Abstract
The importance of CD25+CD4+ regulatory T (Treg) cells in the control of immune responses is established, but their antigen specificity in vivo remains unclear. Understanding Treg-cell specificity requirements will be important if their potential is to be developed for immunotherapy. Pretreatment of recipient mice with donor alloantigen plus anti-CD4 antibody generates CD25+CD4+ Treg cells with the capacity to prevent skin allograft rejection in adoptive transfer recipients. Here we demonstrate that, although this regulation can be antigen-specific, reactivation with the original tolerizing alloantigen allows the Treg cells to suppress rejection of third-party allografts. Aware of the limitations of alloantigen pretreatment, we asked whether graft-protective Treg cells could be generated against unrelated, nongraft antigens. We demonstrate that bystander regulation also extends to CD25+CD4+ Treg cells generated in vivo by exposure to nominal antigens under anti-CD4 antibody cover. Providing these Treg cells are reexposed to the tolerizing antigens before adoptive transfer, they prevent the rejection of fully allogeneic skin grafts. That this might form the basis of a clinically relevant tolerance induction strategy is demonstrated by the fact that, when combined with subtherapeutic anti-CD8 antibody, Treg cells generated in response to nongraft antigens facilitate the acceptance of cardiac allografts in primary recipients. (Blood. 2005;105:4871-4877)
Introduction
The existence of lymphocytes with suppressive capacity was first described more than 30 years ago,1 but in recent years there has been renewed interest in the identification and characterization of regulatory T (Treg) cells that can regulate immune responses particularly with a view to harnessing their potential for immunotherapy. Several surface markers have been identified that can be used to enrich regulatory cells, one of which is CD25, the α subunit of the interleukin 2 (IL-2) receptor. CD25+CD4+ Treg cells with the capacity to regulate responses in vitro have been identified in both mice2-6 and humans.7-12 CD25+CD4+ Treg cells can suppress the proliferation and/or effector activity of both CD4+2,4 and CD8+3,5,13,14 cells, can prevent the development of autoimmune disease,15-17 and have been shown to play a role in tumor immunity18 and transplantation tolerance.13,19-23
We have previously shown that CBA mice pretreated with donor-specific transfusion (DST) under cover of either depleting24 or nondepleting25 anti-CD4 antibody accept fully allogeneic cardiac grafts indefinitely, and, as demonstrated in several other transplantation models,13,19,21,22 they contain CD25+CD4+ Treg cells with the capacity to suppress allograft rejection.20 However, we have recently shown that without further treatment, the nondepleting anti-CD4/DST protocol generates CD25+CD4+ Treg cells from CD25- precursors prior to transplantation that are able to prevent the rejection of donor-type skin allografts in a sensitive adoptive transfer model.26 Significantly, equivalent numbers of CD25+CD4+ cells from naive mice or from mice pretreated with either the anti-CD4-antibody-only or DST-only components of the combined therapy are unable to regulate under these conditions, demonstrating that these Treg cells arise as a consequence of the full pretreatment protocol.23,26 In common with naturally occurring CD25+CD4+ Treg cells,27-29 regulation by these alloantigen-induced cells in vivo is dependent on IL-10 and cytotoxic T lymphocyte-associated antigen 4 (CTLA-4).23
In vitro studies have demonstrated that, although Treg cells require activation via their T-cell receptors to regulate, once activated they can inhibit responses in an antigen-nonspecific or “bystander” manner.2,4,6 In this study we have attempted to exploit this bystander effect in allotransplantation and have developed a strategy in which Treg cells are driven and reactivated by soluble protein antigens completely unrelated to the eventual allograft. CD25+CD4+ T cells generated in this manner have the ability to prevent allograft rejection, suggesting that such an approach might form the basis of protocols with clinical potential.
Materials and methods
Mice
CBA.Ca (CBA, H2k), C57BL/10 (B10, H2b), BALB/c (BALB, H2d), and CBA-recombination-activating gene 1 knockout (CBA-Rag-/-, H2k; kindly provided by Dr D. Kioussis, Division of Molecular Immunology, National Institute for Medical Research, Mill Hill, London, United Kingdom) mice were obtained from and housed in the Biomedical Services Unit, John Radcliffe Hospital (Oxford, United Kingdom). Sex-matched mice between 6 and 12 weeks of age at the time of first experimental procedure were used in all experiments.
Reagents and monoclonal antibodies
The following monoclonal antibodies (mAbs) were used for cell purification, flow cytometry, and in vivo administration. The hybridoma TIB120 (anti-major histocompatibility complex [MHC] class II) was obtained from American Type Culture Collection, Manassas, VA; YTS169 (anti-CD8) and YTS177.9 (anti-CD4)30 were kindly provided by Professor H. Waldmann (Sir William Dunn School of Pathology, Oxford, United Kingdom). RM4-5 (anti-CD4)-cychrome, 16A (anti-CD45RB)-phycoerythrin, 7D4 (anti-CD25)-biotin, and streptavidin-phycoerythrin were purchased from Pharmingen (San Diego, California).
Human gamma globulin (HGG) was purchased from Sigma-Aldrich (St Louis, MO) and was heat aggregated at 63°C for 25 minutes and then incubated overnight on ice prior to use.
In vivo pretreatment protocol
Adult CBA mice received 200 μg anti-CD4 mAb YTS177 intravenously on days -28 and -27. On day -27 they also received 250 μL allogeneic (B10 or BALB) blood or 500 μg HGG intravenously. In some experiments a further dose of allogeneic blood or HGG was administered on day -1. On day 0, either spleens were harvested for isolation of CD25+CD4+ cells or 200 μg anti-CD8 mAb YTS169 was administered intravenously, and a B10 cardiac allograft was performed.
Cell purification
CD45RBhighCD4+ T cells were isolated from lymph nodes and spleens of naive CBA mice, and CD25+CD4+ T cells were obtained from spleens of animals pretreated with YTS177 and allogeneic blood or HGG. Populations were purified by negative selection using magnetic beads followed by fluorescence-activated cell sorting as previously described.23 On reanalysis, all populations were more than 95% pure.
Cell adoptive transfer and skin transplantation
CBA-Rag-/- mice were reconstituted intravenously with 105 CD45RBhighCD4+ cells with or without 2 × 105 CD25+CD4+ cells. The following day full-thickness B10 or BALB tail skin allografts were transplanted onto graft beds prepared on the flanks of the reconstituted mice. Allografts were monitored, and graft survival between groups was compared using the log-rank test31 using software developed and kindly provided by Dr S. Cobbold, Sir William Dunn School of Pathology.
Cardiac transplantation
Heterotopic cardiac allografts were performed as previously described.32 Graft function was followed by abdominal palpation and confirmed at postoperative day 100 by laparotomy. Allograft survival between groups was confirmed by using the log-rank test.
Serum anti-HGG antibody enzyme-linked immunosorbent assay (ELISA)
Plate-bound HGG was used to capture serum anti-HGG antibody, which was then quantified using horseradish peroxidase-conjugated rabbit anti-mouse immunoglobulin G (IgG) and IgM (Jackson ImmunoResearch Laboratories, West Grove, PA) followed by ABTS (2,2′-azino-bis(2-ethyl-benzathiazoline-6-sulfonic) acid).33 Absorbance at 405 nm was read, and results are presented as the mean of duplicate wells ± standard deviation (SD).
Image capture
Skin grafts were photographed 100 days after transplantation using a Kodak DCS 620 camera with a 105-mm focal-length lens (Eastman Kodak, Rochester, NY). Tissue sections were photographed using a Nikon CoolPix 5700 camera (Nikon, Tokyo, Japan) mounted on an Olympus BX40 microscope equipped with a 20 ×/0.5 objective lens (Olympus, Melville, NY). Images were processed with Adobe Photoshop 5.0 software (Adobe, San Jose, CA).
Results
Alloantigen-induced CD25+CD4+ cells regulate the rejection of donor-specific skin grafts
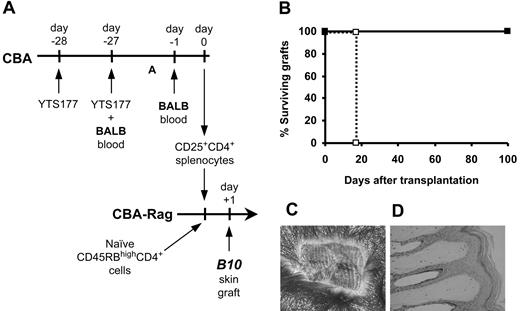
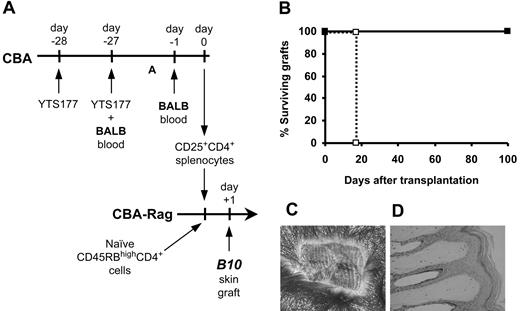
To determine the antigen specificity of CD25+CD4+ cells from pretreated mice, we isolated CD25+CD4+ cells from CBA (H2k) mice pretreated with the anti-CD4 antibody YTS177 and blood from B10 (H2b) mice and transferred them into syngeneic immunodeficient CBA-Rag-/- recipients together with CD45RBhighCD4+ cells from naive CBA donors as an effector population. One day later these recipients received transplants of B10 skin grafts (Figure 1). As we have previously shown,23 animals reconstituted with effector cells alone acutely rejected B10 skin allografts, but the cotransfer of CD25+CD4+ cells isolated from mice pretreated with YTS177 and donor-specific B10 blood prevented this rejection. In contrast, CD25+CD4+ cells isolated from mice pretreated with YTS177 and third-party BALB (H2d) blood had no such protective effect, with all B10 skin allografts being rejected acutely. We postulated that in this model regulation is either donor specific or that pretreatment with BALB blood in combination with anti-CD4 therapy is incapable of generating Treg cells. To distinguish between these 2 possibilities, we harvested CD25+CD4+ cells from mice pretreated with YTS177 and BALB blood and examined their ability to regulate the rejection of donor-specific BALB skin allografts mediated by CD45RBhighCD4+ effector cells in CBA-Rag-/- recipients. As with the B10 model, rejection of these BALB skin grafts was prevented completely with all grafts surviving for more than 100 days (Figure 1) and showing abundant hair growth and normal histology (not shown). The survival of these BALB skin grafts is significant in the context of this model for 2 reasons. First, it shows that the in vivo generation of Treg cells with the capacity to control rejection is not limited to a single donor-recipient strain combination, thereby ruling out a simple defect in generation as a trivial explanation for the inability of CD25+CD4+ cells from mice pretreated with YTS177 and BALB blood to protect B10 (H2b) skin grafts (Figure 1). Second, the data suggest that regulation is alloantigen specific, and it is the specificity of regulation that forms the basis of the remainder of this study.
When specifically rechallenged in vivo, alloantigen-induced CD25+CD4+ cells can regulate the rejection of third-party skin allografts
The generation of Treg cells using protocols that depend on pretreatment with donor-specific alloantigen will likely attract only limited clinical interest because of the difficulty of donor-specific pretreatment in most transplant recipients. However, work from other laboratories has shown that activation of CD25+CD4+ Treg cells by their cognate peptide-MHC ligand can enable them to suppress responses against other antigens in vitro.2,4,6 In at least one of these examples4 it was possible to rule out T-cell cross-reactivity as the mechanism, leaving bystander regulation as the most likely explanation. We, therefore, asked whether it might be possible to exploit deliberately this bystander mechanism in transplantation. Since the data in Figure 1 had shown that it was possible to generate CD25+CD4+ Treg cells in response to BALB alloantigen challenge under anti-CD4 antibody cover, we asked whether reexposure to a second BALB alloantigen challenge would then allow these CD25+CD4+ cells to regulate the rejection of third-party B10 skin allografts. CBA mice were pretreated with BALB blood under the cover of YTS177, and 1 day before purifying CD25+CD4+ cells for adoptive transfer these cell donors were given a second BALB blood transfusion with the objective of reactivating BALB-reactive CD25+CD4+ Treg cells generated by the pretreatment protocol (Figure 2A). Day -1 was chosen for the time of alloantigen rechallenge because in an anti-CD4/DST + DST reactivation protocol in primary heart graft recipients, reactivation of regulatory cells at day -1 was highly effective in protecting the graft from transplant-associated vasculopathy.34 CBA-Rag-/- mice were reconstituted with these CD25+CD4+ T cells together with CD4+CD45RBhigh cells as an effector population and received transplants of B10 (H2b) skin grafts 1 day later. In distinct contrast to the result described in Figure 1, where CD25+CD4+ cells from mice pretreated with YTS177 and BALB blood without reactivation were unable to regulate the rejection of B10 skin grafts administration of a second BALB blood transfusion to the pretreated mice the day before adoptive transfer allowed the transferred CD25+CD4+ T cells to prevent completely the rejection of the third-party B10 skin allografts, with all grafts surviving for more than 100 days (Figure 2B). These skin grafts had good hair growth (Figure 2C) and normal histology (Figure 2D) and were essentially indistinguishable from those B10 skin grafts protected by donor-specific Treg cells (Figure 1C-D), demonstrating the efficiency of this antigen nonspecific regulation.
Alloantigen-induced CD25+CD4+ cells can regulate skin allograft rejection. (A) Pretreatment and adoptive transfer protocol. CBA mice were pretreated with 200 μg YTS177 on days -28 and -27 plus 250 μL allogeneic (B10 or BALB) blood on day -27. On day 0, 2 × 105 CD25+CD4+ spleen cells from these donors were adoptively transferred into CBA-Rag-/- recipients together with 105 CD45RBhighCD4+ cells from naive CBA mice. One day later these reconstituted mice received transplants of B10 or BALB skin grafts. (B) Effect of CD25+CD4+ cells on CD45RBhighCD4+-mediated skin allograft rejection. Reconstitution with CD45RBhighCD4+ cells only (⋄), B10 graft (group i: median survival time [MST], 20 days, n = 4); CD45RBhighCD4+ cells only (□), BALB graft (group ii: MST, 12 days, n = 4); CD45RBhighCD4+ cells plus CD25+CD4+ cells from YTS177/B10 blood pretreated mice (♦), B10 graft (group iii: MST, > 100 days, n = 4; P < .05 versus group i); CD45RBhighCD4+ cells plus CD25+CD4+ cells from YTS177/BALB blood pretreated mice (▪), BALB graft (group iv: MST, > 100 days, n = 4; P < .05 versus group ii); CD45RBhighCD4+ cells plus CD25+CD4+ cells from YTS177/BALB blood pretreated mice (•), B10 graft (group v: MST, 25 days, n = 4; P < .05 versus groups iii and iv). (C) Representative B10 skin graft 100 days after transplantation from group iii, ♦. (D) Histology of graft in panel C (formalin-fixed paraffin section stained with hematoxylin and eosin [H&E]).
Alloantigen-induced CD25+CD4+ cells can regulate skin allograft rejection. (A) Pretreatment and adoptive transfer protocol. CBA mice were pretreated with 200 μg YTS177 on days -28 and -27 plus 250 μL allogeneic (B10 or BALB) blood on day -27. On day 0, 2 × 105 CD25+CD4+ spleen cells from these donors were adoptively transferred into CBA-Rag-/- recipients together with 105 CD45RBhighCD4+ cells from naive CBA mice. One day later these reconstituted mice received transplants of B10 or BALB skin grafts. (B) Effect of CD25+CD4+ cells on CD45RBhighCD4+-mediated skin allograft rejection. Reconstitution with CD45RBhighCD4+ cells only (⋄), B10 graft (group i: median survival time [MST], 20 days, n = 4); CD45RBhighCD4+ cells only (□), BALB graft (group ii: MST, 12 days, n = 4); CD45RBhighCD4+ cells plus CD25+CD4+ cells from YTS177/B10 blood pretreated mice (♦), B10 graft (group iii: MST, > 100 days, n = 4; P < .05 versus group i); CD45RBhighCD4+ cells plus CD25+CD4+ cells from YTS177/BALB blood pretreated mice (▪), BALB graft (group iv: MST, > 100 days, n = 4; P < .05 versus group ii); CD45RBhighCD4+ cells plus CD25+CD4+ cells from YTS177/BALB blood pretreated mice (•), B10 graft (group v: MST, 25 days, n = 4; P < .05 versus groups iii and iv). (C) Representative B10 skin graft 100 days after transplantation from group iii, ♦. (D) Histology of graft in panel C (formalin-fixed paraffin section stained with hematoxylin and eosin [H&E]).
Activated alloantigen-induced CD25+CD4+ cells can regulate third-party skin allograft rejection. (A) Pretreatment and adoptive transfer protocol. CBA-Rag-/- mice were reconstituted with 105 CD45RBhighCD4+ cells with or without 2 × 105 CD25+CD4+ cells from CBA mice pretreated with 200 μg YTS177 and 250 μL BALB blood followed by a further dose of BALB blood the day prior to cell isolation. The reconstituted mice then received a B10 skin graft the following day. (B) Effect of CD25+CD4+ cells on CD45RBhighCD4+-mediated rejection of B10 skin grafts. □ CD45RBhighCD4+ cells only (MST, 17 days, n = 4); ▪ CD45RBhighCD4+ plus CD25+CD4+ cells (MST, > 100 days, n = 4; P < .05). (C) Representative skin graft 100 days after transplantation onto mouse reconstituted with both CD45RBhigh CD4+ and CD25+CD4+ cells. (D) Histology of graft in panel C (H&E).
Activated alloantigen-induced CD25+CD4+ cells can regulate third-party skin allograft rejection. (A) Pretreatment and adoptive transfer protocol. CBA-Rag-/- mice were reconstituted with 105 CD45RBhighCD4+ cells with or without 2 × 105 CD25+CD4+ cells from CBA mice pretreated with 200 μg YTS177 and 250 μL BALB blood followed by a further dose of BALB blood the day prior to cell isolation. The reconstituted mice then received a B10 skin graft the following day. (B) Effect of CD25+CD4+ cells on CD45RBhighCD4+-mediated rejection of B10 skin grafts. □ CD45RBhighCD4+ cells only (MST, 17 days, n = 4); ▪ CD45RBhighCD4+ plus CD25+CD4+ cells (MST, > 100 days, n = 4; P < .05). (C) Representative skin graft 100 days after transplantation onto mouse reconstituted with both CD45RBhigh CD4+ and CD25+CD4+ cells. (D) Histology of graft in panel C (H&E).
CD25+CD4+ T cells tolerized to unrelated soluble-protein antigens in vivo can prevent the rejection of fully allogeneic skin grafts
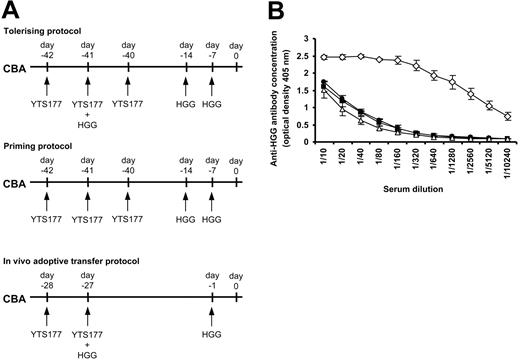
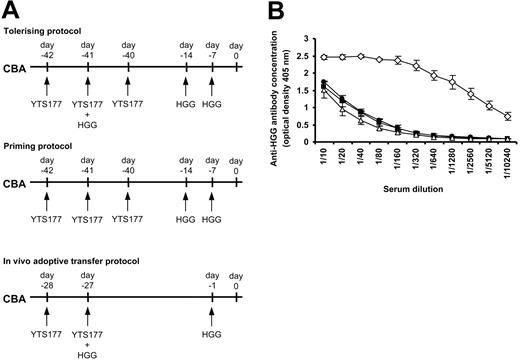
The demonstration that reactivation of Treg cells prior to adoptive transfer prevented the rejection of third-party grafts could have important implications for the design of tolerance induction strategies in humans by allowing more flexibility in the design of pretreatment strategies. However, concerns over the transmission of bloodborne pathogens may limit the utility of blood or other allogeneic cells as a source of tolerizing antigen. Generating Treg cells specific for a noncellular protein antigen or antigens could offer an alternative strategy if it could be shown that such cells can regulate responses to alloantigens in vivo. The ability of CD25+CD4+ T cells to mediate bystander regulation following alloantigen reexposure (Figure 2) led us to ask whether a similar approach might be successful using simple protein antigens. It has previously been demonstrated that tolerance to soluble antigens such as albumin35 or HGG30,36 can be achieved in mice by intravenous administration under the cover of anti-CD4 antibody. We, therefore, chose HGG as a candidate for the tolerizing antigen for these experiments. To determine whether tolerance to HGG could be replicated in our hands we administered HGG under the cover of the anti-CD4 antibody YTS177 based on a previously published protocol30 and then measured serum anti-HGG antibody concentrations by ELISA. Details of the priming and tolerizing protocols are shown in Figure 3A. Positive control mice that received a priming protocol (where HGG was given following but not coincident with YTS177) produced high levels of anti-HGG antibody, whereas mice given HGG under the cover of YTS177 had low antibody titers that were identical to those from naive mice, indicating tolerance to HGG. Having confirmed that tolerance to HGG could be induced, we then asked whether an HGG tolerizing plus reactivation protocol based on that using alloantigen shown in Figure 2 (HGG on day -27 under the cover of YTS177 plus HGG rechallenge on day -1) could also induce tolerance to HGG (Figure 3A, bottom). Importantly, mice pretreated according to this protocol also produced background levels of anti-HGG antibody and were, therefore, judged to be tolerant to HGG (Figure 3B).
Tolerance to HGG can be induced by administration under the cover of anti-CD4 therapy. (A) CBA mice were pretreated as follows and serum harvested at day 0 for ELISA analysis: tolerizing protocol, 200 μg YTS177 on days -42, -41, and -40 and 500 μg HGG on days -41, -14, and -7; priming protocol, 200 μg YTS177 on days -42, -41, and -40 and 500 μg HGG on days -14 and -7; YTS177/HGG + HGG reactivation protocol for in vivo adoptive transfer, 200 μg YTS177 on days -28 and -27 and 500 μg HGG on days -27 and -1. (B) Anti-HGG antibody titer at day 0. ▵ untreated; • tolerizing protocol; ⋄ priming protocol; ▪ in vivo adoptive transfer protocol; n = 2 in per group. Results are presented as mean ± SD.
Tolerance to HGG can be induced by administration under the cover of anti-CD4 therapy. (A) CBA mice were pretreated as follows and serum harvested at day 0 for ELISA analysis: tolerizing protocol, 200 μg YTS177 on days -42, -41, and -40 and 500 μg HGG on days -41, -14, and -7; priming protocol, 200 μg YTS177 on days -42, -41, and -40 and 500 μg HGG on days -14 and -7; YTS177/HGG + HGG reactivation protocol for in vivo adoptive transfer, 200 μg YTS177 on days -28 and -27 and 500 μg HGG on days -27 and -1. (B) Anti-HGG antibody titer at day 0. ▵ untreated; • tolerizing protocol; ⋄ priming protocol; ▪ in vivo adoptive transfer protocol; n = 2 in per group. Results are presented as mean ± SD.
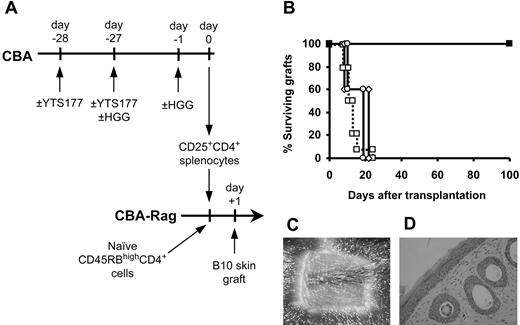
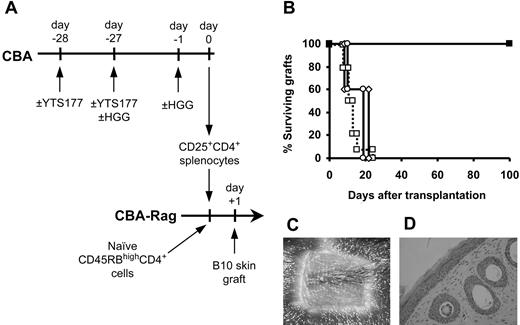
Having established that tolerance to HGG can be induced using this protocol, we asked whether CD25+CD4+ cells generated by pretreatment with HGG under the cover of YTS177 and specifically reactivated with HGG could regulate the rejection of B10 skin allografts (Figure 4A). Animals reconstituted with CD45RBhighCD4+ cells alone all rejected their grafts acutely, but, in contrast, all mice that received cotransfer of CD25+CD4+ cells from animals pretreated with YTS177 and HGG then given a second HGG challenge the day prior to cell isolation (to reactivate the regulatory population) accepted their B10 skin allografts for more than 100 days (Figure 4B). As determined by both macroscopic (Figure 4C) and histologic appearance (Figure 4D), these skin grafts were indistinguishable from those protected by Treg cells driven by donor-specific alloantigen (Figure 1C-D). As important controls, we tested the regulatory capacity of CD25+CD4+ cells from animals pretreated with YTS177 and HGG but without the second (reactivating) dose of HGG and from animals pretreated with HGG in the absence of YTS177 followed by a second dose of HGG prior to cell isolation. In both these groups all B10 skin allografts were rejected acutely.
Activated CD25+CD4+ cells generated against the unrelated antigen HGG can regulate skin allograft rejection. (A) CBA mice were pretreated with the YTS177/HGG + HGG reactivation protocol or with relevant control protocols. At day 0, 2 × 105 CD25+CD4+ T cells from these donors were cotransferred with 105 naive CD45RBhighCD4+ cells into CBA-Rag-/- recipients. These reconstituted mice then received transplants of B10 skin allografts 1 day later. (B) Skin allograft survival. □ CD45RBhighCD4+ cells only (group i: MST, 11.5 days, n = 14); ▪ CD45RBhighCD4+ cells + CD25+CD4+ cells from mice pretreated with 200 μg YTS177 on days -28 and -27 and with 500 μg HGG on days -27 and -1 (group ii: MST, > 100 days, n = 5; P < .05 versus group i); ○ CD45RBhighCD4+ cells + CD25+CD4+ cells from mice pretreated with 500 μg HGG only on days -27 and -1 (group iii: MST, 20 days, n = 5; P = .21 versus group i); ⋄ CD45RBhighCD4+ cells + CD25+CD4+ cells from mice pretreated with 200 μg YTS177 only on days -28 and -27 and 500 μg HGG on day -27 (group iv: MST, 21 days, n = 5; P = .21 versus group i). (C) Representative skin graft from group ii 100 days after transplantation. (D) Histology of graft in panel C (H&E).
Activated CD25+CD4+ cells generated against the unrelated antigen HGG can regulate skin allograft rejection. (A) CBA mice were pretreated with the YTS177/HGG + HGG reactivation protocol or with relevant control protocols. At day 0, 2 × 105 CD25+CD4+ T cells from these donors were cotransferred with 105 naive CD45RBhighCD4+ cells into CBA-Rag-/- recipients. These reconstituted mice then received transplants of B10 skin allografts 1 day later. (B) Skin allograft survival. □ CD45RBhighCD4+ cells only (group i: MST, 11.5 days, n = 14); ▪ CD45RBhighCD4+ cells + CD25+CD4+ cells from mice pretreated with 200 μg YTS177 on days -28 and -27 and with 500 μg HGG on days -27 and -1 (group ii: MST, > 100 days, n = 5; P < .05 versus group i); ○ CD45RBhighCD4+ cells + CD25+CD4+ cells from mice pretreated with 500 μg HGG only on days -27 and -1 (group iii: MST, 20 days, n = 5; P = .21 versus group i); ⋄ CD45RBhighCD4+ cells + CD25+CD4+ cells from mice pretreated with 200 μg YTS177 only on days -28 and -27 and 500 μg HGG on day -27 (group iv: MST, 21 days, n = 5; P = .21 versus group i). (C) Representative skin graft from group ii 100 days after transplantation. (D) Histology of graft in panel C (H&E).
Bystander regulation can facilitate the acceptance of primary cardiac allografts
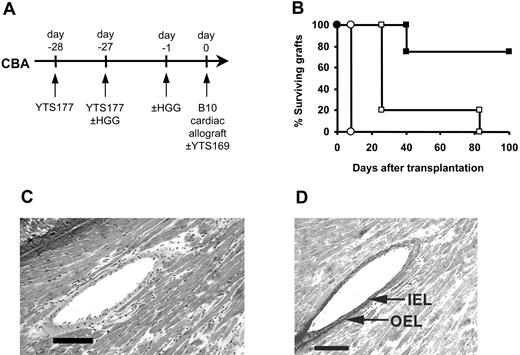
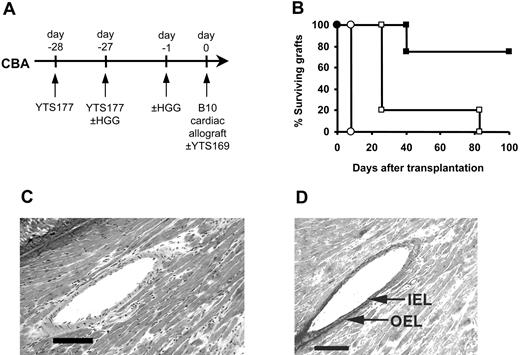
The data presented in Figure 4 clearly demonstrate that CD25+CD4+ T cells generated and reactivated by exposure to nominal antigens have the capacity to prevent the rejection of fully allogeneic grafts in an adoptive transfer system. However, these data were obtained in immunodeficient recipients whereby rejection is mediated by the adoptive transfer of limited numbers of CD4+ effector T cells. To determine whether bystander regulation of allograft rejection is effective in a more clinically applicable model, we asked whether Treg-cell generation and reactivation would allow the survival of fully allogeneic grafts in primary recipients with an intact immune system. Since the anti-CD4/HGG protocol was developed from an anti-CD4/donor-specific blood transfusion protocol which allows the long-term survival of fully mismatched B10 cardiac allografts without additional therapy,25 we used the same heart model to determine the comparative efficacy of the YTS177/HGG + HGG reactivation regimen. CBA mice were pretreated with HGG under the cover of YTS177 on days -28 and -27, reexposed to HGG on day -1, and received transplants of B10 hearts on day 0, but disappointingly these hearts were rejected at control rates (MST, 8 days; Figure 5). We speculated that, although regulation might be occurring to some extent in these recipients, the precursor frequency of the self-restricted HGG-specific Treg cells might be too low to have a significant impact on the alloreactive T-cell population, expected to be far greater in size.37 We have previously shown that in the absence of anti-CD4 antibody Treg cells can also be generated in CBA recipients by successive multiple donor-specific transfusions, while a single DST is not sufficient.38 This incremental effect suggested that multiple challenges with HGG combined with the day -1 reactivating dose might increase the efficacy of the basic YTS177/HGG protocol. Therefore, CBA mice were pretreated with HGG under the cover of YTS177 on days -28 and -27 and rechallenged with HGG on day -1, but they also received additional doses of HGG on days -21, -14, and -7. These mice then received transplants of B10 hearts on day 0. However, these mice also rejected their grafts at control rates (data not shown).
Induction of tolerance to HGG followed by HGG readministration can facilitate the acceptance of primary cardiac allografts. (A) CBA mice were pretreated with the YTS177/HGG + HGG reactivation protocol or with YTS177 only. These mice then received transplants of B10 cardiac allografts on day 0 with or without adjunctive anti-CD8 antibody therapy. (B) Cardiac allograft survival. ○ YTS177/HGG + HGG reactivation protocol (200 μg YTS177 on days -28 and -27 and 500 μg HGG on days -27 and -1; group i: MST, 8 days, n = 5); □200 μg YTS177 on days -28 and -27 and 200 μg YTS169 on day 0 (group ii: MST, 26 days, n = 5; P < .05 versus group i); ▪ YTS177/HGG + HGG reactivation protocol plus anti-CD8 antibody day 0 (200 μg YTS177 on days -28 and -27, 500 μg HGG on days -27 and -1, and 200 μg YTS169 on day 0; group iii: MST, > 100 days, n = 4; P < .05 versus groups i and ii). (C) Histology of long-term surviving graft from group iii, ▪ (H&E). Scale bar = 100 μm. (D) Long-term surviving graft from group iii, ▪, stained with Elastin van Giessen stain to demonstrate internal elastic lamina (IEL) and outer elastic lamina (OEL). Scale bar = 100 μm.
Induction of tolerance to HGG followed by HGG readministration can facilitate the acceptance of primary cardiac allografts. (A) CBA mice were pretreated with the YTS177/HGG + HGG reactivation protocol or with YTS177 only. These mice then received transplants of B10 cardiac allografts on day 0 with or without adjunctive anti-CD8 antibody therapy. (B) Cardiac allograft survival. ○ YTS177/HGG + HGG reactivation protocol (200 μg YTS177 on days -28 and -27 and 500 μg HGG on days -27 and -1; group i: MST, 8 days, n = 5); □200 μg YTS177 on days -28 and -27 and 200 μg YTS169 on day 0 (group ii: MST, 26 days, n = 5; P < .05 versus group i); ▪ YTS177/HGG + HGG reactivation protocol plus anti-CD8 antibody day 0 (200 μg YTS177 on days -28 and -27, 500 μg HGG on days -27 and -1, and 200 μg YTS169 on day 0; group iii: MST, > 100 days, n = 4; P < .05 versus groups i and ii). (C) Histology of long-term surviving graft from group iii, ▪ (H&E). Scale bar = 100 μm. (D) Long-term surviving graft from group iii, ▪, stained with Elastin van Giessen stain to demonstrate internal elastic lamina (IEL) and outer elastic lamina (OEL). Scale bar = 100 μm.
In an attempt to identify adjunctive therapies that might enable Treg cells generated by the YTS177/HGG protocol to influence the outcome of primary heart transplantations we considered 3 separate strategies. The first was to block CD154 using the anti-CD154 antibody MR1,39 which as a monotherapy has been shown to be partially effective in preventing cardiac allograft rejection.40 The second was to use a short course of sirolimus (rapamycin) on the basis that this immunosuppressive agent, unlike calcineurin inhibitors, appears not to inhibit the function of Treg cells.41,42 The third approach was to target CD8+ T cells on the grounds that, in several experimental models, CD8+ T cells have been shown capable of mediating allograft rejection independently of CD4+ help.43-45 The additional possibility of using anti-CD4 antibody as adjunctive therapy was rejected on the theoretical basis that this would almost certainly target the CD25+CD4+ Treg cell population whose regulatory potential we were seeking to augment.
In the anti-CD154 experiments, CBA mice were pretreated with the YTS177/HGG + HGG rechallenge protocol, but in addition they received a subtherapeutic course of the anti-CD154 antibody MR1 (2 mg/kg intraperitoneally on days -27, -25, -23, and -21). These animals then received transplants of B10 cardiac allografts at day 0. The anti-CD154 antibody was administered in the pretreatment rather than the peritransplantation period to avoid the possibility of subjecting the Treg cell population to CD154 blockade. In the rapamycin experiments, CBA mice were pretreated with the YTS177/HGG + HGG rechallenge protocol, received transplants of B10 hearts at day 0, and were then given either of 2 subtherapeutic courses of sirolimus (200 μg/kg/dose intraperitoneally on days +1, +3, +5, and +7 or 200 μg/kg/dose intraperitoneally on days -1, 0, +1, +3, +5, +7, +9, +11, and +13). We were disappointed to find, however, that neither the YTS177/HGG + anti-CD154 nor the YTS177/HGG + rapamycin protocol had any effect on graft outcome compared with relevant controls which received the same therapy in the absence of HGG challenge (data not shown).
In light of the observation that in several transplantation models independent targeting of CD8+ T cells can convert ineffective into effective therapeutic protocols,43-45 we asked whether targeting CD8+ T cells with a suboptimal dose of anti-CD8 antibody would have a similar beneficial effect in the YTS177/HGG + HGG rechallenge protocol. CBA mice were pretreated with YTS 177 on days -28 and -27 with or without HGG at day -27 and day -1, received transplants of B10 cardiac allografts on day 0, and were given a single dose (8 mg/kg on day 0) of the depleting anti-CD8 antibody YTS169. As shown in Figure 5, while addition of this single dose of anti-CD8 antibody to control mice pretreated with anti-CD4 antibody in the absence of HGG had little impact on graft outcome (MST 26 days), adding the anti-CD8 antibody to the YTS177/HGG + HGG rechallenge protocol led to indefinite graft survival in 3 of 4 recipients (MST, > 100 days; Figure 5B), with these long-term surviving hearts showing neither distortion of myocardial architecture (Figure 5C) nor significant intimal proliferation (Figure 5D). Thus, Treg cells generated against nongraft antigens and then reactivated prior to transplantation can, in the presence of subtherapeutic anti-CD8 antibody, prevent allograft rejection in primary recipients with an intact immune system, demonstrating that under appropriate conditions it is possible to exploit the potential of bystander regulation in primary allograft recipients.
Discussion
In recent years, much progress has been made in defining the phenotypic and functional properties of CD25+CD4+ Treg cells. One characteristic that has been demonstrated in vitro by several groups is that these Treg cells require activation via their T-cell receptors to exert regulatory activity, but that, once activated, they are able to regulate in an antigen nonspecific manner.2,4,6 The data presented here are, to our knowledge, the first to demonstrate that, following their generation in response to either alloantigen or nominal antigen given under the cover of anti-CD4 antibody, reexposure of these CD25+CD4+ Treg cells to the original tolerizing antigen allows them to regulate allograft rejection in vivo in a manner that is antigen nonspecific.
The phenomenon of regulation in the specific setting of transplantation is attracting renewed interest because active, self-sustaining regulation of rejection responses may be a route to drug-independent long-term graft survival. A major limiting factor in the clinical use of protocols involving pretreatment of patients with antigen prior to transplantation is that, except for live donor transplantation, neither the timing of the procedure nor the identity of the donor is known in advance. The observation that CD25+CD4+ cells from mice pretreated with YTS177 and unrelated blood can prevent the rejection of third-party skin allografts (Figure 2) demonstrates that regulation can be achieved by the administration of antigens that are not necessarily expressed by the graft itself. The fact that regulation was only observed following rechallenge of the CD25+CD4+ cell donors with the tolerizing antigen is entirely consistent with the in vitro observations of others that naturally occurring CD25+CD4+ Treg cells have the capacity for bystander regulation.2,4,6
The potential for the clinical transmission of infectious agents by administration of human blood or blood products led us to consider alternative antigens for the generation and reactivation of Treg cells. The fact that Treg cells generated by administration of a noncellular antigen such as HGG can prevent the rejection of skin allografts in an adoptive transfer model (Figure 4) and can influence the survival of heart allografts in immunocompetent recipients (Figure 5) appears to support a proof of principle. Clinical protocols could be explored in which patients awaiting transplantation are given a well-defined, quality-controlled noncellular antigen or antigens combined with transient immunotherapy to generate Treg cells. These regulatory cells would be maintained by routine antigen rechallenge36 then reactivated by the same tolerizing antigens immediately prior to transplantation. When combined with a suitable adjunctive therapy such a strategy may facilitate graft acceptance and reduce the need for long-term maintenance immunosuppression. An additional approach being investigated in this laboratory is the generation of CD8+ Treg cells. The ability of CD8+ T cells to undergo priming to exogenous antigen46,47 suggests that, in principle at least, it may be possible to generate CD8+ Treg cells by using an anti-CD8/HGG + HGG reactivation protocol. If, as suggested in Figure 5, targeting the CD8 arm of the response is essential in immunocompetent recipients, then generating Treg cells of both subsets in a combined antibody protocol may be a way of achieving long-term graft survival in primary allograft recipients without additional therapy.
Our observations in this model not only suggest potential strategies for immunotherapy but also shed some light on the mechanisms involved in regulation in vivo. In the situation whereby an allograft expresses the same alloantigens as those in the tolerizing protocol (eg, in the anti-CD4/DST protocol without a DST antigen rechallenge, Figure 1),23 it seems likely that Treg cells will be capable of responding to alloantigens expressed on the graft itself or on resident donor antigen-presenting cells encountered in the draining lymph nodes. This alloantigen-specific reactivation could then lead to the attenuation of alloaggressive responses. However, in the model whereby Treg cells driven by alloantigen under anti-CD4 cover and reactivated by reexposure to the same alloantigens have the ability to regulate the rejection of third-party allografts (Figure 2), both cross-reactivity and bystander regulation seem possible. However, of the 2 possibilities, cross-reactivity seems the less likely in the day -1 alloantigen reactivation protocol because without antigen reexposure no regulation occurs. While it is possible to suggest how reactivation could result in bystander regulation (nonspecific secretion of IL-10 perhaps), it is more difficult to explain why antigen reexposure 1 day before transplantation might enhance cross-reactivity. Finally, in the anti-CD4/HGG + HGG reactivation protocol, CD4+ Treg cells driven by soluble protein antigens will almost certainly have responded to processed peptides presented by self-MHC class II via the indirect pathway. Cross-reactivity between these indirect CD4+ Treg cells and intact alloantigens expressed by the graft or donor antigen-presenting cells via the direct pathway seem extremely unlikely. We propose that these reactivated cells regulate by a bystander mechanism mediated possibly by cytokines, such as IL-10, secreted in a restricted local microenvironment.
The duration of graft protection offered by these non-graft-specific Treg cells is likely to be fairly short-lived since these cells are unlikely to remain activated for a significant length of time in the absence of specific antigen stimulation.48,49 However, it is probable that an initial period of graft protection mediated by bystander regulation could lead to operational tolerance in the alloreactive T-cell population by a variety of established mechanisms such as infectious tolerance, IL-10-mediated immunosuppression, competition for costimulatory ligands, or down-regulation of antigen-presenting cell function.50-53
One potential concern over Treg cells that mediate bystander regulation is the impact that these cells might have on protective immune responses to pathogens. However, in a model in which bystander regulation has been deliberately induced using a protocol analogous to that shown in Figures 2 and 4, we have recently shown that protective responses to influenza virus are undiminished when compared with those in normal unmanipulated mice.34 Clearly this is encouraging, but this conclusion has to be verified with other potential pathogens. This is currently under investigation in our laboratory.
Overall, the data obtained in this study suggest that the ability to generate Treg cell populations by controlled exposure to defined antigens may have important implications for organ transplantation, for bone marrow transplantation, and for autoimmune disease, whereby attenuation of immune responses remains an important goal.
Prepublished online as Blood First Edition Paper, February 15, 2005; DOI 10.1182/blood-2004-10-3888.
Supported by The Wellcome Trust and The Roche Organ Transplantation Research Foundation. M.K. is a Wellcome Trust Research Fellow. K.J.W. holds a Royal Society Wolfson Research Merit Award.
The approach described in this manuscript is subject to patent protection and the intellectual property rights are being commercialized by the University of Oxford.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 1. Alloantigen-induced CD25+CD4+ cells can regulate skin allograft rejection. (A) Pretreatment and adoptive transfer protocol. CBA mice were pretreated with 200 μg YTS177 on days -28 and -27 plus 250 μL allogeneic (B10 or BALB) blood on day -27. On day 0, 2 × 105 CD25+CD4+ spleen cells from these donors were adoptively transferred into CBA-Rag-/- recipients together with 105 CD45RBhighCD4+ cells from naive CBA mice. One day later these reconstituted mice received transplants of B10 or BALB skin grafts. (B) Effect of CD25+CD4+ cells on CD45RBhighCD4+-mediated skin allograft rejection. Reconstitution with CD45RBhighCD4+ cells only (⋄), B10 graft (group i: median survival time [MST], 20 days, n = 4); CD45RBhighCD4+ cells only (□), BALB graft (group ii: MST, 12 days, n = 4); CD45RBhighCD4+ cells plus CD25+CD4+ cells from YTS177/B10 blood pretreated mice (♦), B10 graft (group iii: MST, > 100 days, n = 4; P < .05 versus group i); CD45RBhighCD4+ cells plus CD25+CD4+ cells from YTS177/BALB blood pretreated mice (▪), BALB graft (group iv: MST, > 100 days, n = 4; P < .05 versus group ii); CD45RBhighCD4+ cells plus CD25+CD4+ cells from YTS177/BALB blood pretreated mice (•), B10 graft (group v: MST, 25 days, n = 4; P < .05 versus groups iii and iv). (C) Representative B10 skin graft 100 days after transplantation from group iii, ♦. (D) Histology of graft in panel C (formalin-fixed paraffin section stained with hematoxylin and eosin [H&E]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/12/10.1182_blood-2004-10-3888/5/m_zh80120579650001.jpeg?Expires=1763696724&Signature=syOczC-lDWavPNHg3VYxREHamtyUhhmkBrGS-GIdMD3pH3Wc22Inx8hC6pGJPu7AuGoObGO3vu9H3Le1~6uDhL32taOM2zHPWlfR6LpfW0k3e7G9Deh~2OQ2UOKX6bUArlD-PE6nG7ZdGSWMHm5p~cpi5cX0rvkjIF7gcrjhpHcp~pF5ezn~V8tQNGX5nOKzrVw3fkd9TpYBzFplZ6ED7E4HSRhPJUrF54r3By2cnxB8pyfoD50Uhci~iIg89w7FT2vqSVsH2gBjWDCTKVl8ryC3NM3M1QjvLDm37pYmaHDy5hYEGlYGnQhrVog31l703aC2-mpkuPsVZm22onrf2w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 1. Alloantigen-induced CD25+CD4+ cells can regulate skin allograft rejection. (A) Pretreatment and adoptive transfer protocol. CBA mice were pretreated with 200 μg YTS177 on days -28 and -27 plus 250 μL allogeneic (B10 or BALB) blood on day -27. On day 0, 2 × 105 CD25+CD4+ spleen cells from these donors were adoptively transferred into CBA-Rag-/- recipients together with 105 CD45RBhighCD4+ cells from naive CBA mice. One day later these reconstituted mice received transplants of B10 or BALB skin grafts. (B) Effect of CD25+CD4+ cells on CD45RBhighCD4+-mediated skin allograft rejection. Reconstitution with CD45RBhighCD4+ cells only (⋄), B10 graft (group i: median survival time [MST], 20 days, n = 4); CD45RBhighCD4+ cells only (□), BALB graft (group ii: MST, 12 days, n = 4); CD45RBhighCD4+ cells plus CD25+CD4+ cells from YTS177/B10 blood pretreated mice (♦), B10 graft (group iii: MST, > 100 days, n = 4; P < .05 versus group i); CD45RBhighCD4+ cells plus CD25+CD4+ cells from YTS177/BALB blood pretreated mice (▪), BALB graft (group iv: MST, > 100 days, n = 4; P < .05 versus group ii); CD45RBhighCD4+ cells plus CD25+CD4+ cells from YTS177/BALB blood pretreated mice (•), B10 graft (group v: MST, 25 days, n = 4; P < .05 versus groups iii and iv). (C) Representative B10 skin graft 100 days after transplantation from group iii, ♦. (D) Histology of graft in panel C (formalin-fixed paraffin section stained with hematoxylin and eosin [H&E]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/12/10.1182_blood-2004-10-3888/5/m_zh80120579650001.jpeg?Expires=1763696725&Signature=xoCl0a67EU6oiNImXbPAEZBZxRpzzETimCJNzRND9HWf6C81Wp9lMRo0hKvmWpgwrx6wvBJ~x~HDPoLB8zJmQAOk~R2mBbTjhcN92n0kidBw1YsNINWy~T18VE~PrlWYweiCWwf5dquQbhM~PE1QsPLXX5g7TN4mFB0LrO3DOzRqnph3DkUlTkdb2K7aloZw0L~tNkpsjTO7K0crzpC0Sa6DgZ4apVDj4~bM3jUbF1Kbve7nuTGUaPrCNIDgjF-C6E6pQ0KUAacvlpyLATm3VBPgh4mYQKSRcaY33b-Wv-HrfhrICYmxPdlmxICpbchpgaWU5l6CqCxcPLVr2F3nnw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)