Abstract
G2A is a G-protein–coupled receptor (GPCR) involved in immune regulation. Previous studies have shown that lysophosphatidylcholine (LPC), a bioactive lipid associated with atherosclerosis and autoimmunity, acts through G2A to induce diverse biologic effects. Production of LPC during cell apoptosis serves as a chemotactic signal for macrophage recruitment. Here we demonstrate that macrophage chemotaxis to LPC is dependent on G2A function. Wild-type but not G2A-deficient mouse peritoneal macrophages migrated toward LPC. RNAi-mediated knockdown of G2A in J774A.1 macrophages abolished LPC-induced chemotaxis, whereas overexpression of G2A significantly enhanced this process. Mutation of the conserved DRY motif of G2A resulted in loss of chemotaxis to LPC, suggesting a requirement for G-protein signaling. Unlike most GPCRs, including the chemokine receptors, coupling to Gi is not required for LPC/G2A-mediated chemotaxis, but coupling to Gq/11 and G12/13 is necessary as judged by inhibition with dominant negative forms of these alpha subunits or with regulators of G-protein signaling (RGS) constructs. Collectively, these data establish that pertussis toxin–insensitive G2A signaling regulates macrophage chemotaxis to LPC. Defects in this signaling pathway may be related to the pathogenesis of systemic autoimmune disease.
Introduction
Precise control of immune cell trafficking is complex and essential to the development and function of the immune system. Many types of molecular controls regulate this process, but G-protein–coupled receptors are especially important. The network of chemokines and their receptors has been well studied1 ; however, recent developments have raised interest in nonpolypeptide regulators of immune cell trafficking belonging to a group of related molecules with a lysophospholipid-like structure.2 The mechanism of lysophospholipid-mediated cell migration recently has been shown to require the expression of specific GPCRs, such as the receptors for sphingosine 1-phosphate (S1P), lysophosphatidic acid (LPA), and LPC.3-5 The immunological importance of these lysophospholipid receptors is illustrated by the profound immunodeficiency observed in mice lacking expression of the S1P1 receptor in hematopoietic cells. S1P1-deficient T cells fail to egress from thymus and therefore are completely absent from the periphery.6 The novel immunosuppressive drug FTY720 has been shown to inhibit several S1P receptors, resulting in blocked egress of lymphocytes from lymph nodes and thymus.7,8
Genetic knockout of G2A, one of the receptors for LPC, is associated with a slowly progressive late-onset systemic autoimmune syndrome reminiscent of systemic lupus erythematosus.9 This syndrome is predominantly manifested in mice with the lupus-prone hybrid genetic background. Cell-autonomous enhancement of T-cell proliferation is observed in G2A-deficient mice and could contribute to the autoimmune phenotype. Given the broad expression pattern of G2A in cells of the innate and adaptive immune systems, it is conceivable that this receptor has pleiotropic effects. Macrophages represent an important cellular target for the chemotactic activity of LPC in atherosclerosis.10 Recently, Lauber et al11 showed that during programmed cell death a caspase-3–dependent mechanism activates phospholipase A2 and leads to LPC production, which in turn serves as a chemoattractive signal for phagocytes. Thus, LPC-induced recruitment of macrophages may be important for apoptotic cell clearance and atherogenesis. However, it is not known whether G2A plays a role in macrophage chemotaxis toward LPC and, if so, what G-protein coupling and downstream signals are important for this process.
Many chemotactic factors regulate cell migration via GPCRs. Previous studies have shown that Gi signaling is generally important for cell migration.1 Pertussis toxin (PTX), a specific inhibitor for the Gαi/o family, completely blocks cell migration induced by most chemokines. The complexity of this process is illustrated by the finding that Gαi itself does not seem to be required for cell migration. Instead, the free Gβγ subunit released from the Gi complex is necessary for the transduction of migratory signals.12,13 Recent studies also have shown that functional coupling to Gi is required for cell chemotaxis induced by lysophospholipids such as S1P and LPA.3,4 However, G-protein signaling for LPC/G2A-mediated cell migration has not been examined.
In the current study, we demonstrate that the expression of G2A is required for the chemotaxis of macrophages to LPC. Interestingly, in contrast to most chemokine receptors and other lysophospholipid GPCRs, LPC/G2A-mediated cell migration is transduced through Gi-independent pathways. This observation exemplifies a unique G-protein coupling in lysophospholipid-induced chemotactic signal transduction. Given the involvement of LPC and G2A in inflammatory processes and autoimmunity, LPC/G2A-mediated chemotaxis may play a role in self-antigen clearance and chronic inflammation including atherogenesis.
Materials and methods
Plasmid constructs
Construction of an siRNA specific to mouse G2A has been described previously.5 The retroviral expression construct was generated by cloning the open reading frame of mouse G2A cDNA into the MSCV-IRES-GFP vector. The G2A rescue construct was made by cloning the siRNA-resistant form of G2A5 into a modified pQCXIP vector under the control of the ubiquitin C promoter. The mouse G2A DRY motif mutant was generated by site-directed mutagenesis (Stratagene, La Jolla, CA) to change the residue D136 to A, and the mutated cDNA was cloned into MSCV-IRES-GFP vector. The cDNAs of human dominant-negative G proteins, Gαq Q209L/D277N, Gα11 Q209L/D277N, Gα12 Q231L/D299N, and Gα13 Q226L/D294N were purchased from the Guthrie cDNA Resource Center (Sayre, PA) and verified by DNA sequencing. These cDNAs were cloned into pQCXIP or pQCXIN retroviral vectors (Clontech, Palo Alto, CA). Myctagged bovine GRK2 RGS (1-178 residues) and human p115 RGS (1-252 residues) were kindly provided by Drs Tohru Kozasa and Richard Ye (University of Illinois at Chicago) and subcloned into the pQCXIN retroviral vector.
Reagents
Two types of LPC were used in the experiments with similar results: natural L-α-LPC (chicken egg extract, Avanti Polar Lipids, Alabaster, AL, Catalog #830071) dissolved in water as a 10 mM stock and synthetic 16:0 LPC (Avanti Polar Lipids, Catalog #855675P) dissolved in methanol as a 50 mM stock. Synthetic 14:0, 18:0, and 18:1 LPC also were purchased from Avanti and dissolved in methanol as a 50 mM stock. All lipid stocks were stored under nitrogen at –70°C in glass vials as single-use aliquots and used within a month. Human recombinant C5a (BioVision, Mountain View, CA) was resuspended at 10 μM in 0.2% bovine serum albumin (BSA) and stored at –70°C. Pertussis toxin from 2 different sources (Sigma, St Louis, MO and Calbiochem, San Diego, CA) was used in the experiments and showed the same results; 2′,5′-dideoxyadenosine (DDA) was purchased from Calbiochem and dissolved in dimethyl sulfoxide (DMSO).
Mouse primary macrophages
Wild-type and G2A-deficient9 C57BL/6 mice were intraperitoneally injected with 1.5 mL of 3% Brewer thioglycollate (Difco, Detroit, MI). Peritoneal macrophages were harvested as lavage by cold Hanks balanced salt solution (HBSS) 4 days later. About 85% of harvested cells were macrophages by CD11b and F4/80 flow cytometry staining. PTX treatment of these cells was performed with 100 ng/mL PTX in plastic petri dishes for 1 hour in a 37°C cell culture incubator. After PTX treatment, adherent cells were detached with 5 mM EDTA (ethylenediaminetetraacetic acid) and used directly or combined with suspension cells for further assay.
Cell culture
The mouse macrophage cell line J774A.1 (American Type Culture Collection, Manassas, VA) was cultured in Dulbecco modified Eagle medium (DMEM) with 10% fetal bovine serum (FBS) and 2 mM L-glutamine. Retroviral-infected J774A.1 cells either were marked with enhanced green fluorescent protein (EGFP) or contained puromycin or neomycin drug-resistant gene. Infected cells were sorted by fluorescence-activated cell-sorter (FACS) for EGFP-positive cells or selected with 2 μg/mL puromycin or 500 μg/mL G418.
Transmigration assay
J774A.1 cells were washed twice in serum-free migration buffer (DMEM with 0.1% fatty acid-free BSA and 10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH7.4) and resuspended at 1 × 106 cells/mL in migration buffer. 100 μL cells were added into the upper chambers of a 24-well transwell plate with 8-μm pore size polycarbonate filters (Costar, Corning, NY). The plate was equilibrated at 37°C in a 5% CO2 cell culture incubator for 30 minutes. Chemotactic factors were diluted to the indicated concentrations in serum-free migration buffer, and 600 μL of the mixture was added into the lower chambers of the transwells. After 2 hours at 37°C in a 5% CO2 cell culture incubator, the cells remaining in the upper chambers were wiped off with a cotton swab. Migrated cells attaching to the lower surface of the filters were fixed with 75% ethanol for 30 minutes, washed with water, and stained with hematoxylin. The number of migrated cells was counted under microscope. For each sample, cells in 5 randomly picked fields under 200 × magnification were counted. Migration of peritoneal macrophages was performed following the same procedure, except that these cells were resuspended at 2 × 106 cells/mL in migration buffer, and the assay was performed for 3 hours.
Zigmond chamber assay
Zigmond chambers14 were purchased from Neuro Probe (Gaithersburg, MD), and the assay was performed following the manufacturer's instruction. G2AHIGH J774A.1 cells were washed twice in migration buffer. A drop of cells was placed on the middle of a coverslip and incubated at 37°C for 30 minutes. After the cells adhered, the coverslip was inverted onto a grooved microscope slide so that the cell-covered portion was centered over the bridge, then 100 μLof 20 μM LPC or the control migration buffer was added into one of the 2 grooves, respectively, until they were full. The chamber was then incubated at 37°C for 30 minutes. Cell orientation and morphological changes were examined under a phase contrast microscope with a 20 ×/0.40 NA objective lens (Model ELWD 0.3; Nikon, Garden City, NY). The image was acquired using an Optronics digital camera (Model 60800) and Magna Fire 2.1 software (Optronics, Goleta, CA).
Western blot
Cells were lysed in radioimmunoprecipitation (RIPA) buffer with protease inhibitor cocktail (Roche Diagnostics, Basel, Switzerland). Cell lysates were mixed with sodium dodecyl sulfate (SDS)/2-mercaptoethanol sample buffer and loaded without boiling into a Novex 8%-16% Tris (tris(hydroxymethyl)aminomethane)-glycine gel (Invitrogen, Carlsbad, CA). Proteins in the gel then were transferred onto nitrocellular membranes and hybridized with a hamster monoclonal antibody against a mouse G2A amino-terminal epitope (1-40 amino acids).
Reverse transcriptase–polymerase chain reaction (RT-PCR)
RNA was extracted using RNAqueous 4 PCR kit (Ambion, Austin, TX). All RNA samples were treated with DNaseI to remove genomic DNA contamination. Reverse transcription was performed using SuperScript II kit (Invitrogen). The PCR reaction was performed using these gene-specific primers: Gαs 5′ GAGCTGGCCAACCCTGAGAA 3′ and 5′ CGATTTGCCAGCGAGGACTT 3′, Gαolf 5′ AACTCACCGCCTGCTGCTTC 3′ and 5′ CAGGCCGCCACGTAAATGAT 3′, Gαi1 5′ GGTGTGGGAGGGAGGAGTGA 3′ and 5′ GGCAGGTGCATCCAACCTCT 3′,Gαi2 5′ CGCCTTGAGCGCCTATGACT 3′ and 5′ GCAATCCTGCCAGGTCCACT 3′, Gαi3 5′ GAACCAGTGGGCTTGCTGCT 3′ and 5′ ATCCGAAAGCCGGATTGTGA 3′, Gαo 5′ ACCAGCCCACTGAGCAGGAC 3′ and 5′ GTTGGGTGAGCGGTTTTTGC 3′, Gαt1 5′ CAGCATCTGCTTCCCCGACT 3′ and 5′ TGGAATGGGGATCTGCTGGT 3′, Gαt2 5′ TGCTGGTGGAGGATGACGAA 3′ and 5′ TCACCAACAGGATGGGCTGA 3′, Gαz 5′ AAGCCGCGGTCTACATCCAA 3′ and 5′ GGCCAACTCAGGAAGGCAGA 3′, Gαq 5′ GCAGGGTGTGAACCGGAAAC 3′ and 5′ CGTTCGGCACAGTTTGCATC 3′, Gα11 5′ ACCGCATGGAGGAGAGCAAG 3′ and 5′ TCAGCTGCAGGATGGTGTCC 3′,Gα14 5′ CTCCTGCCTGGCTGTTCCAT 3′ and 5′ GCGCGCACAGTTCAAACAAC 3′,Gα15 5′ GCCTTCCGGCTGCTCATCTA 3′ and 5′ TCTCCTCCATGCGGTTCTCC 3′, Gα12 5′ AGCGCCAGAAGTGGTTCCAG 3′ and 5′ AGCAGAGGGAGGGGCTGTCT 3′, Gα13 5′ ACCACCGCGATCAACACAGA 3′ and 5′ GGGCCGAGACTCCTCCCTAA 3′ and β actin 5′ CACAGGCATTGTGATGGACT 3′ and 5′ CTTCTGCATCCTGTCAGCCAA 3′.
Results
Requirement of G2A for the chemotaxis of mouse primary macrophages to LPC
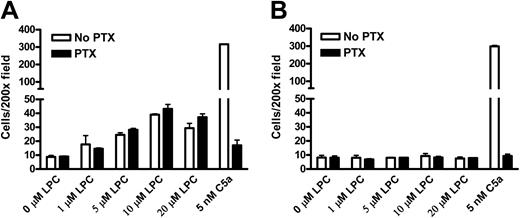
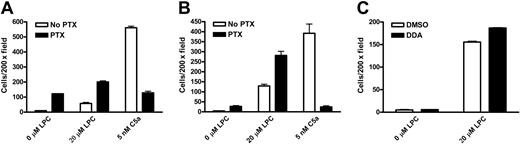
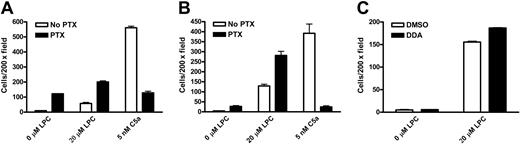
Chemotaxis of wild-type and G2A-deficient9 mouse peritoneal macrophages to LPC and the complement C5a was examined in transwell assays. Macrophage cells migrating through the polycarbonate membrane remained attached to the lower surface and were stained with hematoxylin and counted at 200 × magnification. Wild-type macrophages were chemotactic to different concentrations of LPC, with about 4-fold more migration above background at 10 μM (Figure 1A). In contrast, G2A–/– macrophages did not migrate to LPC (Figure 1B). As a control for general chemotactic capability, the migration of wild-type and G2A–/– macrophages to C5a was at the same level (Figure 1A,B). Absence of G2A expression in G2A–/– macrophages was verified by RT-PCR and Western blot (data not shown). These results demonstrate that the chemotaxis of mouse primary macrophages to LPC is dependent on G2A function. To assess whether LPC/G2A-mediated macrophage chemotaxis is transduced through Gi, we treated the cells with PTX, which adenosine diphosphate (ADP)–ribosylates the Gαi/o subunits to specifically inhibit Gi activation.1 Interestingly, unlike most chemoattractants, LPC-induced chemotaxis of mouse primary macrophages was resistant to PTX treatment (Figure 1A). As a control, chemotaxis of these cells to C5a was completely abolished (Figure 1A,B).
G2A is required for the chemotaxis of mouse peritoneal macrophages to LPC. Thioglycollate-elicited peritoneal macrophages were pretreated with 100 ng/mL PTX or control buffer (No PTX) at 37°C for 1 hour. Chemotaxis of these cells to LPC and C5a was assessed by transwell assays. Migrated macrophages crossing the polycarbonate membrane were stained and counted under microscope at 200 × magnification. (A) Migration of wild-type (WT) mouse peritoneal macrophages to LPC and C5a. (B) Migration of G2A-deficient (G2A–/–) mouse peritoneal macrophages to LPC and C5a. The results are representative of 5 independent experiments.
G2A is required for the chemotaxis of mouse peritoneal macrophages to LPC. Thioglycollate-elicited peritoneal macrophages were pretreated with 100 ng/mL PTX or control buffer (No PTX) at 37°C for 1 hour. Chemotaxis of these cells to LPC and C5a was assessed by transwell assays. Migrated macrophages crossing the polycarbonate membrane were stained and counted under microscope at 200 × magnification. (A) Migration of wild-type (WT) mouse peritoneal macrophages to LPC and C5a. (B) Migration of G2A-deficient (G2A–/–) mouse peritoneal macrophages to LPC and C5a. The results are representative of 5 independent experiments.
Genetic modulation of G2A expression in J774A.1 macrophage cell line
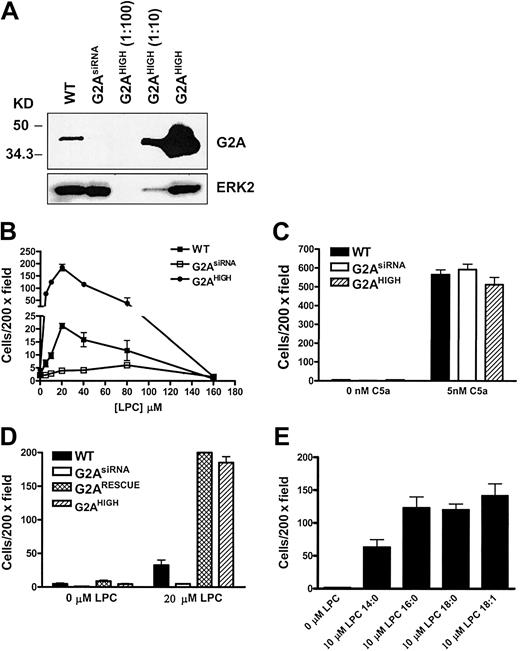
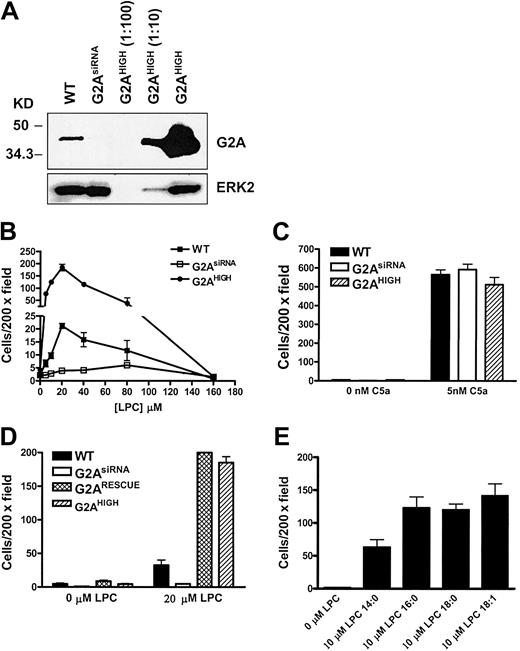
To further delineate G2A migratory signaling, the mouse J774A.1 macrophage cell line was chosen as an in vitro system to study LPC-induced chemotaxis. Small interfering RNA (siRNA) and retroviral overexpression were used to inhibit or enhance G2A expression, respectively. Expression of G2A siRNA was driven by a human H1 RNA promoter in a retroviral vector as previously reported.5 Overexpression of G2A was achieved by MSCV-G2A-IRES-GFP retroviral infection (named G2AHIGH J774A.1 cells). In J774A.1 cells transduced with G2A siRNA retrovirus (named G2AsiRNA J774A.1 cells), the expression of G2A was stably inhibited by more than 90% (Figure 2A). With retroviral overexpression, G2A protein was about 20-fold above the endogenous level (Figure 2A).
The requirement of G2A in J774A.1 cell chemotaxis to LPC. (A) Western blot of G2A expression in wild-type (WT), G2AsiRNA, and G2AHIGH J774A.1 cells. The lysate of G2AHIGH J774A.1 cells was diluted 10- and 100-fold before loading. (B) Comparison of LPC-induced chemotaxis of wild-type, G2AsiRNA, and G2AHIGH J774A.1 cells. Transwell migration assays were used to examine the role of G2A in J774A.1 cell chemotaxis to different concentrations of LPC. (C) As a control for cell motility, 5 nM C5a was used as a chemoattractant for J774A.1 cell migration under the same experimental condition. (D) Rescue of G2AsiRNA J774A.1 cell chemotaxis to LPC by reconstitution of G2A expression. An siRNA-resistant form of G2A cDNA was cloned into retroviral vector under the control of the ubiquitin C promoter, and G2AsiRNA J774A.1 cells were infected with this construct to reconstitute the expression of G2A. The chemotactic response to LPC of these cells (G2ARESCUE) was compared with wild-type, G2AsiRNA, and G2AHIGH J774A.1 cells. (E) Effects of different species of LPC on J774A.1 cell chemotaxis. LPC with different length of acyl chain was used as a chemoattractant to induce G2AHIGH J774A.1 cell migration. These results are representative of at least 3 independent experiments.
The requirement of G2A in J774A.1 cell chemotaxis to LPC. (A) Western blot of G2A expression in wild-type (WT), G2AsiRNA, and G2AHIGH J774A.1 cells. The lysate of G2AHIGH J774A.1 cells was diluted 10- and 100-fold before loading. (B) Comparison of LPC-induced chemotaxis of wild-type, G2AsiRNA, and G2AHIGH J774A.1 cells. Transwell migration assays were used to examine the role of G2A in J774A.1 cell chemotaxis to different concentrations of LPC. (C) As a control for cell motility, 5 nM C5a was used as a chemoattractant for J774A.1 cell migration under the same experimental condition. (D) Rescue of G2AsiRNA J774A.1 cell chemotaxis to LPC by reconstitution of G2A expression. An siRNA-resistant form of G2A cDNA was cloned into retroviral vector under the control of the ubiquitin C promoter, and G2AsiRNA J774A.1 cells were infected with this construct to reconstitute the expression of G2A. The chemotactic response to LPC of these cells (G2ARESCUE) was compared with wild-type, G2AsiRNA, and G2AHIGH J774A.1 cells. (E) Effects of different species of LPC on J774A.1 cell chemotaxis. LPC with different length of acyl chain was used as a chemoattractant to induce G2AHIGH J774A.1 cell migration. These results are representative of at least 3 independent experiments.
G2A expression is critical for the LPC-induced chemotaxis of J774A.1 macrophages
Migration of wild-type, G2AsiRNA, and G2AHIGH J774A.1 cells to LPC was evaluated by transwell assay. Wild-type and G2AHIGH J774A.1 cells showed a bell-shaped dose response to LPC with a maximum effect at about 20 to 30 μM in migration buffer (Figure 2B). However, when G2A expression was suppressed in G2AsiRNA J774A.1 cells, chemotaxis of these cells to LPC was significantly reduced (Figure 2B). When G2A was overexpressed, cell chemotaxis toward LPC increased by about 8-fold (Figure 2B). G2A overexpression alone did not confer migratory ability to the cells, because G2AHIGH J774A.1 cells did not migrate to the control buffer (0 μM LPC) (Figure 2B). To assess whether modulation of G2A expression affected overall cell motility, we examined the migration of wild-type, G2AsiRNA, and G2AHIGH J774A.1 cells to C5a, which induces chemotaxis through the C5a receptor.15 As shown in Figure 2C, the migration of these cells to C5a was not significantly affected by alteration in the expression of G2A.
The specificity of gene silencing by siRNA depends on sequence homology between the siRNA and the target sequence. To rule out the possibility of “off-target” gene silencing,16 an siRNA-resistant form of G2A5 was expressed in G2AsiRNA J774A.1 cells. Reconstitution of G2A rescued the chemotaxis of J774A.1 cells toward LPC to similar levels seen in G2AHIGH J774A.1 cells (Figure 2D), suggesting that the biologic effect observed in G2AsiRNA J774A.1 cells is specifically due to the loss of G2A expression. In conclusion, our results demonstrate that similar to mouse primary macrophages, LPC serves as a chemoattractant for J774A.1 macrophages, and G2A is required for LPC-induced chemotaxis of these cells.
Previous studies have demonstrated that LPC species with different acyl chain lengths have distinct binding affinities for G2A.17 Here we were prompted to examine whether binding affinity is correlated with functional activity in stimulating cell migration. The chemotactic effects of 14:0, 16:0, 18:0, and 18:1 LPC were tested in G2AHIGH J774A.1 cells. Compared with control medium, these forms of LPC induced significant cell chemotaxis. However, the activity of 14:0 LPC was about 50% lower than that of 16:0, 18:0, and 18:1 LPC (Figure 2E).
LPC induces J774A.1 cell chemotaxis but not chemokinesis
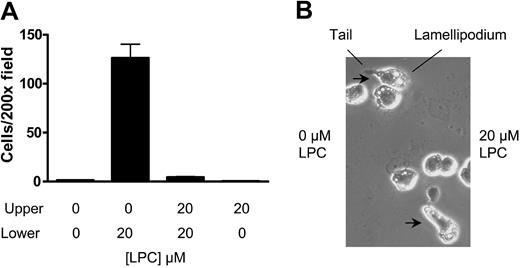
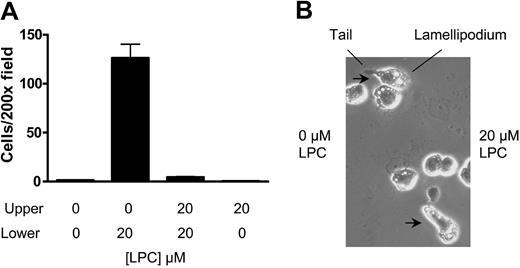
Chemotaxis and chemokinesis are 2 types of cell locomotion that exhibit directional and random movement, respectively.18 To assess whether LPC triggers chemotaxis or chemokinesis in J774A.1 cells, we added the same concentration of LPC to the upper chamber to abolish the chemical gradient. This treatment abrogated the migration of G2AHIGH J774A.1 cells to the lower chamber (Figure 3A). Moreover, adding LPC to the upper chamber alone did not trigger cell migration (Figure 3A). These results indicate that a concentration gradient of LPC is required for J774A.1 cell migration, and therefore the migration is of a chemotactic nature.
LPC induces J774A.1 cell chemotaxis but not chemokinesis. (A) An LPC gradient is required for J774A.1 cell migration. The migration of G2AHIGH J774A.1 cells was tested in transwell assay. LPC-induced J774A.1 cell migration was abolished by adding the same concentration of LPC into the upper chamber of the transwell. LPC alone in the upper chamber did not stimulate J774A.1 cell migration. The results are representative of 3 independent experiments. (B) Zigmond chamber assay. Morphological orientation of G2AHIGH J774A.1 cells toward the chemoattractant LPC was examined in a Zigmond chamber. Migrating cells with extending lamellipodia and retracting tails were observed.
LPC induces J774A.1 cell chemotaxis but not chemokinesis. (A) An LPC gradient is required for J774A.1 cell migration. The migration of G2AHIGH J774A.1 cells was tested in transwell assay. LPC-induced J774A.1 cell migration was abolished by adding the same concentration of LPC into the upper chamber of the transwell. LPC alone in the upper chamber did not stimulate J774A.1 cell migration. The results are representative of 3 independent experiments. (B) Zigmond chamber assay. Morphological orientation of G2AHIGH J774A.1 cells toward the chemoattractant LPC was examined in a Zigmond chamber. Migrating cells with extending lamellipodia and retracting tails were observed.
To directly visualize LPC-induced chemotaxis, cell orientation to LPC was observed microscopically in a Zigmond chamber.14 As shown in Figure 3B, G2AHIGH J774A.1 cells were orientated to the chemoattractant, with the leading edge of the lamellipodium facing toward LPC and the retracting tail on the side of control medium.
G-protein signaling is important for LPC/G2A-mediated chemotaxis
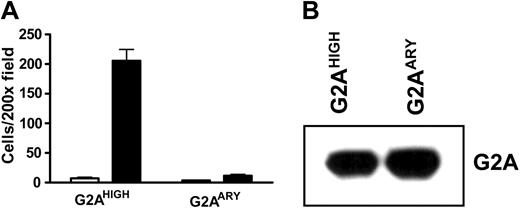
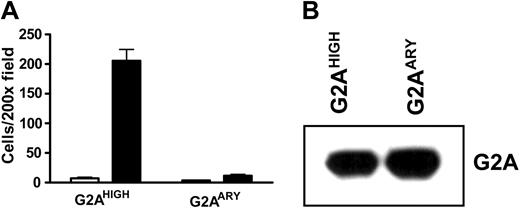
GPCRs generally transduce signals through heterotrimeric G-protein complexes. However, some biologic processes are mediated through G-protein–independent pathways, such as the activation of mitogen-activated protein (MAP) kinases through arrestin-Src complexes from the β2 adrenergic receptor.19 To examine whether G-protein signaling is important for LPC/G2A-mediated chemotaxis, we generated a DRY motif mutant of G2A, with a DRY to ARY mutation. As reported, the DRY motif of GPCRs is important for G-protein coupling, activation, and downstream signal transduction.20,21 Overexpression of the DRY mutant G2A did not stimulate J774A.1 cell migration to LPC (Figure 4A); this defect was not due to reduced protein expression levels (Figure 4B). These results indicate that G-protein signaling is important for LPC/G2A-mediated cell chemotaxis.
G-protein signaling in LPC/G2A-mediated chemotaxis. (A) Chemotaxis of J774A.1 cells overexpressing wild-type (G2AHIGH) or DRY mutant G2A (G2AARY). J774A.1 cells were transduced with wild-type or DRY mutant (DRY to ARY) G2A to overexpress these proteins. Effects of the mutation were assessed by examining cell migration toward LPC. The results are representative of 4 independent experiments. (B) Western blot of G2A proteins. Wild-type and DRY mutant G2A proteins were detected at similar level with G2A antibody.
G-protein signaling in LPC/G2A-mediated chemotaxis. (A) Chemotaxis of J774A.1 cells overexpressing wild-type (G2AHIGH) or DRY mutant G2A (G2AARY). J774A.1 cells were transduced with wild-type or DRY mutant (DRY to ARY) G2A to overexpress these proteins. Effects of the mutation were assessed by examining cell migration toward LPC. The results are representative of 4 independent experiments. (B) Western blot of G2A proteins. Wild-type and DRY mutant G2A proteins were detected at similar level with G2A antibody.
Gi and Gs signaling in LPC/G2A-mediated chemotaxis
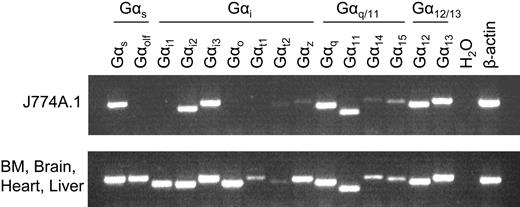
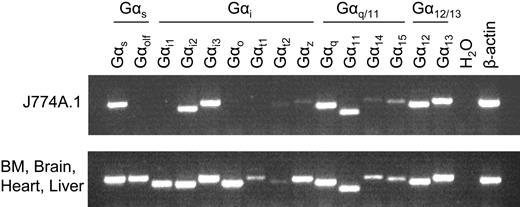
To further identify which types of G-protein coupling are important for LPC/G2A-mediated chemotaxis, we used RT-PCR to profile the expression of Gα genes in J774A.1 cells. Based on sequence homology, Gα genes can be divided into 4 subfamilies: Gαs, Gαi, Gαq/11, and Gα12/13. RT-PCR results showed that J774A.1 cells express mRNA of representative members from all 4 Gα subfamilies, predominantly Gαs, Gαi2, Gαi3, Gαq, Gα11, Gα12, and Gα13 (Figure 5).
Expression profile of Gα genes in J774A.1 cells. RNA was extracted from wild-type J774A.1 cells and treated with DNaseI to remove genomic DNA contamination. Gα gene expression in J774A.1 cells was examined by RT-PCR using gene-specific primers. The cDNA mixture of bone marrow, brain, heart, and liver was used as a positive control.
Expression profile of Gα genes in J774A.1 cells. RNA was extracted from wild-type J774A.1 cells and treated with DNaseI to remove genomic DNA contamination. Gα gene expression in J774A.1 cells was examined by RT-PCR using gene-specific primers. The cDNA mixture of bone marrow, brain, heart, and liver was used as a positive control.
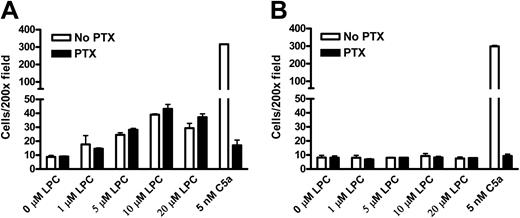
Gi signaling is necessary for cell chemotaxis induced by a variety of chemoattractants.1 Wild-type and G2AHIGH J774A.1 cells were pretreated with PTX, and chemotaxis to LPC and C5a was compared. Consistent with previous studies,13 the chemotaxis of J774A.1 cells to C5a was completely blocked by PTX treatment (Figure 6A,B). In contrast, PTX treatment did not inhibit the chemotaxis of wild-type and G2AHIGH J774A.1 cells toward LPC (Figure 6A,B), which corroborates with the results in mouse primary macrophages (Figure 1). We also noticed that the migration of J774A.1 cells toward the control buffer and LPC was enhanced upon PTX treatment (Figure 6A,B). Similar observations that PTX treatment increases cell migration have been reported previously,22,23 but the exact mechanisms are not known, which may be due to an increase of basal motility or an inhibitory role of Gi in cell migration in these cells. In addition to mouse primary and J774A.1 macrophages, we also showed that LPC/G2A-mediated chemotaxis of DO11.10 T cells and G2A-overexpressing U937 monoblastic cells was resistant to PTX treatment (Figure S1, available on the Blood website; see the Supplemental Figure link at the top of the online article). These results suggest that LPC, distinct from most chemoattractants, induces J774A.1 cell migration through Gi-independent pathways.
Gi and Gs signaling in LPC/G2A-mediated chemotaxis. (A, B) Effects of pertussis toxin on chemotaxis of wild-type and G2AHIGH J774A.1 cells to LPC and C5a. J774A.1 cells were pretreated with 100 ng/mL PTX for 16 hours. 20 μM LPC was used as a chemoattractant to induce migration of PTX-treated or untreated J774 cells. Chemotaxis toward C5a was used as a control that is sensitive to PTX. The results are representative of 9 independent experiments. (C) Gs signaling in LPC/G2A-mediated chemotaxis. G2AHIGH J774 cells were pretreated with 100 μM of2′,5′-dideoxyadenosine (DDA) for 1 hour to specifically inhibit adenylate cyclase or with the same volume of DMSO as a control. 20 μM LPC was used as a chemoattractant to induce migration of DDA-treated or untreated J774 cells. The results are representative of 3 independent experiments.
Gi and Gs signaling in LPC/G2A-mediated chemotaxis. (A, B) Effects of pertussis toxin on chemotaxis of wild-type and G2AHIGH J774A.1 cells to LPC and C5a. J774A.1 cells were pretreated with 100 ng/mL PTX for 16 hours. 20 μM LPC was used as a chemoattractant to induce migration of PTX-treated or untreated J774 cells. Chemotaxis toward C5a was used as a control that is sensitive to PTX. The results are representative of 9 independent experiments. (C) Gs signaling in LPC/G2A-mediated chemotaxis. G2AHIGH J774 cells were pretreated with 100 μM of2′,5′-dideoxyadenosine (DDA) for 1 hour to specifically inhibit adenylate cyclase or with the same volume of DMSO as a control. 20 μM LPC was used as a chemoattractant to induce migration of DDA-treated or untreated J774 cells. The results are representative of 3 independent experiments.
To assess whether Gs-mediated cAMP elevation is involved in LPC/G2A-induced chemotaxis, we treated G2AHIGH J774A.1 cells with 2′,5′-dideoxyadenosine (DDA) to specifically inhibit adenylate cyclase.24 As previously reported,25 preincubation with 100 μM DDA decreased the basal level of intracellular cAMP measured by cAMP enzymeimmunoassay kit from Amersham Pharmacia Biotech (Piscataway, NJ) (data not shown). However, DDA treatment did not block the chemotaxis of J774A.1 cells to LPC (Figure 6C), suggesting that cAMP increase by Gs signaling is not necessary for this process. But the results do not exclude that other downstream signals triggered by Gs might be involved in LPC/G2A-mediated chemotaxis.
Coupling of LPC/G2A-mediated chemotaxis to Gq/11 and G12/13
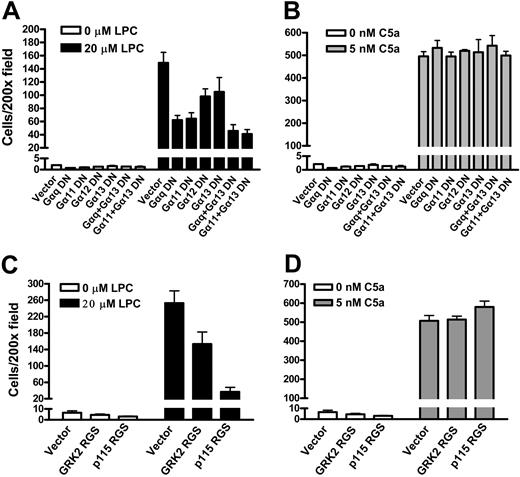
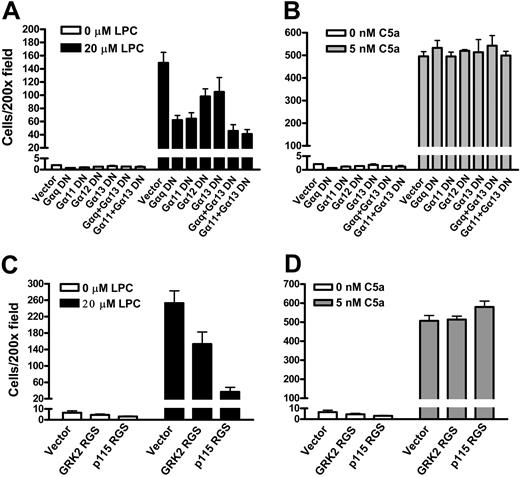
Two different approaches were used to examine whether Gq/11 or G12/13 signaling is associated with LPC/G2A-mediated cell migration. G2AHIGH J774A.1 cells were infected with dominant negative human Gα subunits26,27 or with regulators of G-protein signaling (RGS) constructs.28,29 The dominant negative xanthine nucleotide-binding Gα mutants can bind to cognate receptors but cannot bind GTP or Gβγ and therefore block the receptor-mediated G-protein signaling.26 RGS domains of GRK2 and p115 negatively regulate Gq/11 and G12/13 signaling, respectively, by acting as GTPase-activating proteins.28,29 Northern blot analysis showed that the expression of these dominant negative G-protein mRNAs was about 3- to 10-fold higher than the corresponding endogenous G proteins (data not shown). As shown in Figure 7A, expression of dominant negative mutants of Gαq/11 or Gα12/13 inhibited the migration of G2AHIGH J774A.1 cells to LPC, and the inhibition of both Gq/11 and G12/13 led to further reduction. As a control, the migration of G2AHIGH J774A.1 cells to C5a was not altered by Gαq/11 or Gα12/13 dominant-negative mutants (Figure 7B). Similarly, expression of GRK2 and p115 RGS constructs resulted in a decrease of G2AHIGH J774A.1 cell migration to LPC (Figure 7C), but chemotaxis of these cells to C5a was not affected (Figure 7D). These results suggest that Gq/11 and G12/13 signaling is important for LPC/G2A-mediated J774A.1 cell chemotaxis.
Gq/11 and G12/13 signaling in LPC/G2A-mediated chemotaxis. (A) Effects of Gα dominant-negative mutants on J774A.1 cell migration to LPC. G2AHIGH J774A.1 cells were transduced with dominant negative Gα subunits, Gαq Q209L/D277N, Gα11 Q209L/D277N, Gα12 Q231L/D299N, and Gα13 Q226L/D294N to specifically inhibit Gq/11 and G12/13 signaling. Migration of these cells to 20 μM LPC was compared. The results are representative of 3 independent experiments. (B) Effects of Gα dominant negative mutants on J774A.1 cell migration to C5a. (C) Inhibition of J774A.1 cell migration to LPC by RGS constructs. G2AHIGH J774A.1 cells were infected with GRK2 RGS or p115 RGS, and chemotaxis to LPC was examined. The results are representative of 3 independent experiments. (D) Effects of RGS constructs on C5a induced J774A.1 cell migration.
Gq/11 and G12/13 signaling in LPC/G2A-mediated chemotaxis. (A) Effects of Gα dominant-negative mutants on J774A.1 cell migration to LPC. G2AHIGH J774A.1 cells were transduced with dominant negative Gα subunits, Gαq Q209L/D277N, Gα11 Q209L/D277N, Gα12 Q231L/D299N, and Gα13 Q226L/D294N to specifically inhibit Gq/11 and G12/13 signaling. Migration of these cells to 20 μM LPC was compared. The results are representative of 3 independent experiments. (B) Effects of Gα dominant negative mutants on J774A.1 cell migration to C5a. (C) Inhibition of J774A.1 cell migration to LPC by RGS constructs. G2AHIGH J774A.1 cells were infected with GRK2 RGS or p115 RGS, and chemotaxis to LPC was examined. The results are representative of 3 independent experiments. (D) Effects of RGS constructs on C5a induced J774A.1 cell migration.
Discussion
G2A and LPC-induced cell chemotaxis
LPC can stimulate chemotaxis of T lymphocytes,30 macrophages,10 vascular smooth muscle cells,31 and mesangial cells.32 However, molecular mechanisms of LPC-induced chemotaxis have just begun to be elucidated. G2A has been identified as a receptor for LPC, and Jurkat T cells overexpressing G2A exhibit chemotaxis to LPC.17 More recently, siRNA silencing and retroviral overexpression of G2A in the mouse DO11.10 T-cell hybridoma demonstrated the requirement of G2A in LPC-induced T-cell migration.5 In this report, we show that the chemotaxis of mouse primary and J774A.1 macrophages to LPC is dependent on G2A expression and that the G2A-mediated cell migratory signals are transduced through Gi-independent pathways. Taken together, these results demonstrate that G2A is required for LPC-induced chemotaxis of immune cells.
In addition to immune cells, LPC also can induce chemotaxis of other cell types, such as vascular smooth muscle cells.31 However, the expression level of G2A mRNA is very low in vascular smooth muscle cells as measured by RT-PCR,33 suggesting that a different receptor or mechanism may be required for LPC-induced chemotaxis of these cells. Although the identity of the receptor(s) remains elusive, GPR4 may be a candidate. Previous studies have shown that GPR4 is another receptor for LPC at lower affinity, and LPC and sphingosylphosphorylcholine (SPC) induce cell chemotaxis in a GPR4-dependent manner in Swiss 3T3 cells overexpressing GPR4.34 While a recent report argues against SPC and LPC as ligands for GPR4,35 another line of evidence from competition binding assays shows that LPC binds to microvascular endothelial cells where GPR4 but not G2A is expressed.36 Given the distinct expression patterns of G2A and GPR4, one may speculate that these 2 receptors are responsible for LPC-induced chemotaxis differentially in various cell contexts. In this respect, it is interesting to note that although expression of GPR4 is lower than G2A in mouse primary and J774A.1 macrophages by RT-PCR (L.V.Y. and O.N.W., unpublished observations, June 2003), its expression level is similar to G2A in the human THP-1 monocytic cell line and human primary monocytes and macrophages as measured by quantitative RT-PCR.11
Although it has been shown previously that high level of G2A overexpression can exert some biologic activities without exogenous addition of ligand37,38 or in a ligand-independent manner,39 G2A-mediated cell chemotaxis is ligand-dependent. Overexpression of G2A alone is not sufficient to trigger cell migration in the absence of LPC (Figure 2B). Additionally, G2A-mediated chemotaxis is sensitive to variations in the acyl chain lengths of LPC. While 16:0, 18:0, and 18:1 LPC show similar levels of activity in stimulating cell migration, 14:0 LPC is about 50% less active (Figure 2E). As previously reported,17 14:0 LPC fails to compete the binding of [3H]-16:0 LPC to G2A, but 16:0, 18:0, and 18:1 LPCs are potent competitors with similar binding affinities. These results suggest that the biologic activity of different LPC species in chemotaxis may be correlated with their receptor binding affinities.
Intriguingly, recent studies demonstrated that G2A, as well as 3 related GPCRs—OGR1, GPR4, and TDAG8—can sense pH change to elicit downstream biologic activities such as inositol phosphate (IP) and cAMP formation.40-42 LPC functions like an antagonist to decrease the pH-dependent activation of G2A in the IP assay.41 A histidine (H174) in the human receptor plays a role in the proton-sensing function of G2A.41 It appears that G2A is responsive to 2 different signals: LPC and protons. We previously showed that change of pH alone does not induce DO11.10 cell migration, and LPC-mediated chemotaxis is not significantly affected by pH from 7.0 to 8.0 but reduced at pH 6.5.5 Based on these observations, we speculate that LPC and protons modulate G2A conformational change and activities through putative LPC binding sites and histidines, respectively, and then trigger distinct downstream events such as chemotaxis and IP accumulation. Crosstalk between the actions of LPC and protons on G2A may exist.
Gi-independent pathways in cell migration
Directional chemotaxis of immune cells to sites of inflammation or targeted cells is regulated by a variety of molecules including chemokines, lipid metabolites, extracellular nucleotides, complement factors, and bacterial components.1,43-45 In many cases, GPCRs serve as sensors to detect and transduce the chemotactic signals. Binding of chemoattractants to GPCRs activates the receptors and triggers the dissociation of heterotrimeric G-protein complexes to release GTP-bound Gα subunit and free Gβγ subunit, which in turn elicit downstream migratory signaling. Based on a large body of research evaluating chemokines, lipids, and other chemoattractants, Gi signaling has been commonly linked to chemotaxis. Cell migration induced by most chemoattractants is blocked by treatment with PTX acting to inhibit Gαi/o.1
Despite the prevailing view of the requirement of Gi signaling in GPCR-mediated chemotaxis, exceptions have been reported. For example, chemotaxis induced by monocyte chemotactic protein-2 (MCP-2) and macrophage inflammatory protein-1α (MIP-1α) is resistant to PTX in human monocytes and interleukin-2–activated natural killer (IANK) cells, respectively.22,46 Some evidence suggests that other G proteins, in addition to Gi, are involved in cell migration. As shown by al-Aoukaty et al,47 MIP-1α–induced IANK cell migration is inhibited by permeabilization with streptolysin-O and then introduction of antibodies against Gs,Go, and Gz into these cells. It also is reported that Gq/11 and Gβγ-mediated pathways are required for human umbilical vein endothelial cell (HUVEC) cell migration stimulated by vascular endothelial growth factor (VEGF).48 G-protein coupling for chemotaxis also may be cell-type dependent. For example, S1P and LPA-induced chemotaxis is resistant to PTX in T-helper 1 cells,23 while it is sensitive to PTX in many other cell types.3,4
G2A has been identified as a new member of a GPCR subfamily recognizing bioactive lipids as their ligands. These bioactive lipids, including LPA, S1P, platelet-activating factor (PAF), and leukotrienes, can induce cell migration through PTX-sensitive Gi-dependent pathways in many cell types.3,4,49 Distinct from other lipid chemoattractants, LPC/G2A-mediated chemotaxis is resistant to PTX treatment. By using dominant-negative forms of Gα subunits and RGS constructs, we have demonstrated that Gq/11 and G12/13 are involved in LPC/G2A-mediated cell movement. Interestingly, it has been shown that G2A couples promiscuously with various G proteins during different biologic processes in different cell types. For example, Gi coupling for calcium flux in MCF10A cells and extracellular signal-regulated kinase (ERK) activation in Chinese hamster ovary (CHO) cells,17 G13 coupling for stress fiber formation in fibroblasts,38 Gq and G13 coupling for NF-κB activation, and Gs and G13 coupling for apoptosis in Hela cells39 all have been reported in these ectopic overexpression systems. While important for calcium flux and ERK activation, Gi coupling is not required for LPC/G2A-mediated chemotaxis. These observations illustrate the diversity of G-protein coupling in GPCR-mediated cell migration, which may be important for the control of specificity and efficacy of chemotactic signaling induced by a variety of chemoattractants.
Recent studies have shown that the PTX-sensitive chemoattractant formyl-methionyl-leucine-phenylalanine (fMLP) acts on different G proteins to trigger 2 divergent “frontness” and “backness” signaling pathways necessary for neutrophil polarity and chemotaxis.50 The “frontness” signaling is transduced via Gi, PI3Ps, Rac, and F-actin, and the “backness” signaling is mediated through G12/13, Rho, p160-ROCK, and myosin.50 Although we have not delineated the molecular mechanisms downstream of Gq/11 and G12/13 in LPC/G2A-mediated chemotaxis, one may speculate that G12/13 coupling is similarly involved in the “backness” pathway, and Gq/11 is involved in the “frontness” pathway. In this respect, Gq/11 has been shown to transmit signals to activate PI3K.51,52 Furthermore, inhibition of PI3K with LY294002 reduces LPC/G2A-mediated chemotaxis (L.V.Y. and O.N.W., unpublished observations, October 2003).
Potential roles of LPC/G2A-mediated chemotaxis in apoptotic cell clearance and atherogenesis
The demonstration that macrophages require G2A for sensing chemotactic LPC gradients may potentially connect this GPCR to a number of physiological and pathological processes. LPC is a major lysolipid component of oxidized low-density lipoprotein (oxLDL) particles that accumulate in atherosclerotic lesions and contribute to macrophage recruitment.10,31,53 LPC also has been identified as an early chemotactic signal released by apoptotic cells to attract phagocytes.11,54 A connection between defective apoptotic cell clearance and accelerated autoimmunity and atherosclerotic plaque formation recently has been documented in a mouse model generated by crossing atherosclerosis-susceptible apoE–/– mice with autoimmune-prone gld mice.55 Continuous infusion of LPC further impairs apoptotic cell clearance in these mice.55 These observations suggest that LPC and G2A may play a role in the complex interaction between apoptotic cell clearance, autoimmunity, and atherogenesis.
In conclusion, we have demonstrated that G2A is required for LPC-induced chemotaxis of macrophage cells and that the signaling for LPC/G2A-mediated chemotaxis is transduced through Gi-independent pathways in these cells. Given the involvement of LPC and G2A in autoimmunity, atherosclerosis, and inflammation, further studies are warranted to determine the role of LPC/G2A interaction in these pathological processes.
Prepublished online as Blood First Edition Paper, September 21, 2004; DOI 10.1182/blood-2004-05-1916.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Donghui Chen, James Johnson, and Shirley Quan for excellent technical assistance; Drs Tohru Kozasa and Richard Ye for kindly providing RGS constructs; Drs Mel Simon, Jong-Ik Hwang, Amar Nijagal, and Gregory Ferl for critically reading the manuscript; and Barbara Anderson for help with manuscript preparation.
L.V.Y. is an associate of the Howard Hughes Medical Institute. C.G.R. is supported by a fellowship from the Cancer Research Institute. L.W. is a fellow of the Leukemia and Lymphoma Society. O.N.W. is an investigator of the Howard Hughes Medical Institute.
We gratefully acknowledge support from the Plum Foundation.