the percentage of infected cells compared with a matched heat-inactivated control. ▪ indicates type O serum; □, type A serum, and ▦, type B serum. Error bars indicate ± SEM.
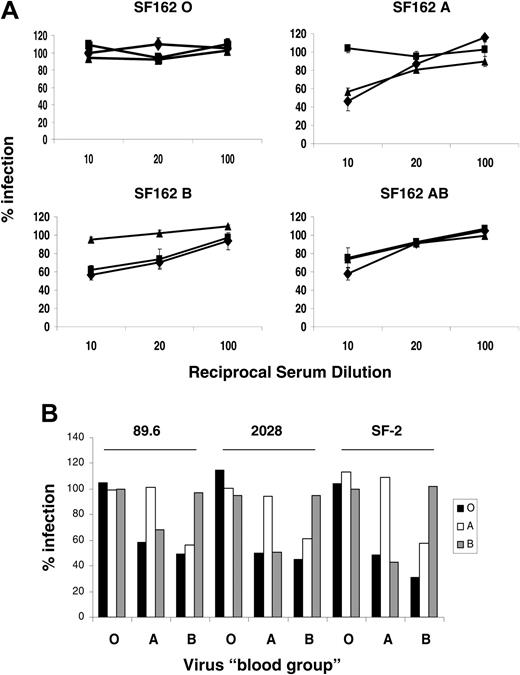
The R5-using isolate SF162, the dual-tropic R5 × 4 isolates 2028 and 89.6, and the X4-using laboratory strain SF-2 of HIV-1 were then propagated in PBMCs cultured in autologous serum, and fresh serum was supplemented every 2 days. We assessed 200 FFU of each virus for serum sensitivity in ABO sera as in Figure 2. A similar pattern of serum sensitivity was observed for all the isolates (Figure 4A-B). Viruses propagated in cells from an A donor were sensitive in O and B sera, whereas virus from a B donor was inactivated by O and A sera. As expected, virus from O donors was insensitive to all sera, whereas virus from AB donors was sensitive in all test sera. We saw no ABO-dependent virus inactivation by heat-inactivated sera (not shown), implying that all the inactivation was complement dependent. Therefore, HIV-1 derived from donor PBMCs, a primary cell target of the virus, acquired the “blood group” of the donor.
Anti-ABO antibodies are required for viral serum sensitivity
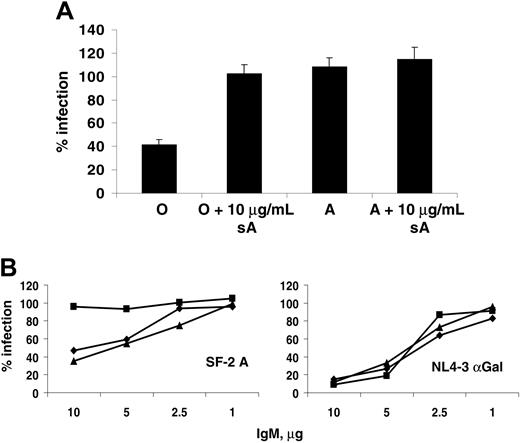
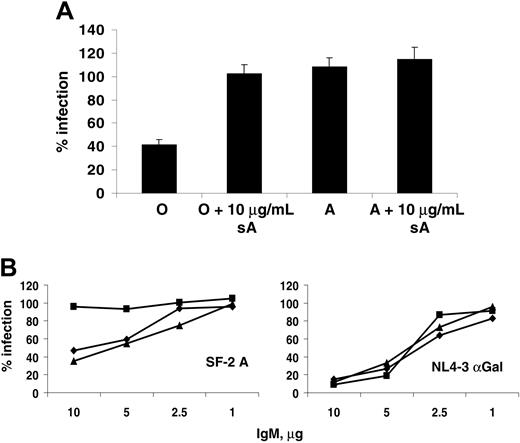
To demonstrate that the serum sensitivity observed was anti-ABO antibody dependent, the assays were repeated with soluble A-trisaccharide as a competitive inhibitor. HIV-1 SF-2 derived from an A donor was sensitive in O serum but not in A, as expected. However, addition of excess soluble A abrogated this serum sensitivity in O serum without any effect in A serum (Figure 5A). This finding demonstrated that an A binding factor in O serum was responsible for the viral inactivation by complement.
Anti-ABO and anti-αGal antibodies found in sera are mainly IgM and IgG, with IgM being the most efficient at fixing complement. Total IgM was purified from donor serum and diluted to 10 μg/mL, 5 μg/mL, 1 μg/mL, and 0.1 μg/mL and added to SF2-A in the presence of active or inactive guinea pig complement (Figure 5B). While none of the IgMs neutralized virus in the presence of inactive complement (not shown), IgM from O and B donors, but not A, inactivated up to 60% of SF2-A when mixed with active complement, and this effect titrated out with IgM concentration. By contrast, virus derived from αGalT-transfected cells was sensitive to IgM from all sera when mixed with complement, and this inactivation was stronger, a reflection of the levels of anti-αGal IgM in human serum. These results showed that both anti-carbohydrate antibody and complement were required for the observed viral inactivation, and thus incorporation of ABO antigens by viral particles sensitizes them to serum inactivation analogous to transfusion incompatibility.
Passive acquisition of ABO antigens from human serum by PBMCs. PBMCs isolated from O, A, or B donors were cultured in 10% human serum from individuals of blood group A or B. The cells were stained for blood group antigen expression using the H-specific lectin (Ulex), a blood group A-specific monoclonal antibody, or the B-selective lectin BSI-B4 (open curves) compared with negative controls (shaded curves), no lectin (H antigen), isotype antibody (anti-A), or the same cells cultured in O serum (B lectin). Both lectins were FITC-conjugated, and a secondary anti-mouse FITC was used for the anti-A staining.
Passive acquisition of ABO antigens from human serum by PBMCs. PBMCs isolated from O, A, or B donors were cultured in 10% human serum from individuals of blood group A or B. The cells were stained for blood group antigen expression using the H-specific lectin (Ulex), a blood group A-specific monoclonal antibody, or the B-selective lectin BSI-B4 (open curves) compared with negative controls (shaded curves), no lectin (H antigen), isotype antibody (anti-A), or the same cells cultured in O serum (B lectin). Both lectins were FITC-conjugated, and a secondary anti-mouse FITC was used for the anti-A staining.
Discussion
In this report we demonstrate that HIV-1 virions can incorporate ABO blood group antigens, both in an artificial transfection system and, more importantly, when primary strains are propagated in human PBMCs. In cells expressing the transferases that synthesize the antigen, progeny virions incorporated the blood group associated in part with the envelope protein gp120. In PBMCs, which do not usually express these enzymes, the antigens could be acquired passively from the serum. The presence of these antigens sensitizes the virus to the serum of ABO distinct individuals by the action of heat labile complement in conjunction with anti-AB antibodies. The sensitivity, while weak, resembles transfusion incompatibility and may have implications for the relative risk of transmission between individuals of different blood groups.
Serum sensitivity of HIV-1 propagated in PBMCs from ABO-defined donors. (A) Serum sensitivity of SF162 grown in PBMCs from ABO-secreting donors in the presence of autologous serum. Virus (200 FFU) was incubated for 1 hour at 37°C with varying concentrations of donor serum diluted against a constant background of matched heat-inactivated serum so that total serum concentration was 10%. The viruses were then plated onto target NP2CD4CCR5 cells, and virus infection was scored by in situ p24 staining 72 hours later. The results are expressed as the percentage of viral infection compared with the matched HI control. Diamonds (♦), squares (▪), and triangles (▴) represent viral infections in the presence of O, A, or B sera, respectively. Error bars represent ± SEM. (B) HIV-1 89.6, 2028, or SF-2 from ABO-defined PBMCs were treated as in panel A. Results represent the percentage infection of these viruses in 10% O (▪), A(□), and B (▦) serum compared with a matched HI control.
Serum sensitivity of HIV-1 propagated in PBMCs from ABO-defined donors. (A) Serum sensitivity of SF162 grown in PBMCs from ABO-secreting donors in the presence of autologous serum. Virus (200 FFU) was incubated for 1 hour at 37°C with varying concentrations of donor serum diluted against a constant background of matched heat-inactivated serum so that total serum concentration was 10%. The viruses were then plated onto target NP2CD4CCR5 cells, and virus infection was scored by in situ p24 staining 72 hours later. The results are expressed as the percentage of viral infection compared with the matched HI control. Diamonds (♦), squares (▪), and triangles (▴) represent viral infections in the presence of O, A, or B sera, respectively. Error bars represent ± SEM. (B) HIV-1 89.6, 2028, or SF-2 from ABO-defined PBMCs were treated as in panel A. Results represent the percentage infection of these viruses in 10% O (▪), A(□), and B (▦) serum compared with a matched HI control.
Arendrup et al21 demonstrated neutralization of HIV-1 from an A donor with a mouse monoclonal anti-A. With the monoclonals available to us, however, we failed to detect any neutralization in our system (not shown), and, furthermore, heat-inactivated serum failed to neutralize the viruses in our assays. Our data are consistent with a recent report showing that measles virus raised in HeLa cells, transfected with A or B transferases, was serum sensitive in an ABO-dependent manner.22 The level of inactivation (around 40%-50%) was similar in this study.
Importantly, we have extended our studies to show that HIV-1 grown in primary lymphocytes supplemented with autologous serum acquire ABO antigens. While lymphocytes do not normally express ABO transferases, they can acquire the antigens passively from serum.3-5 Consistent with this, viruses derived from PBMCs grown in autologous sera displayed ABO sensitivity. Arendrup et al21 have also speculated that blood group expression may be induced during viral replication in T cells. We are currently investigating this possibility.
It is interesting to note that, while much research has been devoted to searching for genetic factors that confer resistance to HIV-1 transmission, the most notable being the MHC and CCR5 loci,33,34 little is known about the role that some of the most well-studied blood group polymorphisms may play in viral transmission. The sensitivity of cells expressing α(1-3)α-galactosyltransferase, and viruses derived from them, to human sera is a major barrier to xenotransplantation and zoonosis because up to 5% of total IgM reacts against this antigen.19 Similarly, anti-ABO responses are strong enough to lead to life-threatening hemolytic shock and transplant rejection. ABO antigens can be added to both N- and O-linked glycans. While many N-linked glycans of gp120 are highly mannosylated, the majority of the O-linked glycans have mature structures.15-17 The HIV envelope is known to contain cellular glycoproteins and glycolipids,18 which may also carry sugar antigens. It should be borne in mind that the incorporation of the complement regulatory proteins, CD55 and CD59, are known to help protect virions from complement attack,35 and the relative weakness of the serum sensitivity observed might reflect this.
Purified IgM confers ABO serum sensitivity to virions. (A) Inhibition of serum inactivation by excess soluble blood group antigens. SF-2 virus grown in PBMCs from an A donor was assayed for serum sensitivity in O and A sera as before, in the presence or absence of 10 μg soluble A trisaccharide. (B) IgM from ABO sera differentially sensitizes virus to guinea pig complement. SF-2 derived from an A donor was incubated with varying concentrations of IgM purified from ABO-defined donor sera in the presence of active guinea pig complement (C′) or a heat-inactivated control. Diamonds (♦), squares (▪), and triangles (▴) represent viral infections in the presence of IgM from O, A, or B sera, respectively. HIV-1 NL4-3 raised in 293T cells transfected with αGal(1-3)galactosyltransferase was treated similarly as a control for antibody-dependent serum sensitivity. Both viruses were plated on NP2CD4X4 cells and processed as before.
Purified IgM confers ABO serum sensitivity to virions. (A) Inhibition of serum inactivation by excess soluble blood group antigens. SF-2 virus grown in PBMCs from an A donor was assayed for serum sensitivity in O and A sera as before, in the presence or absence of 10 μg soluble A trisaccharide. (B) IgM from ABO sera differentially sensitizes virus to guinea pig complement. SF-2 derived from an A donor was incubated with varying concentrations of IgM purified from ABO-defined donor sera in the presence of active guinea pig complement (C′) or a heat-inactivated control. Diamonds (♦), squares (▪), and triangles (▴) represent viral infections in the presence of IgM from O, A, or B sera, respectively. HIV-1 NL4-3 raised in 293T cells transfected with αGal(1-3)galactosyltransferase was treated similarly as a control for antibody-dependent serum sensitivity. Both viruses were plated on NP2CD4X4 cells and processed as before.
The question these results raise is how relevant are “natural” immune responses to the transmission of HIV-1 between individuals? The reason for the evolution of the ABO blood group system is at present unknown. Higher primates and humans have acquired polymorphisms in highly conserved enzymes that lead to transfusion incompatibility by antibodies generated in response to molecular mimicry of sugar antigens by gut flora.36 The maintenance of these polymorphisms, and their variable frequencies in different populations, implies that a variety of selective pressures act on these genes. A current theory is that ABO polymorphisms are maintained by the actions of infectious agents.6 Variation in ABO transferases may be driven by parasites that use specific sugar moieties, highlighted by the discovery that this family of sugar antigens serve as attachment factors for Norwalk-like viruses9,10,37 and H pylori11,12 on the gastric mucosa.
We postulate an additional role for infectious agents to maintain these polymorphisms. Selective transfer of pathogens between individuals of different ABO blood groups may have to surmount a natural immune barrier to transmission.38 Pathogens that have a glycosylation pattern, or acquire one from their host, that resembles that given by one ABO group, will be at an inherent disadvantage when transmitted to individuals of a different blood group. This may act at the level of restricting pathogen entry to the host by direct neutralization/serum inactivation. In addition, opsonization of pathogens by natural antibodies and complement may induce more rapid phagocytosis by antigen-presenting cells and induction of adaptive immunity.23,39 Interestingly, in some studies of respiratory pathogens, most notably influenza, transmission between individuals varied with ABO blood group,40 as did mean antibody titers and duration of symptoms.41
Blood group secretors express ABO antigens on mucosal epithelia, including those of the male and female reproductive tracts, and these antigens are released into fluid secretions.2 Our results suggest that viral replication in these mucosal tissues could lead to either direct envelope glycosylation from an antigen-expressing infected cell or the acquisition of these antigens from the mucosal secretion by infected lymphocytes. Either way, this blood group phenotype could be carried by these virions during transfer to the next host. In this case the possession of a functional fut2 (Secretor transferase) gene will govern the ABO incorporation. Interestingly, secretor status has been shown to have an affect on HIV-1 transmission risk in a cohort of Senegalese sex workers,42 although ABO was not tested in this study.
Opsonization by natural antibodies may also lead to antibody- and complement-mediated enhanced infection of phagocytes.43 Since phagocytic macrophages and dendritic cells are HIV-1 targets and express antibody and complement receptors, opsonization of virions by anti-A or -B antibodies may enhance infection of these cells. In this case, the potential effect of ABO incompatibility would be detrimental to the host. This possibility requires in vitro and epidemiologic investigations.
Should ABO polymorphisms play a protective role, one might expect to see underrepresentation of a particular blood group among infected patients. For example, individuals of blood group O may be less likely to acquire HIV from an A partner due to circulating anti-A. Thus, small effects, such as antibody/complement reactions against discordant ABO antigens on incoming virions, may allow more efficient control of primary viral infections. This may also be apparent in parenteral infections, whereby virus is injected directly into the blood stream. Protection from infection solely by this route seems unlikely. Where ABO has been speculated to influence viral infections, the difference in representations has been low, but the mean duration of symptoms has differed. This raises another scenario. It is now well established that effective control of primary HIV-1 replication in the host determines the viral load set point and the time to disease progression.44 That this is mainly due to the action of cytotoxic T cells may imply that the faster the adaptive immune response recognizes the virus in the context of inflammatory activators such as complement components, the more effective the control of primary replication. Innate immune responses play a determining role in the efficient induction of adaptive immunity.23,45 We are currently determining whether any ABO group is overrepresented among long-term nonprogressors or multiply exposed seronegative individuals.
Prepublished online as Blood First Edition Paper, February 22, 2005; DOI 10.1182/blood-2004-11-4267.
Supported by the Medical Research Council, United Kingdom.
The authors declare they have no competing financial interests.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Keith Aubin technical assistance and Elaine Thomas, Mary Collins, and Colin Porter for helpful advice and gifts of reagents.