Abstract
We identified regulator of G-protein signaling-5 (RGS-5) as an angiogenic pericyte marker at sites of physiologic and pathologic angiogenesis. In a mouse model of pancreatic islet cell carcinogenesis, RGS-5 is specifically induced in the vasculature of premalignant lesions during the “angiogenic switch” and further elevated in tumor vessels. Similarly, RGS-5 is overexpressed in highly angiogenic astrocytomas but not in hypoxia-inducible factor-1α (HIF-1α)–deficient tumors, which grow along preexisting brain capillaries without inducing neovessels. Elevated levels of RGS-5 in pericytes are also observed during wound healing and ovulation indicating a strong correlation between RGS-5 expression and active vessel remodeling beyond tumor angiogenesis. Moreover, antitumor therapy, which reverses tumor vasculature to an almost normal morphology, results in down-regulation of RGS-5 transcription. Taken together, these data demonstrate for the first time a factor that is specific for “activated” pericytes. This further supports the notion that pericytes, like endothelial cells, undergo molecular changes during neovascularization that makes them a novel target for antiangiogenic therapy.
Introduction
Neovascularization is a fundamental process that ensures adequate metabolic supply to tissues in numerous biologic and pathologic states, including tumorigenesis. It is tightly regulated during embryogenesis, leading to the establishment of a hierarchical vascular network. In contrast to the high proliferative activity in the prenatal stage, the vasculature in the adult is relatively quiescent.1,2 Exceptions are phases of active vessel remodeling in female reproductive organs and the formation of granulation tissue during wound healing. The earliest stages of angiogenesis are defined by vasodilation and increased vascular permeability of capillaries or postcapillary venules in response to proangiogenic factors such as vascular endothelial growth factor (VEGF).3,4 This is accompanied by detachment of support cells (pericytes) from the vessel wall and basement membrane and extracellular matrix degradation. The underlying endothelial cells then migrate into the perivascular space, multiply, and form new vascular sprouts. Pericytes are again recruited to the vasculature in response to growth factors such as platelet derived growth factor B (PDGF-B), and in turn, reduce endothelial cell proliferation and help to mature and stabilize the newly formed vessels.5 Angiogenesis is also a discrete and essential step during tumor formation and progression,6 but here, the quiescent microvasculature undergoes an “angiogenic switch” and is converted into a chaotic network of proliferating tumor vessels. In contrast to physiologic angiogenesis, in which new vessels rapidly mature and become stable, tumors have lost the control mechanisms to terminate vessel growth. Consequently, the tumor vasculature develops unique characteristics and becomes distinct from the normal blood supply system. Tumor vessels are not organized into the definitive venules, arterioles, and capillaries of their normal counterparts, but instead, share chaotic features of all of them. Furthermore, they are dilated and tortuous, often leading to hemorrhages. Due to the constant vascular remodeling, pericytes, which are usually in close contact with the endothelium, become more loosely associated or less abundant.7,8 In addition to such morphologic changes, tumor endothelial cells have been shown to express genes and surface markers that are selective for angiogenic blood vessels, supporting the notion that these endothelial cells change to become distinct from normal endothelium.9-11
To understand the progressive angiogenic alterations leading to the profound differences in tumor vasculature, we have used a mouse model of pancreatic islet carcinogenesis in which the “angiogenic switch” is a discrete step in tumorigenesis and occurs prior to tumor formation.12 In RIP1-Tag5 transgenic mice, the oncogene SV40 large T antigen (Tag) is expressed under the control of the rat insulin promoter (RIP) in β cells of the pancreas when mice are approximately 10 weeks of age.13 Consequently, transgenic mice develop islet carcinomas in a multistep pathway beginning with hyperplastic islets at about 14 weeks of age. A subset of these hyperplastic islets develops an angiogenic phenotype by the age of 16 weeks and progresses into highly vascularized, solid tumors over a period of 15 weeks. Strikingly, transformation of the microangioarchitecture, predominantly vessel dilatation, becomes apparent in hyperplastic/early angiogenic islets prior to the expansion of the tumor mass. This first phase of neovascularization is then followed by extensive vessel sprouting and total loss of vessel hierarchy in angiogenic islets and hemorrhaging in end-stage tumors.3 Although we have a reasonable understanding of vessel remodeling during tumor progression, and can arrest tumorigenesis by interfering with angiogenesis in RIP-Tag mice,14,15 it remains unclear which molecular changes in the vasculature are associated with tumor progression or regression during therapy.
In the course of characterizing the vasculature of progressively growing tumors at a molecular level, we defined markers that were associated with the angiogenic phenotype in RIP1-Tag5 mice. One of these marker genes is a member of the regulator of G-protein signaling family, RGS-5. RGS proteins comprise a family of molecules with a unifying catalytic function but varying tissue distribution. They stimulate the intrinsic guanosine triphosphatase (GTPase) activity of activated Gα subunits and thereby accelerate G-protein inactivation. Thus, RGS molecules inhibit signaling downstream of G-protein–coupled receptors.16 Although much is known about the biochemistry of RGS molecules, their physiologic role in vivo is largely unknown. Here, we report that RGS-5 is induced in angiogenic tumor vasculature of RIP1-Tag5 mice and during wound healing and ovulation. Surprisingly, RGS-5 colocalizes with pericytes in the vascular bed and not with endothelial cells. This finding supports increasing evidence that pericytes, like endothelial cells, change their phenotype within the tumor microenvironment and may thus be novel targets for antiangiogenic therapy.17 Therefore, RGS5 is the first gene described that is up-regulated in pericytes in vivo during the early phases of physiologic and pathologic neovascularization.
Materials and methods
Mice
RIP1-Tag5 mice (kindly provided by D. Hanahan, University of California at San Francisco, San Francisco, CA) were generated in the C3HeB/Fe background. In these transgenic mice expression of the Tag oncogene in pancreatic islets starts at approximately week 10 and leads to premature death between weeks 30 and 35. C3HeB/Fe wild-type mice were used to study ovarian angiogenesis and wound healing. Generation of hypoxia-inducible factor-1α (HIF-1α) wild-type and HIF-1α knock-out astrocytomas and intracranial implantation into athymic mice has been published previously.18 Irradiation of RIP1-Tag5 mice, bone marrow reconstitution, and adoptive transfers have been described.15 All mice in the treatment group received 3 adoptive transfers with CD4+ Tag-specific effector cells (TagTCR1). After therapy, tumor nodules with a “white” appearance were isolated from pancreatic tissue and compared to “red” tumors in untreated RIP1-Tag5 mice.
Northern blotting
RNA from blood vessels of normal islets of Langerhans and RIP1-Tag5 insulinomas were compared using the polymerase chain reaction (PCR)–select cDNA subtraction kit (Clontech, Heidelberg, Germany). A total of 2000 cDNAs were analyzed for differential expression. A 1080–base pair (bp) fragment with a perfect match to mouse RGS-5, including the 550-bp open reading frame, was identified as highly up-regulated in tumor samples. The 1080-bp DNA fragment (200 ng) was labeled with [α-32P]dCTP (Amersham, Freiburg, Germany) using a random-primed labeling kit (Roche, Mannheim, Germany). Total RNA (20 μg) was separated on a 1% formaldehyde gel, blotted on Hybond N (Amersham) membrane, and hybridized overnight at 42°C in 50% formamide, 6 × SSPE (saline sodium phosphate EDTA [ethylene diamine-tetraacetic acid]), 5 × Denhardt, 0.5% sodium dodecyl sulfate (SDS), 100 μg salmon sperm DNA, and radioactive probe. The filter was washed in 7 × SSPE and 0.1% SDS at room temperature, followed by 1 × SSPE, 0.5% SDS at 37 °C and 0.1 × SSPE, 1% SDS at 65 °C and exposed for autoradiography.
Quantitative reverse transcription–PCR analysis
RNA and cDNA from islets/insulinomas and skin samples were prepared as described.15 Total RNA from sorted cells was isolated using the Absolutely RNA Microprep Kit (Stratagene, Amsterdam, The Netherlands). RNA was translated into single-stranded cDNAusing the Superscript cDNASynthesis kit (Invitrogen, Groningen, The Netherlands) and random hexamers (Amersham Biosciences, Freiburg, Germany). Relative gene expression levels were determined using real-time PCR TaqMan technology (Applied Biosystems, Weiterstadt, Germany) and SYBR green incorporation. The mouse hypoxanthine phosphoribosyltransferase (HPRT) gene served as an internal standard. The following mouse-specific primers (5′ to 3′) were used: CD146: CACGACATTGGCTTGAATAGTTG, CACGTCACTCCCCATGATGA; claudin-5: GCCCAGCTCGTACTTCTGTGA, TTAAGGCACGGGTAGCACTCA; desmin: GACGCTGTGAACCAGGAGTTC, GCGTTCTGCTGCTCCAAG; HPRT: ACACCTGCTAATTTTACTGGCAACA, TGGAAAAGCCAAATACAAAGCCTA; PDGF-B: AAGTTTAAGCACACCCATGACAAG, ATTAAATAACCCTGCCCACACTCT; PDGF receptor β (PDGFR-β): GCTCACGGTCTGAGCCATTC, GCTCGGACATTAAGGCTTGCT; RGS-5: GCTTTGACTTGGCCCAGAAA, CCTGACCAGATGACTACTTGATTAGCT; αSMA (smooth muscle actin): GATCCACAAAACGTTCACAGTTG, GCTGTCTACCTTCCAGCAGATGT; Tie2–: TTTACAACAGCGTCTATCGGACTC, TCGCCTCTCGACTTCCACAT; vascular endothelial (VE)–cadherin: CAGAATTAAGCACTGACACATCATAGC, GCGCATGCTCATAGCAAGAGT; and VEGF receptor 2 (VEGFR2): GATGCAGGAAACTACACGGTCAT, GGTCCCATACTGGTAGGAATCCA.
Immunohistochemistry
Tissue was freshly frozen in OCT compound (Tissue Tek, Vogel, Germany). Alternatively, mice hearts were perfused with phosphate-buffered saline (PBS) and 4% paraformaldehyde, and organs were immersed in 30% sucrose overnight. Prefixed tissue was then frozen in OCT compound. Staining was performed as described13 on 7- to 10-μm sections with anti-CD31 (rat IgG2a, MEC 13.3; 5 μg/mL; BD PharMingen, Heidelberg, Germany), followed by cyanin 3 (Cy3)–conjugated IgG F(ab′)2 fragment goat antirat (3 μg/mL; Dianova, Hamburg, Germany) as secondary reagent. Stainings for antidesmin (mouse IgG1, D33, 1:300; Dako, Hamburg, Germany) and anti-PDGFR-β (mouse IgG2a, 28D4, 10 μg/mL; BD PharMingen) were performed using the fluorescein isothiocyanate (FITC)–M.O.M. kit (Alexis, Grünberg, Germany). Tissues were covered with Vectashield mounting medium containing DAPI (4,6-diamidino-2-phenylindole; Alexis). All histology was analyzed using the Axioplan 2 microscope (Carl Zeiss, Hallbergmoos, Germany) Plan-Neofluar objective lenses and 2.5 ×/0.075, 10 ×/0.30, and 25 ×/0.80 oil (Zeiss). AxioCAM camera and AxioVision 3.1 (Zeiss) were used for image recording. All images were processed using Adobe Photoshop software (San Jose, CA).
Nonradioactive in situ hybridization
A 1000-bp fragment encoding full-length RGS-5 and a 680-bp PDGFR-β fragment were cloned into Bluescript Vector with flanking T3 and T7 promoters. Digoxigenin-labeled riboprobes were generated from linearized plasmid DNA using digoxigenin-11-UTP and T3 or T7 enzyme mix (Roche) in an in vitro transcription reaction. Tissue was frozen in OCT compound, cryosectioned at 7 μm, and fixed in acetone at 4°C. Alternatively, tissue was fixed in 4% paraformaldehyde overnight and embedded in paraffin, and 7-μm sections were deparaffinized in xylene and rehydrated through an alcohol series. In situ hybridization was performed as published previously10,19 with some modifications. Sections were fixed in 4% paraformaldeyde, permeabilized with 0.2% (frozen sections) or 1.5% (paraffin sections) pepsin (Dako) in 0.2 N HCl, acetylated with acetic anhydride, and prehybridized for 2 hours at 55°C in 40% formamide, 10% dextran, 1 × Denhardt, 4 × saline sodium citrate (SSC), 10 mM dithiothreitol (DTT), and 1 mg/mL tRNA (Sigma, Taufkirchen, Germany). Denatured riboprobes were added at a final concentration of 150 ng/mL and sections were incubated at 55°C overnight. After washing twice with 2 × SSC at 45°C, sections were rinsed in TNE buffer (10 mM Tris [tris(hydroxymethyl)aminomethane]–HCl, pH 7.5, 500 mM NaCl, 1 mM EDTA [ethylenediaminetetraacetic acid]) and incubated with RNase A/T1 cocktail (diluted 1:35 in TNE buffer; Ambion, Huntington, United Kingdom) at 37°C for 1 hour. Subsequently, slides are washed in 2 × SSC, 50% formamide at 55°C, followed by 0.1 × SSC at 55°C. Slides were rinsed in Tris-buffered saline (TBS) and incubated with peroxidase blocking reagent (Roche), followed by 1% blocking reagent (purified casein; Roche) containing rabbit immunoglobulin fraction (1 μg/mL; Dako). After blocking, slides were incubated with horseradish peroxidase (HRP)–antidigoxigenin Fab fragments (1 U/mL in 1% blocking reagent; Roche) for 45 minutes. Biotin tyramids (GenPoint kit, Dako) were added for 8 minutes, followed by 20 minutes of incubation with HRP-antibiotin antibodies (diluted 1:150 in 1% blocking reagent; Dako). For higher signal amplification, biotin tyramids were added a second time for 5 minutes, followed by incubation with alkaline phosphatase (AP)–antibiotin antibodies (diluted 1:75 in 1% blocking reagent; Dako) for 20 minutes. In between staining steps, sections were washed in TBS. Signal was detected by incubation in chromogenic substrate (0.45 mg/mL 4-nitroblue tetrazolium chloride and 0.18 mg/mL 5-bromo-4-chloro-3-indolyl-phosphate; Roche) in buffer containing 0.1 M Tris (pH 9.5), 1 M NaCl, 0.05 M MgCl2, 0.1% Tween 20, and 0.24 mg levamisole (Sigma) for 30 minutes in the dark. Sections were counter-stained with 1% methyl green. In some experiments, AP-antibiotin antibodies and subsequent color development was replaced by mouse monoclonal Cy3-conjugated antibiotin IgG fraction (3 μg/mL; Dianova). In double-labeling experiments, in situ hybridization was followed by immunohistochemistry using anti-CD31 antibodies and FITC-conjugated IgG F(ab′)2 fragment goat antirat (3 μg/mL; Dianova) or antidesmin antibodies and the FITC-M.O.M. kit.
Fluorescence-activated cell sorting
Preparation of single-cell suspensions from solid RIPTag tumors has been described previously.17 Briefly, isolated tumors from 5 to 7 30-week-old RIP1-Tag5 mice were minced with a razor blade and digested in a 20 mL cocktail of 1 × PBS containing 10 mg/mL bovine serum albumin (BSA), 550 U/mL collagenase II (Worthington, Cell Systems Biotechnology, St. Katharinen, Germany), 500 U/mL collagenase IV (Worthington), and 3 U/mL DNase I (Worthington) for 15 minutes in a 37°C waterbath equipped with a submersible stirrer. Digestion was stopped by adding Dulbecco Modified Eagle Medium (DMEM)/5% fetal calf serum (FCS). Cells were passed through a 70-μm cell strainer and washed at 720g for 5 minutes. Erythrocytes were then lysed in 1 mL PharmLyse buffer for 20 seconds (BD PharMingen) and washed in DMEM/5% FCS. The single-cell suspension was incubated with Fc block (CD16/CD32, 2.4G2; 2.5 μg/μL; BD PharMingen) prior to specific staining. For the isolation of endothelial cells, tumor cells were stained with anti–CD31-phycoerythrin (PE; rat IgG2a, MEC 13.3, 4 μg/mL; BD PharMingen) and ME-9F1-FITC (rat IgG2a, 30 μg/mL; kindly provided by A. Hamann, Deutsches Rheuma-Forschungszentrum, Berlin, Germany). For the isolation of perivascular cells, tumor cells were incubated with anti-CD140b (rat IgG, 30 μg/mL; e-bioscience, THP Medical Products, Vienna, Austria) which was allophycocyanin (APC)–labeled with the Phycolink Allophycocyanin Conjugation Kit (Prozyme, Europa Bioproducts, Ely, United Kingdom). Propidium iodide (1 μg/mL) was added for the exclusion of dead cells. Cells were sorted using a FACSVantage SE flow cytometer (Becton Dickinson, Heidelberg, Germany).
Skin wounds
Wounds of about 5 mm in length were introduced on the back of C3HeB/Fe mice by cutting the skin and panniculus carnosus. At 1, 3, 6, 9, and 14 days after wounding, 5 to 9 skin samples per group were harvested. The complete wound including 2 mm of the margins was excised at each time point. A similar amount of skin from untreated animals was collected. Tissue was snap frozen in liquid nitrogen for RNA analysis.
Isolation of pancreatic normal islets, angiogenic islets, and solid tumors
After retrograde perfusion with 1 × Hanks balanced salt solution (Invitrogen)/25 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.4), tissue was excised and 4 pancreata digested in 13 mL 1 × Hanks/HEPES containing collagenase type XI (1200 U/mL; Sigma) for 12 minutes at 37°C. Collagenase digestion was terminated by washing cells twice in ice-cold 1 × Hanks/HEPES/1% BSA. Cells were resuspended in 27% Ficoll (Sigma) in 1 × Hanks/0.1% BSA and overlaid by 23% and 11% Ficoll solutions. Islets were enriched in the first and second interphase after 20 minutes of centrifugation at 800g. Single islets were isolated under a dissecting microscope. Angiogenic islets can be discriminated from normal pancreatic islets by size and the appearance of red patches due to neovascularization and hemorrhaging. Solid tumors were dissected from pancreatic tissue with scissors.
Western blotting
Protein extracts from isolated islets of Langerhans and dissected tumors were prepared as described.13 Protein extracts (20 μL) were separated on 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE), blotted, and incubated with anti–PDGFR-β (rabbit polyclonal, 1:500; BD PharMingen), anti-VEGFR2 (rat IgG2a, Avas12a1, 1 μg/mL; BD PharMingen) and anti–glyceraldehyde-3-phosphate dehydrogenase (anti-GAPDH; IgG1, 3.3 μg/mL; Chemicon International, Hofheim, Germany), followed by HRP-labeled secondary antibodies (10 ng/mL; Alexis) and Super Signal chemiluminescent substrate (Pierce, Rockford, IL).
Results
RGS-5 up-regulation in RIP1-Tag5 tumors coincides with the “angiogenic switch”
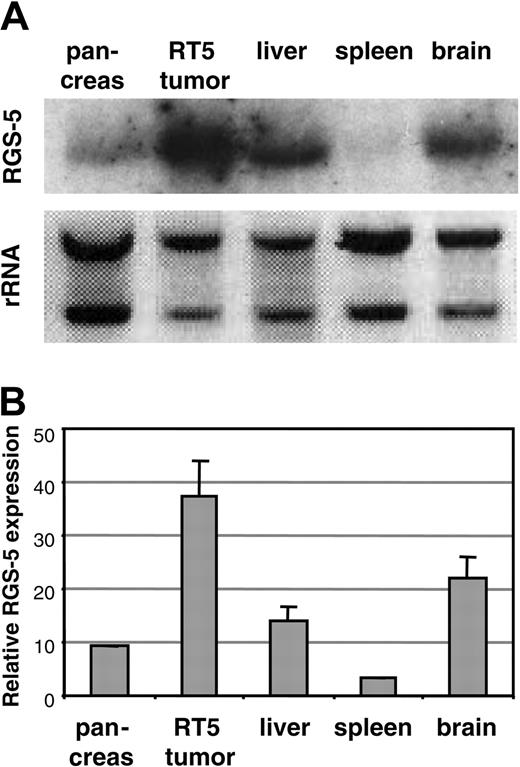
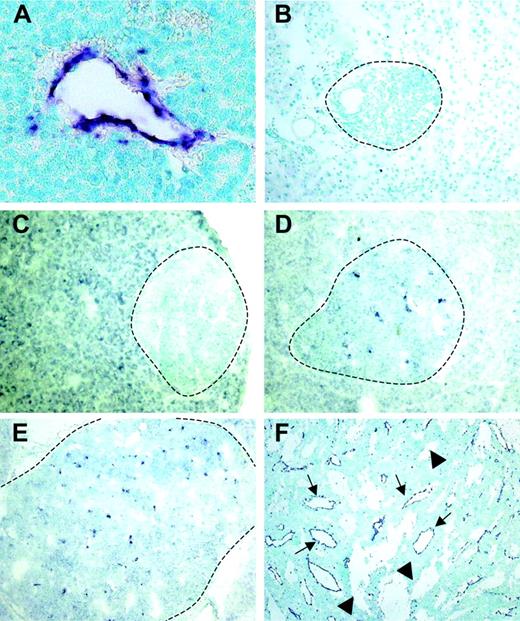
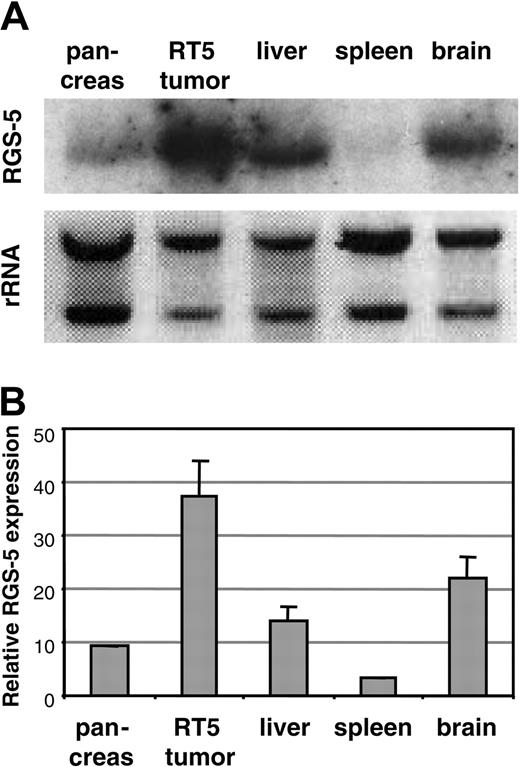
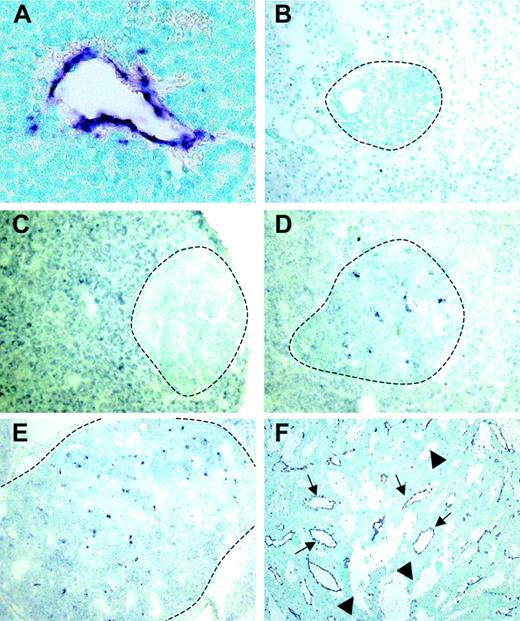
Using a differential cDNA cloning approach between blood vessels of normal islets of Langerhans and pancreatic tumors of RIP1-Tag5 mice, we found RGS-5, a known member of a larger family of regulators of G-protein signaling, to be highly up-regulated in tumors (R.G., unpublished results, June 2000). Northern blot (Figure 1A) and quantitative reverse transcription (RT)–PCR (Figure 1B) analyses showed that RGS-5 transcription was induced 4- to 5-fold in pancreatic islet tumors when compared to normal pancreas, which exhibited very low levels of RGS-5 RNA. This is in agreement with the finding that RGS-5 is constitutively expressed in a variety of murine organs, specifically in brain but also in heart, aorta, skeletal muscle, liver, and kidney.20 To define the cell source and the onset of RGS-5 transcription, we performed in situ hybridization analyses on transgenic pancreatic tissues of 25- to 30-week-old RIP1-Tag5 mice, which display all stages of tumor progression. We found RGS-5 staining in cells that are closely associated with tumor blood vessels (Figure 2A), confirming our initial findings using the differential cDNA cloning approach. RGS-5 signals were not detectable in normal and hyperplastic islets or in the adjacent exocrine pancreas of transgenic mice (Figure 2B-C). However, we observed spotty RGS-5 staining in a subset of early angiogenic islets (Figure 2D) that are characterized by the first appearance of dilated blood vessels as we have previously shown with intravital microscopy.3 This stage is defined as the “angiogenic switch.”21 Larger and more advanced angiogenic islets displayed a further increase in the number of RGS-5+ cells in close conjunction to vessels (Figure 2E). The most dominant expression of RGS-5, however, was detected in small and large vessels of late-stage tumors (Figure 2F). Thus, RGS-5 up-regulation coincides with the “angiogenic switch” in vessel-associated cells and increases during tumor progression, exhibiting highest levels in solid, end-stage tumors.
Murine RGS-5 is up-regulated in insulinomas. RGS-5 mRNA expression in normal mouse organs (brain, spleen, pancreas, and liver) and a pool of isolated solid tumors from 30-week-old RIP1-Tag5 (RT5) mice was examined by Northern blot (A) or RT-PCR analysis (B). The graph represents data from 3 to 5 organ samples. Error bars in all histograms represent SDs.
Murine RGS-5 is up-regulated in insulinomas. RGS-5 mRNA expression in normal mouse organs (brain, spleen, pancreas, and liver) and a pool of isolated solid tumors from 30-week-old RIP1-Tag5 (RT5) mice was examined by Northern blot (A) or RT-PCR analysis (B). The graph represents data from 3 to 5 organ samples. Error bars in all histograms represent SDs.
RGS-5 expression is restricted to angiogenic vessels and is up-regulated early during multistep tumorigenesis. RGS-5 transcripts are visualized using in situ hybridization on paraffin (A) or frozen tissue sections (B-F) and counterstained with methyl green. (A) RGS-5+ tumor blood vessel (original magnification × 25). In the same transgenic animal a normal islet of Langerhans (B, original magnification × 16) and a hyperplastic islet (C, original magnification × 12.5) are negative for RGS-5. (D) First RGS-5 signals are detected in early angiogenic islets (original magnification × 12.5) and the number of signals increases continuously in late angiogenic islets (E, × 10) and highly vascularized, solid tumors (F, original magnification × 5). Tumor vessels (arrows) can be distinguished from “blood lakes” (arrowheads), tumor cell–lined cavities, which do not participate in the blood circulation. Dotted lines indicate islet boundaries.
RGS-5 expression is restricted to angiogenic vessels and is up-regulated early during multistep tumorigenesis. RGS-5 transcripts are visualized using in situ hybridization on paraffin (A) or frozen tissue sections (B-F) and counterstained with methyl green. (A) RGS-5+ tumor blood vessel (original magnification × 25). In the same transgenic animal a normal islet of Langerhans (B, original magnification × 16) and a hyperplastic islet (C, original magnification × 12.5) are negative for RGS-5. (D) First RGS-5 signals are detected in early angiogenic islets (original magnification × 12.5) and the number of signals increases continuously in late angiogenic islets (E, × 10) and highly vascularized, solid tumors (F, original magnification × 5). Tumor vessels (arrows) can be distinguished from “blood lakes” (arrowheads), tumor cell–lined cavities, which do not participate in the blood circulation. Dotted lines indicate islet boundaries.
RGS-5 is induced in physiologic angiogenesis
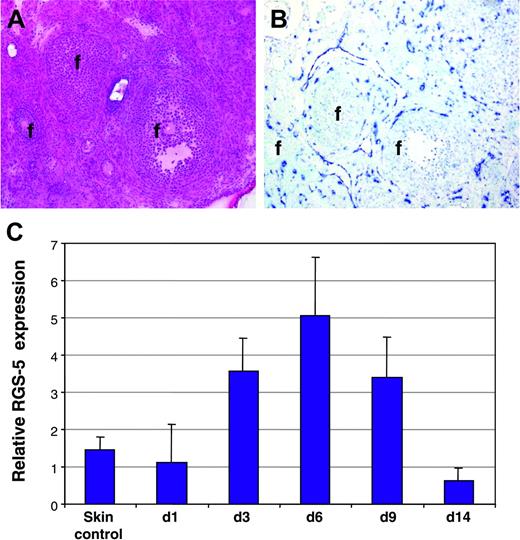
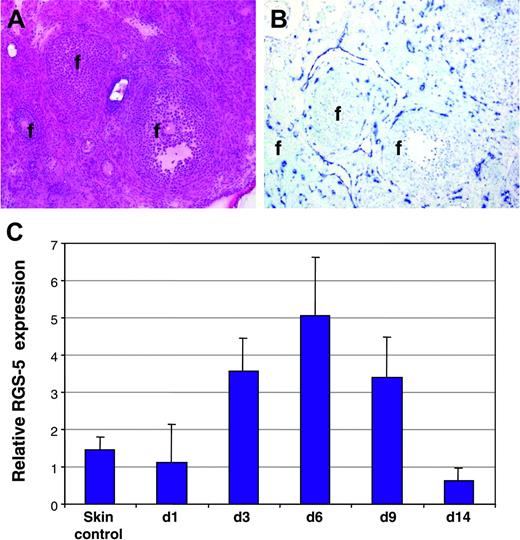
To reveal whether RGS-5 is also up-regulated in physiologic angiogenesis, we monitored RGS-5 expression in hormone-induced ovarian angiogenesis22 and in granulation tissue of cutaneous wounds.23 Figure 3A depicts the histology of a mouse ovary after hormone-induced ovulation in which follicles of different maturation stages are surrounded by the highly vascularized ovarian stroma. In situ hybridization analysis displayed strong RGS-5 expression in close association with blood vessels (Figure 3B). Skin wounds were introduced on the back of C3H mice and samples collected at various time points thereafter (days 1, 3, 6, 9, 14). RGS-5 transcription levels in total RNA preparations of wounds and healthy skin were compared using quantitative RT-PCR. RGS-5 expression exactly follows the kinetics of wound healing, where granulation tissue builds up to reach a maximum at around day 6 and steadily declines thereafter (Figure 3C). By day 14, most of the granulation tissue has resolved and the wounded skin is hardly discernible from undamaged skin. Thus, in cutaneous wound healing, RGS-5 expression rises due to increased neovascularization in granulation tissue.
RGS-5 expression during physiologic neovascularization. (A-B) Consecutive frozen tissue sections of a mouse ovary stained with (A) hematoxylin and eosin or (B) after in situ hybridization with a RGS-5–specific riboprobe (f indicates follicles; original magnification × 12.5). (C) Relative RGS-5 expression levels during dorsal wound healing. RNA from 5 to 9 independent samples of untreated skin (control) and wounds at days 1, 3, 6, 9, and 14 was quantified by RT-PCR.
RGS-5 expression during physiologic neovascularization. (A-B) Consecutive frozen tissue sections of a mouse ovary stained with (A) hematoxylin and eosin or (B) after in situ hybridization with a RGS-5–specific riboprobe (f indicates follicles; original magnification × 12.5). (C) Relative RGS-5 expression levels during dorsal wound healing. RNA from 5 to 9 independent samples of untreated skin (control) and wounds at days 1, 3, 6, 9, and 14 was quantified by RT-PCR.
RGS-5 is overexpressed in PDGFR-β+ perivascular cells
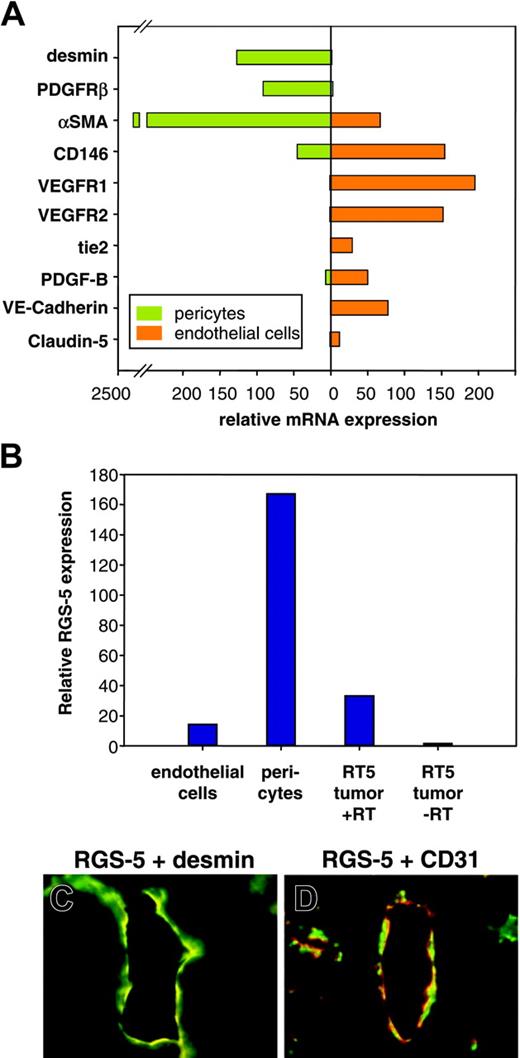
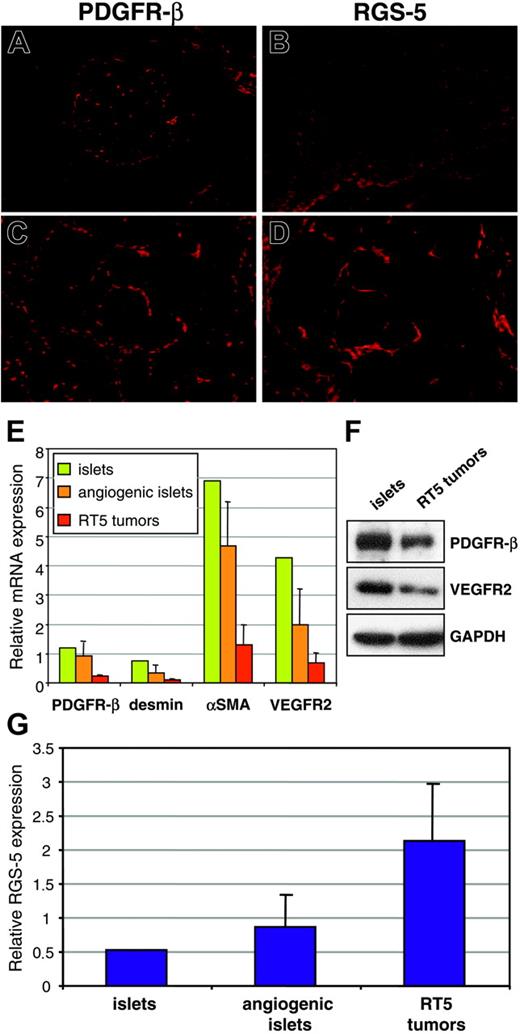
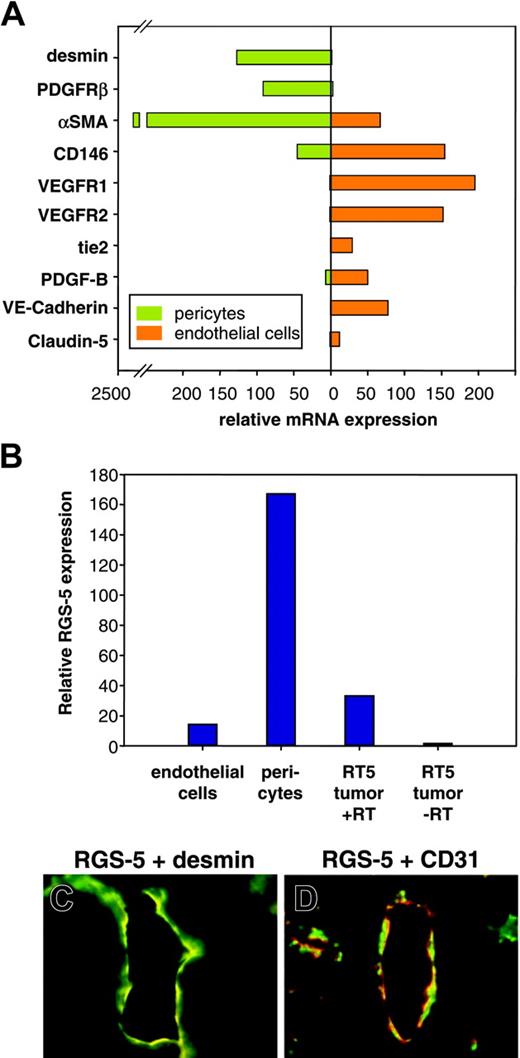
Although we demonstrated RGS-5 mRNA expression in the lining of ovarian and tumor vessels, the close proximity of endothelial and perivascular cells makes it difficult to resolve cell-type specificity by in situ hybridization. However, because RGS-5 expression has been reported in developing pericytes in the embryo,24,25 we aimed to analyze RGS-5 expression separately in endothelial cells and pericytes to define its cell-type specificity. Tumors from 30-week-old RIP1-Tag5 mice were isolated and single-cell suspensions were sorted by flow cytometry. Endothelial cells were discriminated by CD31 (platelet endothelial cell adhesion molecule [PECAM]) expression and immunoreactivity for the hybridoma ME-9F1. ME-9F1 has been described to selectively react with mouse endothelial cells.26 Perivascular cells were sorted as CD31– and PDGFR-β+ cells (data not shown). PDGFR-β has previously been shown to be a suitable marker for pericytes in RIP-Tag tumors and is not expressed by tumor cells.17 To further assess specificity and purity of the sorted cell populations, quantitative RT-PCR analyses of various markers were performed. Selective expression of the known endothelial cell markers CD146, VEGFR1, VEGFR2, tie2, PDGF-B, VE-cadherin, and claudin-5 were found in CD31/ME-9F1+ cells, whereas the perivascular cell markers desmin and αSMA were expressed in PDGFR-β+ cells (Figure 4A). Most interestingly, however, RGS-5 expression is dramatically up-regulated in the perivascular cell population (Figure 4B). Double-labeling experiments using desmin as a perivascular marker confirm the colocalization with RGS-5 transcripts in RT5 tumor vessels (Figure 4C). In contrast, the endothelial cell marker CD31 is in close vicinity to RGS-5, but the expression does not overlap (Figure 4D).
RGS-5 is specifically up-regulated in perivascular cells of RIP1-Tag5 tumors. Endothelial cells (CD31+, ME-9F1+) and PDGFR-β+ cells were separated from end-stage RIP1-Tag5 tumors by flow cytometry. (A) Total RNA from isolated cells was analyzed for marker gene expression to confirm endothelial cell or pericyte phenotypes. αSMA expression in the endothelial cell fraction most likely indicates the presence of some perivascular cells, which remain attached to endothelial cells during purification. (B) Relative RGS-5 expression was quantified for each cell population and compared to RGS-5 expression in total RNA prepared from whole tumors (–RT indicates minus reverse transcriptase control). Expression levels in panels A and B were normalized to HPRT as an internal standard. In situ hybridization, followed by immunohistochemistry shows colocalization of the pericyte marker desmin (green) with RGS-5 transcripts (red) in panel C, but in panel D no perfect match was seen with RGS-5 (red) and the endothelial cell marker CD31 (green) in RIP1-Tag5 tumor vessels (original magnification × 16).
RGS-5 is specifically up-regulated in perivascular cells of RIP1-Tag5 tumors. Endothelial cells (CD31+, ME-9F1+) and PDGFR-β+ cells were separated from end-stage RIP1-Tag5 tumors by flow cytometry. (A) Total RNA from isolated cells was analyzed for marker gene expression to confirm endothelial cell or pericyte phenotypes. αSMA expression in the endothelial cell fraction most likely indicates the presence of some perivascular cells, which remain attached to endothelial cells during purification. (B) Relative RGS-5 expression was quantified for each cell population and compared to RGS-5 expression in total RNA prepared from whole tumors (–RT indicates minus reverse transcriptase control). Expression levels in panels A and B were normalized to HPRT as an internal standard. In situ hybridization, followed by immunohistochemistry shows colocalization of the pericyte marker desmin (green) with RGS-5 transcripts (red) in panel C, but in panel D no perfect match was seen with RGS-5 (red) and the endothelial cell marker CD31 (green) in RIP1-Tag5 tumor vessels (original magnification × 16).
PDGFR-β+ cells selectively up-regulate RGS-5 in the tumor microenvironment
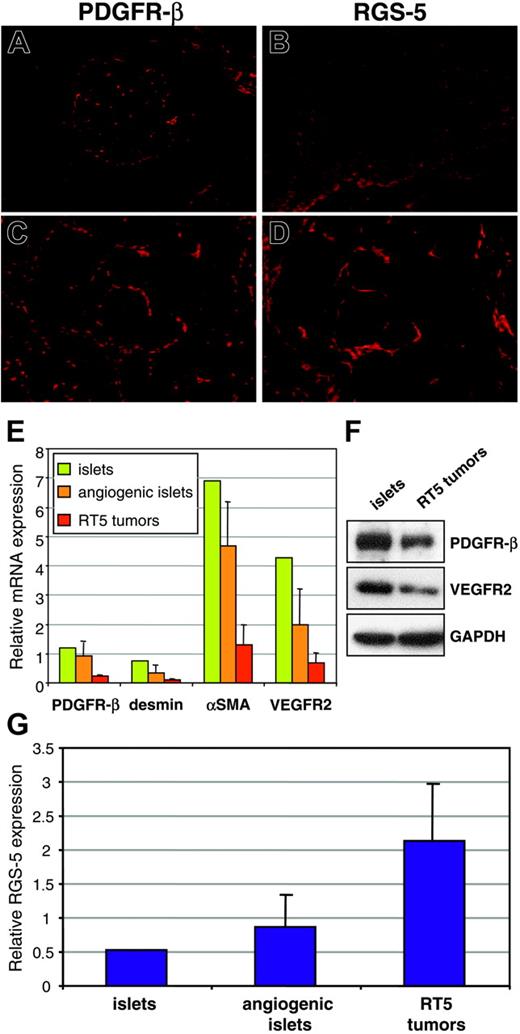
Previous studies have shown that pericyte numbers and the degree of pericyte coverage of tumor vessels vary considerably between different tumor models.8,27 Therefore, 2 possible explanations for elevated RGS-5 levels in RIP1-Tag5 tumors are an increased recruitment of pericytes into the tumor bed or, alternatively, a true up-regulation of RGS-5 expression per pericyte. To resolve this issue, we compared PDGFR-β and RGS-5 expression between normal and tumor tissue. In normal islets, PDGFR-β+ cells are found in the vascular network (Figure 5A), whereas RGS-5 expression is absent (Figure 5B). This is consistent with RGS-5 expression data shown in Figure 2B. In contrast, in solid tumors PDGFR-β+ and RGS-5+ cells exert comparable staining patterns
Intratumoral PDGFR-β+cells selectively up-regulate RGS-5. (A) Immunoreactivity of anti–PDGFR-β antibodies in normal pancreatic islets and (C) RIP1-Tag5 tumors is compared to RGS-5 in situ hybridization signals in islets (B) and tumors (D; for all frozen sections, original magnification × 10). (E) RNA from normal islets (1 pool of 200 islets), angiogenic islets (3 pools of 10 angiogenic islets), and tumors (12 independent tumor samples) was analyzed for the expression of known pericyte markers (PDGFR-β, desmin, αSMA) and for VEGFR2 as a marker for endothelial cells. (F) Western blot analysis of pools of islets and RT5 tumors. (G) Relative expression of RGS-5 in normal islets, angiogenic islets, and tumors is shown. Expression levels in panels E and G were normalized to HPRT.
Intratumoral PDGFR-β+cells selectively up-regulate RGS-5. (A) Immunoreactivity of anti–PDGFR-β antibodies in normal pancreatic islets and (C) RIP1-Tag5 tumors is compared to RGS-5 in situ hybridization signals in islets (B) and tumors (D; for all frozen sections, original magnification × 10). (E) RNA from normal islets (1 pool of 200 islets), angiogenic islets (3 pools of 10 angiogenic islets), and tumors (12 independent tumor samples) was analyzed for the expression of known pericyte markers (PDGFR-β, desmin, αSMA) and for VEGFR2 as a marker for endothelial cells. (F) Western blot analysis of pools of islets and RT5 tumors. (G) Relative expression of RGS-5 in normal islets, angiogenic islets, and tumors is shown. Expression levels in panels E and G were normalized to HPRT.
on consecutive sections (Figure 5C-D). These findings imply that pericytes qualitatively change by up-regulating RGS-5 expression during tumor progression. However, from our histologic analyses (Figure 5A-D) we cannot exclude a simultaneous increase in pericyte numbers. Because negative results from in situ hybridization should be interpreted with caution, we complemented our histologic studies with quantitative RT-PCR analyses of RGS-5 and endothelial/pericyte markers at different tumor stages. Total RNA was prepared from separate pools of normal islets and angiogenic islets as well as from end-stage insulinomas. Surprisingly, we found that the relative expression of pericyte (PDGFR-β, desmin, αSMA) and endothelial (VEGFR2) markers decreases with tumor progression (Figure 5E). These RNA data were confirmed in Western blot analyses using PDGFR-β and VEGFR2 as representative markers (Figure 5F). This result implies that overall vessel density and pericyte number decrease during tumor progression. Indeed, lower vessel density has been reported in a variety of cancers and is a function of the reduced oxygen requirement of malignant cells.3,18,28 In contrast, the reciprocal, high RGS-5 expression in insulinomas (Figure 5G) suggests up-regulated RGS-5 expression by tumor pericytes and not increased pericyte recruitment.
Differential RGS-5 expression in brain tumors with high and low angiogenic potential
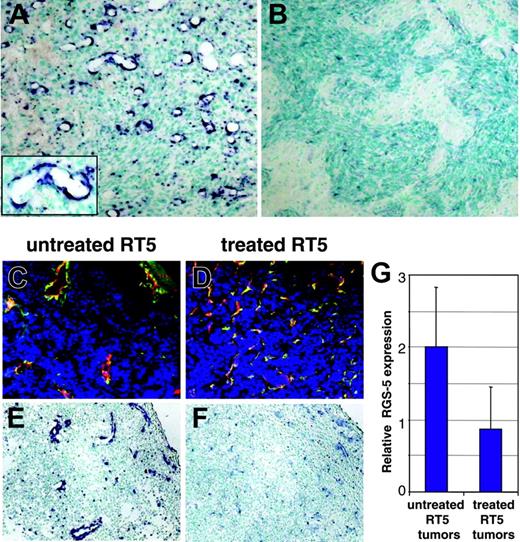
Having demonstrated progressive RGS-5 up-regulation in pericytes during the “angiogenic switch” in pancreatic islet carcinogenesis, we aimed to determine if this phenomenon also occurred in other tumor models. Interestingly, 2 orthotopic astrocytoma models were recently developed that differ in their ability to switch on angiogenesis.18 On transplantation into mouse brain, SV40 Tag/H-ras transformed astrocytes (WT-AST) develop into hypoxic, necrotic, and hemorrhagic tumors with a typical activated tumor vasculature that resembles the RIP1-Tag5 tumor phenotype.18 Astrocytomas that are deficient in the HIF-1α gene (HIFko-AST) are, in contrast to WT-AST, unable to respond to hypoxia and do not display the typical features of tumor vessel remodeling but instead invade into the brain parenchyma.18 This elegant system therefore allows a comparison of RGS-5 expression in strongly (WT-AST) versus weakly (HIFko-AST) angiogenic variants of the same tumor type. WT-AST expresses high levels of RGS-5 around the dilated tumor blood vessels, similar to blood vessels in RIP1-Tag5 tumors, suggesting pericyte origin (Figure 6A). Indeed, RGS-5 has been reported to colocalize with pericytes in developing brain microvessels24 and is expressed in certain areas of normal brain.20,29,30 In contrast, HIFko-AST, which does not induce tumor neovascularization, exhibits significantly lower levels of RGS-5 (Figure 6B). HIFko-AST resembles nonangiogenic stages of tumor progression in RIP1-Tag5 mice prior to the “angiogenic switch.” Thus, RGS-5 expression strongly correlates with the degree of vascular remodeling and angiogenesis in 2 independent tumor models.
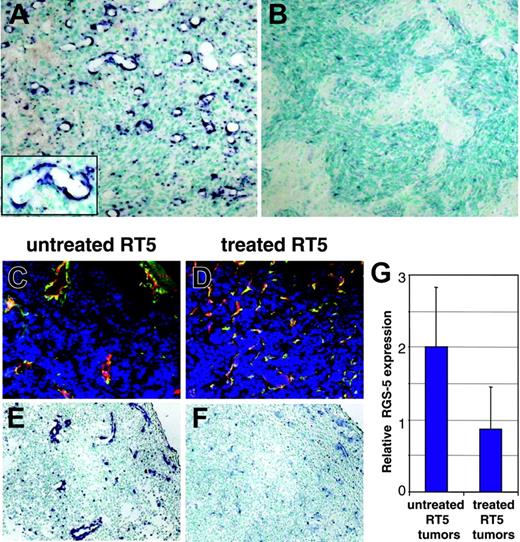
RGS-5 expression correlates with the degree of vascular remodeling in hypoxia-responsive and nonresponsive astrocytomas. (A) WT-AST (original magnification × 10; insert, original magnification × 25) and (B) HIFko-AST (original magnification × 10) were probed with a RGS-5–specific antisense riboprobe to demonstrate differential RGS-5 expression in the tumor vasculature. (C) Tumor samples from untreated 26-week-old RIP1-Tag5 mice with a hemorrhagic “red” appearance and enlarged tumor vessels were stained with anti-CD31 (red) and antidesmin (green) antibodies and the merge is shown (original magnification × 16). (D) Littermates were irradiated with 1000 rad, followed by bone marrow reconstitution and 3 adoptive transfers. At 26 weeks of age, “white” tumors were isolated, which display capillary-like vessels with no signs of hemorrhaging. Costaining for CD31 (red) and desmin (green) is shown (original magnification × 16). Differential expression of RGS-5, assessed by in situ hybridization, is shown for untreated tumors (E) and for regressing tumors after therapy (F). Original magnifications × 16 (E-F). (G) Histologic analyses were complemented by quantitative RT-PCR. Expression levels were normalized to HPRT.
RGS-5 expression correlates with the degree of vascular remodeling in hypoxia-responsive and nonresponsive astrocytomas. (A) WT-AST (original magnification × 10; insert, original magnification × 25) and (B) HIFko-AST (original magnification × 10) were probed with a RGS-5–specific antisense riboprobe to demonstrate differential RGS-5 expression in the tumor vasculature. (C) Tumor samples from untreated 26-week-old RIP1-Tag5 mice with a hemorrhagic “red” appearance and enlarged tumor vessels were stained with anti-CD31 (red) and antidesmin (green) antibodies and the merge is shown (original magnification × 16). (D) Littermates were irradiated with 1000 rad, followed by bone marrow reconstitution and 3 adoptive transfers. At 26 weeks of age, “white” tumors were isolated, which display capillary-like vessels with no signs of hemorrhaging. Costaining for CD31 (red) and desmin (green) is shown (original magnification × 16). Differential expression of RGS-5, assessed by in situ hybridization, is shown for untreated tumors (E) and for regressing tumors after therapy (F). Original magnifications × 16 (E-F). (G) Histologic analyses were complemented by quantitative RT-PCR. Expression levels were normalized to HPRT.
RGS-5 is down-regulated during therapeutic tumor regression
The strong correlation of RGS-5 expression and angiogenesis suggests that it may be used to monitor the effectiveness of antiangiogenic therapy. However, potent antiangiogenic drugs that induce vessel death do not allow assessment of RGS-5 expression levels.14,17 In contrast, our previously reported antitumor strategy, which combines irradiation and adoptive transfers of activated, tumor-specific lymphocytes, leads to complete tumor rejection by remodeling the vasculature into an almost normal appearance without inducing endothelial cell apoptosis.15 RGS-5 expression was therefore assessed in tumors treated with this regimen. Histopathology of RIP1-Tag5 tumors after irradiation/adoptive transfer therapy was compared to untreated littermates (Figure 6C-F). Untreated tumors show the typical heterogeneous appearance with predominantly large vessels being located next to small vessels and extensive hemorrhaging (Figure 6C). In contrast, treated tumors display a high vessel density with capillary-sized vessels resembling normal pancreatic vasculature (Figure 6D).15 Importantly, RGS-5 signals are significantly reduced in treated tumors, in concert with the vascular remodeling (Figure 6E-F). In situ hybridization results are supported by quantitative analyses of RGS-5 transcripts in treated and untreated tumors (Figure 6G). Thus, these data demonstrate that RGS-5 expression in pericytes is strongly associated with the complex and tightly regulated process of tumor angiogenesis and becomes less important once the vasculature acquires a more stable, quiescent state.
Discussion
RGS-5 is a negative regulator of heterotrimeric G-protein signaling pathways although its function in vivo remains elusive. Its organ distribution suggests involvement in fundamental regulatory processes and recent gene profiling experiments have shown that RGS-5 expression responds to changing tissue environments. For instance, RGS-5 is down-regulated in brain capillaries and choroid plexus of stroke-prone spontaneously hypertensive rats, indicating a possible link between G-protein regulation and hypertension.30 Similarly, down-modulation of RGS-5 has been found in neointimal smooth muscle cells (SMCs) after bypass grafting in monkeys, a finding that associates RGS-5 with SMC pathology.31 Its expression is also repressed in cytokine-stimulated vascular endothelial cells (human umbilical vein endothelial cells [HUVECs]) in models for atherosclerosis32 and capillary tube formation.33 In contrast, RGS-5 expression is up-regulated in a human neuroblastoma cell line after exposure to hydrostatic pressure.34 Furthermore, increased expression of RGS-5 occurs in human renal cell carcinoma although the precise location within the tumor was not investigated.35 Here, we report that murine RGS-5 is strongly overexpressed in autochthonous pancreatic insulinomas in a well-studied transgenic mouse model of tumorigenesis. Furthermore, overexpression is restricted to the tumor vasculature and, most excitingly, temporally and quantitatively coincident with tumor-induced angiogenic activity, being first detected in progenitor lesions undergoing the “angiogenic switch.” Therefore our data demonstrate for the first time an intimate association between RGS-5 expression and vessel remodeling during tumor-induced neovascularization.
The microvasculature is a complex system and fundamentally comprised of endothelial cells and stabilizing periendothelial cells, for example, pericytes, which are embedded in the basal membrane of capillaries and postcapillary venules.2 RGS-5 transcription has previously been detected in both vascular cell types, endothelial cells30,32 and perivascular SMC/pericytes.24,31 However, the close association of these cells in the vasculature has made precise cellular localization of RGS-5 technically difficult, even in double-labeling experiments. By successfully isolating both cell types from insulinomas, we showed that RGS-5 transcripts are enriched in PDGFR-β+ cells, which correspond to the perivascular cell compartment. This finding is consistent with a previous report demonstrating RGS-5 expression in pericytes in perinatal brain.24 In that study, mice deficient in PDGF-B or PDGFR-β lacked most of the pericytes in the developing blood vessels of the brain, with a corresponding absence of RGS-5 expression.24 These results univocally demonstrate that RGS-5 is a marker for pericytes in the developing brain. Moreover, RGS-5 expression is reminiscent of the expression pattern of PDGFR-β during embryonic development, strongly suggesting a role of RGS-5 during fetal vascular maturation.25 The data presented here complement these studies by revealing novel insights about RGS-5 in adult pathologic neovascularization. Due to the high homology between RGS family members and the lack of RGS-5–specific antibodies, all in vivo studies rely on RNA expression data.
Strong RGS-5 expression is not only found during tumor angiogenesis, but also during ovarian angiogenesis. Moreover, RGS-5 mRNA is transiently up-regulated in skin wounds during the formation of granulation tissue and declines on wound closure (Figure 3). Colocalization of RGS-5 with PDGFR-β+ perivascular cells during tumor angiogenesis, physiologic neovascularization, and embryonic development24,25 may indicate mechanistic overlaps between these processes and conservation of RGS-5 function. In this context, it is of interest that RGS-5 has been shown to attenuate PDGFR-β signaling in vitro.25 Because PDGF-B binding to its cognate receptor, PDGFR-β, is essential for pericyte recruitment to endothelial cells during development,36 this may indicate a role of RGS-5 in pericyte migration. Up-regulation of RGS-5 during vascular maturation may inhibit G protein-coupled receptor signaling by accelerating the transition from guanosine triphosphate (GTP)– to guanosine diphosphate (GDP)–bound Gα subunits and render pericytes less responsive to migratory signals. Thus, regulated RGS-5 expression may fine-tune pericyte motility during vessel growth and stabilization. Intriguingly, it has been shown that some RGS proteins interfere with chemoattractant receptors, thereby regulating cell migration and adhesion during cerebellar development and immune function.37,38
Phenotypic changes in perivascular cells have been reported for some tumor types, including the RIPTag model.8,27 Moreover, pericytes have been described as pluripotent cells, which can change appearance and marker expression on cytokine activation.39 Therefore, it is feasible that factors within the tumor environment induce “activated” or “immature” perivascular cells, and in turn, such phenotypic variation may result in differential sensitivity to extrinsic signals. For instance, only immature, PDGFR-β+ pericytes involved in neovascularization respond to PDGF-B injection by detaching from endothelial cells, whereas mature vessels remain inert.40 Similarly, inhibitors of PDGFR-β signaling selectively target pericytes in RIPTag tumors, but have no effect on the vasculature in normal tissue.17 It is generally accepted now that endothelial cells change their morphology and gene expression profile during tumor angiogenesis.10,19 However, molecular analyses have been lacking for tumor-resident pericytes. Thus, our data, for the first time, demonstrate molecular changes in perivascular cells, which are specifically induced during tumor angiogenesis and are potentially involved in regulating vascular angiogenic activity.
An understanding of the molecular mechanisms of neovascularization and the contribution of distinct vascular cell types will be essential for the design of novel antiangiogenic therapies. For instance, a combined therapeutic approach interfering with VEGFR signaling on endothelial cells and PDGFR signaling on perivascular cells has been proven to be highly effective. It targets 2 distinct cell populations at different stages of tumor progression and thus produces synergistic effects.17 This preclinical study also highlights the importance of pericytes in anticancer strategies. Strikingly, RGS-5 expression mimics the dynamics of neovascularization for the perivascular compartment by being up-regulated in the transition from prevascular hyperplastic to angiogenic islets. Similar to the RIPTag model, RGS-5 expression is low in nonangiogenic brain tumors lacking responsiveness to hypoxia (HIFko-AST) and high in brain tumors with ongoing vessel remodeling (WT-AST). Most important, however, these angiogenic changes are reversible with antitumor therapy and RGS-5 expression decreases as the tumor vasculature returns to a more “normal” phenotype (Figure 6). We demonstrate here that vessel “normalization” is not limited to the endothelial compartment but also includes perivascular cells as reflected in the reduction of RGS-5 expression. This finding has direct clinical application. Growing evidence indicates that “normalization” of tumor vessels will be an essential part of a new generation of combination therapies,15,41 and RGS-5 might be indicative of a window where intratumoral pericytes are responsive to therapy. Furthermore, elucidating the function of RGS-5 in pericyte pathobiology will not only lend important insights into tumor angiogenesis, but RGS-5 itself may also become a new target for anti-angiogenic drug development.
Prepublished online as Blood First Edition Paper, September 30, 2004; DOI 10.1182/blood-2004-06-2315.
Supported by grants from the Deutsche Forschungsgemeinschaft (SFB 405) and the European Community (QLG1-CT-1999-00202).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank Ludmila Umansky, Christine Schmitt, Kathrin Frank, and Steven Song for excellent technical assistance; Klaus Hexel and Manuel Scheuermann for operating the FACSVantage SE flow cytometer; Alf Hamann for antibodies; and Douglas Hanahan for transgenic mice.