Abstract
Tumor antigen–specific T-cell tolerance imposes a significant barrier to the development of effective therapeutic cancer vaccines. Bone marrow–derived antigen-presenting cells (APCs) are critical in the induction of this unresponsive state. Here we show that in vitro treatment of APCs with the tyrosine kinase inhibitor, imatinib mesylate (STI-571), enhances the activation of naive antigen-specific T cells and restores the responsiveness of tolerant T cells from tumor-bearing hosts. Furthermore, in vivo treatment with STI-571 not only prevented the induction of tolerance in tumor-specific CD4+ T cells, preserving their responsiveness to a subsequent immunization, but also resulted in enhanced vaccine efficacy. These findings demonstrate that tolerance to tumor antigens is not an insurmountable obstacle and points to modulation of APC function as a promising strategy in the immunotherapy of cancer.
Introduction
A fundamental problem in the field of cancer immunotherapy relates to the ability of tumor cells to induce T-cell tolerance.1,2 The induction of this state of unresponsiveness is antigen specific, occurs early during the progression of either hematologic or solid malignancies, and imposes a remarkable barrier to effective immunization of the tumor-bearing host.3,4 More recent studies using bone marrow chimeras have unambiguously demonstrated that antigen processing and presentation by bone marrow–derived antigen-presenting cells (APCs) represent the dominant mechanisms in the induction of antigen-specific CD4+ T-cell tolerance in vivo.5,6
The requirement for APCs in the induction of T-cell tolerance to tumor antigens, together with their well-known role in priming antitumor T-cell responses, not only has placed these cells at the crossroads of immune tolerance versus immune activation, but also points to therapeutic manipulation of APCs as an enticing strategy to modulate T-cell responses against tumors. Defining the intracellular mechanism(s) by which APCs can induce unresponsiveness or activation of antitumor T-cell responses has become therefore a critical step for the continued development of effective cancer immunotherapy.
Recently, a number of studies have provided evidence that tyrosine phosphorylation of intracellular targets in APCs may play an important role in determining T-cell activation versus T-cell priming in response to cognate antigen. For instance, Lu and Lemke have demonstrated that APCs genetically devoid of the Tyro-3 family of receptor tyrosine kinases, Tyro-3, Axl, and Mer, display increased expression of major histocompatibility complex (MHC) class II molecules, produce elevated amounts of interleukin-12 (IL-12), and induce strong lymphocyte activation in vivo.7 Similarly, using the tyrosine kinase inhibitor, Tyrphostin AG490, we have found that in vitro inhibition of signal transducer and activator of transcription 3 (Stat3) in dendritic cells (DCs) and macrophages resulted in inflammatory cells capable of effectively priming naive antigen-specific T cells and restored the responsiveness of anergic CD4+ T cells from tumor-bearing hosts. Conversely, increased phosphorylation of Stat3 in APCs led to an opposite effect (ie, impaired antigen-specific T-cell responses to cognate antigen).8 Given these findings, APC function, and more specifically the ability to prime rather than tolerize tumor-specific T cells, might very well be amenable for modulation with pharmacologic agents targeting tyrosine phosphorylation of intracellular targets in APCs.
Imatinib mesylate (STI-571) is a well-known inhibitor of the c-abl, bcr-abl, c-kit, and platelet-derived growth factor receptor (PDGFR) tyrosine kinases. Several studies in experimental tumor models as well as in recently completed clinical trials have shown that STI-571 is highly effective in blocking the tyrosine kinase activity of the bcr/abl fusion protein, leading to remarkable hematologic as well as cytogenetic remissions in patients with chronic myelogenous leukemia (CML).9,10 Because of its additional inhibitory activity upon c-kit11 and PDGFR,12 this drug is also being evaluated in clinical trials for patients with tumors displaying aberrant activation of these signaling pathways. This potent tyrosine kinase inhibitory effect of STI-571, together with the emerging view that tyrosine phosphorylation of intracellular targets can negatively regulate immune responses, led us therefore to evaluate the in vitro and in vivo effects of this drug on APCs' function and antigen-specific T-cell responses to cognate antigen.
Materials and methods
Mice
Male BALB/c mice (6- to 8-weeks old) were obtained from the National Institutes of Health (Frederick, MD). T-cell receptor (TCR) transgenic mice expressing an αβ T-cell receptor specific for amino acids 110 to 120 from influenza hemagglutinin presented by I-Ed were a generous gift of H. von Boehmer (Harvard University).13 Transgenic mice used in these experiments were heterozygous for the transgene. All experiments involving the use of mice were performed in accordance with protocols approved by the Animal Care and Use Committee of the University of South Florida College of Medicine.
Tumor cells
A20 lymphoma cells expressing hemagglutinin (HA) as a model tumor antigen (A20HA) were selected and grown in vitro as previously reported.3 For in vivo tumor challenge experiments, transfected tumor cells were washed in sterile Hanks buffered salt solution (HBSS) and injected via the tail vein into BALB/c mice, in a total volume of 0.2 mL, 1 × 106 tumor cells per mouse.
Isolation of peritoneal elicited macrophages (PEMs)
BALB/c mice were injected intraperitoneally with 1 mL thioglycollate (DIFCO Laboratories, Detroit, MI). Then 4 days later, PEMs were obtained by peritoneal lavage as previously described.14
Generation of bone marrow–derived dendritic cells
Generation of DCs was performed as described before.15 Briefly, DCs were generated from murine bone marrows using RPMI 1640 medium supplemented with 10% fetal calf serum (FCS), 20 ng/mL murine recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF), and 10 ng/mL IL-4 (both from RDI, Flanders, NJ). The cultures were maintained at 37°C in 5% CO2-humidified atmosphere in 24-well plates. On day 3 of culture, floating cells were gently removed and fresh medium with cytokines was replaced. On day 5, cells were collected and DCs were enriched by centrifugation over a 13.5% metrizamide gradient (Accurate Chemicals, Westbury, NY). The purity of the DC fraction was higher than 80% as determined by fluorescence-activated cell sorter (FACS) analysis of surface molecule expression.
Antigen-presentation studies
PEMs (1 × 105/well) or BM-derived DCs (1 × 104/well) were cultured with 5 × 104 purified T cells isolated from either the spleen of TCR transgenic mice (naive T cells) or the spleen of A20HA-bearing mice previously transferred with anti-HA CD4+ T cells (tolerized T cells). HA-peptide110-120SFERFEIFPKE or irrelevant OVA-peptide323-339 (ISQAVHAAHAEINEAGR) was then added—or not—to the APC–T-cell cultures. After 48 hours, supernatants were collected and stored at –70°C until assayed for IL-2 and interferon-gamma (IFN-gamma) production by enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN). Values for T cells cultured with irrelevant peptide are usually less than 5% of the values for antigen-stimulated T cells. Data from T cells cultured with media alone or with cognate HA-peptide are displayed as the amount of cytokine produced by 100 clonotype-positive T cells/well.
Treatment with imatinib mesylate (STI-571)
For in vitro studies, STI-571 (kindly provided by Dr E. Buchdunger, Novartis Pharma, Basel, Switzerland) was diluted in HBSS to the concentrations indicated under each experimental design. PEMs or BM-derived DCs were incubated with STI-571 in the presence or not of lipopolysaccharide (LPS) for 24 hours. Then, supernatants were collected and purified T cells were added to the APC culture as indicated above. Collected supernatants were used for determination by ELISA of the levels of IL-1α, tumor necrosis factor α (TNF-α) regulated on activation normal T cell expressed and secreted (RANTES), IL-4, IL-6, IL-10, and IL-18 produced by APCs.
For in vivo studies, the following experimental design was used: single-cell suspensions were made from peripheral lymph nodes and spleen that were harvested from TCR transgenic donors. The percentage of lymphocytes double positive for CD4 and the clonotypic TCR was determined by flow cytometry as described under “Flow cytometric analysis.” Cells were washed 3 times in sterile Hanks balanced salt solution (HBSS), and injected into the tail vein of tumor-free or A20HA lymphoma–bearing recipients such that a total of 2.5 × 106 CD4+ anti-HA TCR+ T cells was transferred to each recipient. Half the mice in each group then received treatment with STI-571 (12.5 mg/kg intraperitoneally per day) for 10 days. On day +9 after T-cell transfer, all the animals were immunized subcutaneously with 1 × 107 plaque-forming units (pfu) of a recombinant vaccinia virus encoding influenza hemagglutinin (vacc-HA). In all experiments, 4 mice per subgroup were used and mice were analyzed individually. Mice were killed 6 days after immunization and spleen cells were obtained by passage over nylon mesh and centrifugation on a Ficoll gradient (Ficoll-Paque; Pharmacia Biotech, Uppsala, Sweden). Then, splenocytes were passed over nylon wool for enrichment of T cells. Optimization of this technique has allowed us to obtain at least 5 × 106 highly purified T cells per spleen, an amount sufficient to perform the following studies:
Flow cytometric analysis. T cells were stained with fluorescein isothiocyanate (FITC)–conjugated goat antimouse CD4 (Caltag, Burlingame, CA) and biotinylated rat anticlonotypic TCR monoclonal antibody (MAb) 6.5 followed by phycoerythrin (PE)–conjugated streptavidin (Caltag). Gated events (50 000) were collected on a FACSCAN (Becton Dickinson, San Jose, CA) and analyzed using FlowJo software (Treestar, Ashland, OR). Background staining of splenocytes or lymph node cells from naive BALB/c mice is routinely less than 0.10%.
Cytokine release. Purified T cells (5 × 104 /well) isolated from the different experimental groups were mixed with fresh splenocytes (1 × 105/well) from naive BALB/c mice, to which 12.5 μg/mL of synthetic HA peptide was or was not added. Then 48 hours later, supernatants were collected and stored at –70°C until assayed for IL-2 and IFN-gamma by ELISA (R&D Systems). Values for T cells cultured in media alone were less than 10% of the values for HA-stimulated T cells. Data are displayed as picograms per milliliter of the specific cytokine/100 clonotype+ T cells per well.
For analysis of the NK-cell, B-cell, and myeloid populations in the spleen of STI-571–treated mice or untreated controls, we followed an experimental design similar to the one described under “Treatment with imatinib mesylate (STI-571),” the only difference being that no T-cell purification was performed. Briefly, mice were killed on the day of analysis and their spleens were harvested. Splenocytes (1 × 106) were then stained with PE-conjugated antimouse NK1.1 or PE-conjugated antimouse B220, along with APC-conjugated antimouse CD11b and FITC-conjugated antimouse CD11c (all antibodies from BD Pharmingen, San Diego, CA). Events (50 000) were collected on a FACSCAN (Becton Dickinson) and analyzed using FlowJo software.
In vivo priming with vacc-HA
A recombinant vaccinia virus encoding hemagglutinin from the 1934 PR8 strain of influenza was a generous gift of Dr Hyam Levitsky (Johns Hopkins University). On the days indicated for each particular experimental design, mice were primed by subcutaneous inoculation with 1 × 107 pfu recombinant vaccinia encoding HA suspended in 0.1 cc HBSS.
Western blot and immunoprecipitation studies
APCs were treated with LPS (5 μg/mL), STI-571 (10 μM), or LPS + STI-571, or were left untreated. After 24 hours, whole cell lysates were obtained and Western blot and immunoprecipitation studies were performed. Briefly, equal amounts of total cellular proteins were separated by sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis followed by immunoblotting with either mouse monoclonal anti–c-abl antibody or with a goat polyclonal anti–c-kit antibody (both from Santa Cruz Biotechnology, Santa Cruz, CA). Phosphorylation of c-abl or c-kit was determined by immunoprecipitation with anti–c-abl or anti–c-kit antibodies, respectively, followed by immunoblotting with a mouse monoclonal antiphosphotyrosine antibody (Upstate Biotechnology, Lake Placid, NY).
RNA isolation and RNase protection assays (RPA)
Total RNA was isolated from untreated or STI-571–treated APCs by TRIzol reagent (Life Technologies, Grand Island, NY). RPAs were carried out using BD Pharmingen's cytokine and chemokine multiprobe templates according to the manufacturer's protocol (BD Pharmingen). Briefly, the multiprobe template was synthesized by in vitro transcription with incorporation of [a-32P] uridine triphosphate (UTP) and purified on a G50 Sephadex column. Purified probe was hybridized with 6 μg total RNA for 16 hours, followed by RNase digestion at 37°C for one hour. Protected RNA fragments were separated on a 5% polyacrylamide denaturing gel and quantified with Image Quant Software (Molecular Dynamics, Sunnyvale, CA).
Statistical analysis
A 2-way analysis of variance (ANOVA) was used to evaluate the magnitudes of LPS (or tumor in the in vivo experiments) and imatinib mesylate–induced effects on cytokine production by clonotypic T cells.
Results
Imatinib mesylate enhances the antigen-presenting function of APCs
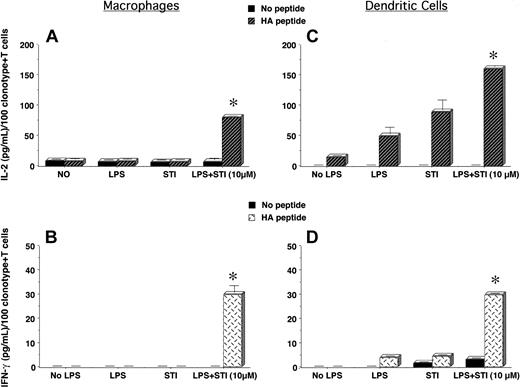
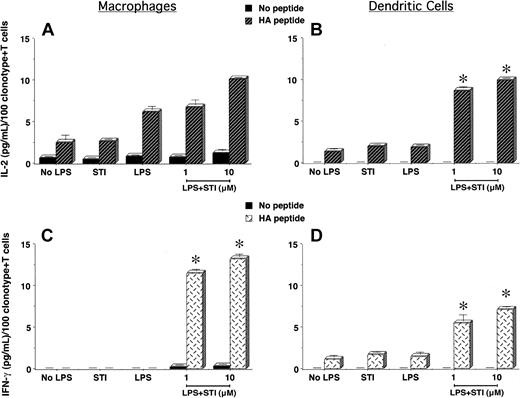
To determine the in vitro effects of imatinib mesylate on APCs' function and regulation of antigen-specific CD4+ T-cell responses, we treated PEMs or bone marrow–derived DCs with LPS in the presence or not of STI-571 for 24 hours. Following this treatment, supernatants were removed and naive CD4+ T cells specific for an MHC class II–restricted epitope of influenza hemagglutinin (HA) were added to the APC cultures and stimulated or not with cognate HA-peptide. As shown in Figure 1A, clonotypic T cells encountering cognate peptide on PEMs treated with LPS + 10 μM STI-571 displayed an enhanced IL-2 production compared with those T cells encountering HA-peptide on either untreated PEMs (No LPS) or PEMs treated with either STI-571 or LPS alone. In spite of the ability of untreated or LPS-treated PEMs to trigger IL-2 production by T cells, these APCs failed to elicit a full effector function of antigen-specific CD4+ T cells, as determined by the lack of IFN-gamma production by clonotypic T cells in response to cognate peptide (Figure 1C, No LPS or LPS). In sharp contrast, clonotypic T cells encountering HA-peptide on PEMs treated with LPS plus either 1 or 10 μM STI-571 were capable of producing IFN-gamma (Figure 1C, LPS + STI, P < .0001), indicative that STI-571 enabled PEMs to elicit full effector function of naive antigen-specific CD4+ T cells.
Antigen-specific CD4+ T-cell responses to cognate antigen presented by STI-571–treated APCs. PEMs (1 × 105/well) or BM-derived DCs (1 × 104/well) from BALB/c mice were cultured for 24 hours in media alone (No LPS), STI-571 (STI, 10 μM), LPS (5 μg/mL), or LPS plus STI-571 as indicated (1 or 10 μM). Then, supernatants were collected and 5 × 104 purified naive T cells from the spleen of anti-HA TCR mice were added to the APCs in the presence or not of 12.5 μg HApeptide110-120. After 48 hours, IL-2 and IFN-gamma production by antigen-specific T cells in response to HA-peptide presented by either PEMs (A,C) or BM-derived DCs (B,D) was determined by ELISA. Data represent mean ± SE of triplicate cultures and are expressed as the amount of cytokine produced per 100 clonotype+ T cells/well. Shown is a representative experiment of 4 independent experiments with similar results (*P statistically significant for the difference in cytokine production between treatment with LPS + STI-571 and LPS alone).
Antigen-specific CD4+ T-cell responses to cognate antigen presented by STI-571–treated APCs. PEMs (1 × 105/well) or BM-derived DCs (1 × 104/well) from BALB/c mice were cultured for 24 hours in media alone (No LPS), STI-571 (STI, 10 μM), LPS (5 μg/mL), or LPS plus STI-571 as indicated (1 or 10 μM). Then, supernatants were collected and 5 × 104 purified naive T cells from the spleen of anti-HA TCR mice were added to the APCs in the presence or not of 12.5 μg HApeptide110-120. After 48 hours, IL-2 and IFN-gamma production by antigen-specific T cells in response to HA-peptide presented by either PEMs (A,C) or BM-derived DCs (B,D) was determined by ELISA. Data represent mean ± SE of triplicate cultures and are expressed as the amount of cytokine produced per 100 clonotype+ T cells/well. Shown is a representative experiment of 4 independent experiments with similar results (*P statistically significant for the difference in cytokine production between treatment with LPS + STI-571 and LPS alone).
Unlike PEMs, antigen presentation by bone marrow–derived DCs induced effector function of naive antigen-specific CD4+ T cells. As shown in Figure 1B,D, clonotypic T cells encountering antigen in either untreated (No LPS) or LPS-treated DCs not only produced IL-2 (Figure 1D), but were also capable of secreting lower amounts of IFN-gamma in response to cognate peptide (Figure 1D). This intrinsic ability of DCs to prime antigen-specific CD4+ T cells was further enhanced following treatment with STI-571. Clonotypic CD4+ T cells encountering cognate peptide on DCs treated with LPS plus STI-571 produce higher levels of IL-2 (Figure 1B, LPS + STI, P < .0001) and IFN-gamma (Figure 1D, LPS + STI, P < .0001) than those T cells encountering antigen on LPS-treated DCs. However, when compared with T-cell responses to cognate antigen presented by treated PEMs, the production of IFN-gamma by clonotypic CD4+ T cells encountering antigen on treated DCs was of a lesser magnitude (Figure 1D versus 1C, LPS + STI). This difference could relate to either the higher number of PEMs used as APCs relative to the number of DCs (1 × 105 PEMs versus 1 × 104 DCs/well) or could be indicative of a different effect of STI-571 on particular subsets of APCs. In any case, in vitro treatment of PEMs or DCs with STI-571 enhanced their antigen-presenting capabilities leading to effective activation of naive antigen-specific CD4+ T cells.
In vivo treatment with imatinib mesylate enhances the response of antigen-specific CD4+ T cells to immunization
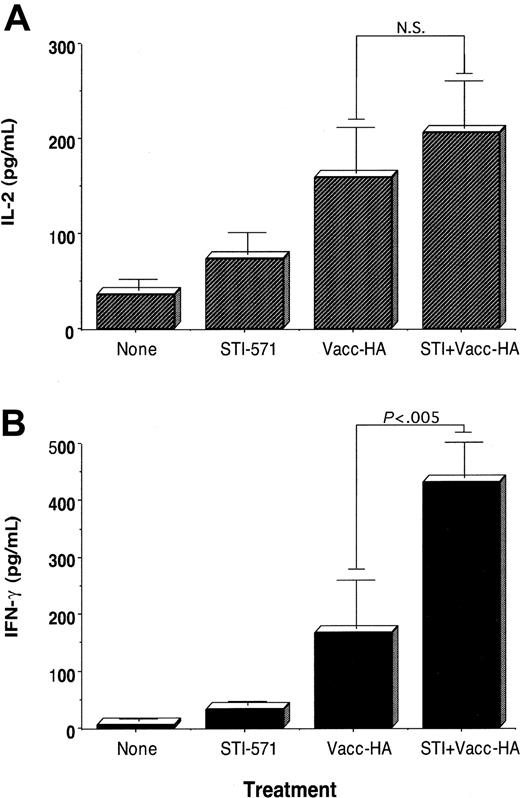
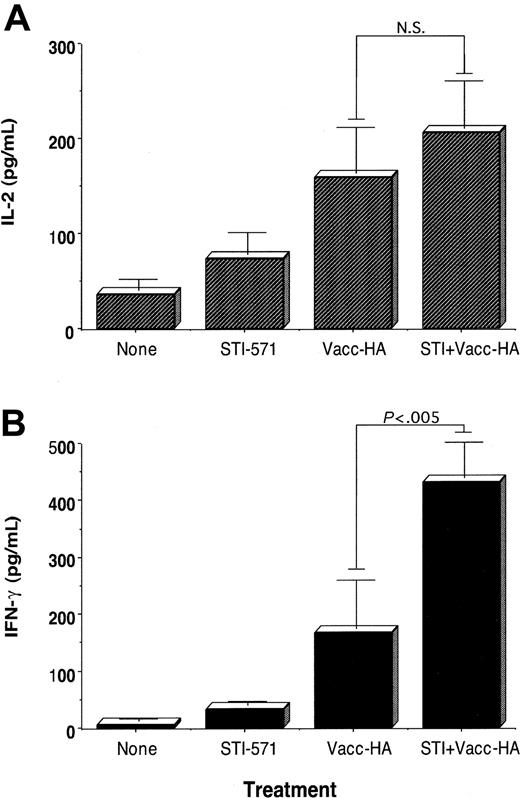
Given the results in Figure 1, we determined next whether in vivo treatment with STI-571 may enhance the response of antigen-specific CD4+ T cells to immunization. Using an adoptive transfer system of anti-HA CD4+ T cells into naive BALB/c mice, we have previously demonstrated that vaccination of these mice with a recombinant vaccinia encoding-HA (vacc-HA) resulted in antigen-specific T cells that fulfill several functional criteria indicative of effective priming: clonal expansion, increased IL-2 production, and differentiation into effector cells capable of producing IFN-gamma.3,4 Reminiscent of those findings, antigen-specific CD4+ T cells isolated from vaccinated BALB/c mice produce higher levels of IL-2 (Figure 2A, vacc-HA) as well as IFN-gamma (Figure 2B, vacc-HA) relative to unvaccinated mice (Figure 2B, None), following in vitro restimulation with HA-peptide. In the absence of vaccination, antigen-specific CD4+ T cells isolated from animals treated with STI-571 (12.5 mg/kg intraperitoneally per day for 10 days) produce slightly higher levels of IL-2 (Figure 2A, STI-571) and IFN-gamma (Figure 2B, STI-571) than those clonotypic T cells isolated from untreated mice. The magnitude of this antigen-specific T-cell response was further augmented in mice treated with STI-571 in combination with antigen-specific vaccination. As a reflection of enhanced priming in vivo, clonotypic T cells from these mice produce the highest level of IFN-gamma in response to in vitro restimulation with HA-peptide (Figure 2B, vacc-HA + STI, P < .005 compared with vacc-HA alone). No enhanced production of IL-4 or IL-10 by these same clonotypic T cells was observed following their stimulation with cognate peptide (data not shown). Additional experiments have indicated that at least 7 days of in vivo treatment with STI-571 are required to significantly enhance T-cell responses to vaccination (data not shown).
Effect of in vivo treatment with STI-571 on the functional responses of antigen-specific CD4+ T cells to immunization. BALB/c mice were given STI-571 (12.5 mg/kg per day) or vehicle alone intraperitoneally for 10 days. On day 10, all the animals received 2.5 × 106 anti-HA TCR+ transgenic CD4+ T cells intravenously. Then 9 days later, half of the mice in each group were immunized subcutaneously with 1 × 107 pfu vacc-HA. Animals were killed 6 days later, and T cells were purified from their spleens as indicated in “Materials and methods.” T cells were then cultured with media alone or HA-peptide (12.5 μg/mL) plus fresh splenocytes for 48 hours. Supernatants were collected and assayed for IL-2 (A) and IFN-gamma production (B) by ELISA. Data represent mean ± SE of triplicate cultures from 3 mice in each group and are representative of 3 independent experiments with similar results. Values for T cells cultured without HA-peptide were below the limit of detection (NS indicates not statistically significant)
Effect of in vivo treatment with STI-571 on the functional responses of antigen-specific CD4+ T cells to immunization. BALB/c mice were given STI-571 (12.5 mg/kg per day) or vehicle alone intraperitoneally for 10 days. On day 10, all the animals received 2.5 × 106 anti-HA TCR+ transgenic CD4+ T cells intravenously. Then 9 days later, half of the mice in each group were immunized subcutaneously with 1 × 107 pfu vacc-HA. Animals were killed 6 days later, and T cells were purified from their spleens as indicated in “Materials and methods.” T cells were then cultured with media alone or HA-peptide (12.5 μg/mL) plus fresh splenocytes for 48 hours. Supernatants were collected and assayed for IL-2 (A) and IFN-gamma production (B) by ELISA. Data represent mean ± SE of triplicate cultures from 3 mice in each group and are representative of 3 independent experiments with similar results. Values for T cells cultured without HA-peptide were below the limit of detection (NS indicates not statistically significant)
STI-571–treated APCs restore the responsiveness of tumor-specific T cells
Tumor antigen–specific T-cell tolerance imposes a remarkable barrier to the development of effective therapeutic cancer vaccines. Using the HA-specific TCR-transgenic model we have previously demonstrated that CD4+ T cells specific for an MHC class II epitope of influenza hemagglutinin (HA) are rendered unresponsive during the progression of A20 B-cell lymphoma expressing HA as a model tumor antigen (A20HA). In this system, clonotype-positive T cells undergo a transient clonal expansion and have a phenotype (CD44high, CD45Rblow, and CD62Llow) associated with antigen recognition. In spite of these changes however, functional analysis demonstrated that these antigen-specific CD4+ T cells were unable to be primed in vivo and display a significantly diminished IL-2 and IFN-gamma production in response to in vitro restimulation with cognate antigen presented by APCs.3,5
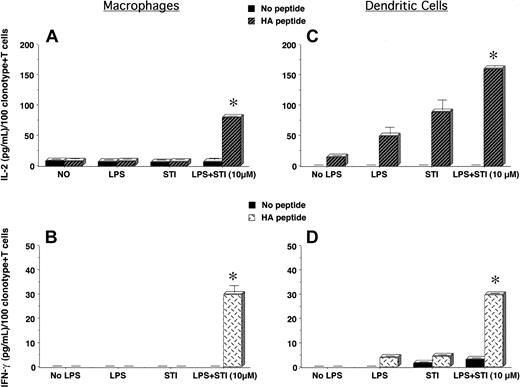
Given the enhanced T-cell stimulatory capabilities of STI-571–treated APCs (Figure 1), we sought to determine whether these APCs might restore the responsiveness of tolerant T cells isolated from A20 lymphoma–bearing mice. As shown in Figure 3A-B, PEMs treated with LPS plus 10 μM STI-571 were capable of triggering IL-2 (Figure 3A, P < .0001) and IFN-gamma (Figure 3B, P < .0001) production by tolerized CD4+ T cells. In sharp contrast, tolerant T cells encountering cognate antigen on either untreated (Figure 3B, No LPS) or LPS-treated PEMs remained fully unresponsive. A significant improvement in the functional capabilities of antigen-specific CD4+ T cells was also observed when T cells isolated from tumor-bearing hosts encountered cognate antigen on DCs treated with LPS + 10 μM STI-571(Figure 3C-D, P < .001 for IL-2 production and P < .0001 for IFN-gamma production, compared with LPS alone).
STI-571–treated APCs restore the responsiveness of tolerant CD4+ T cells from tumor-bearing mice. PEMs (1 × 105/well) or BM-derived DCs (1 × 104/well) from BALB/c mice were treated as in Figure 1 and then cultured with 5 × 104 T cells isolated from the spleen of A20HA-bearing mice, in the presence or not of 12.5 μg HA peptide110-120. After 48 hours, supernatants were collected, and IL-2 and IFN-gamma production by clonotype-positive T cells in response to HA-peptide presented by either PEMs (A-B) or BM-derived DCs (C-D) was determined by ELISA. Data represent mean ± SE of triplicate cultures and are expressed as the amount of cytokines produced per 100 clonotype+ T cells/well. Shown is a representative experiment of 4 independent experiments with similar results (*P statistically significant for the difference in cytokine production between treatment with LPS + STI-571 and LPS alone).
STI-571–treated APCs restore the responsiveness of tolerant CD4+ T cells from tumor-bearing mice. PEMs (1 × 105/well) or BM-derived DCs (1 × 104/well) from BALB/c mice were treated as in Figure 1 and then cultured with 5 × 104 T cells isolated from the spleen of A20HA-bearing mice, in the presence or not of 12.5 μg HA peptide110-120. After 48 hours, supernatants were collected, and IL-2 and IFN-gamma production by clonotype-positive T cells in response to HA-peptide presented by either PEMs (A-B) or BM-derived DCs (C-D) was determined by ELISA. Data represent mean ± SE of triplicate cultures and are expressed as the amount of cytokines produced per 100 clonotype+ T cells/well. Shown is a representative experiment of 4 independent experiments with similar results (*P statistically significant for the difference in cytokine production between treatment with LPS + STI-571 and LPS alone).
Of note, tolerized T cells produce more IL-2 and IFN-gamma on a per-cell basis than naive antigen-specific T cells in response to cognate antigen presented by STI-571–treated APCs. As shown in Figure 3A, IL-2 production by tolerized T cells was approximately 7-fold higher relative to the response of naive T cells to cognate antigen presented by PEMs treated with LPS + 10 μM STI-571 (70 pg/mL versus 10 pg/mL per 100 clonotype+ T cells, respectively, Figure 3A versus 1A). A 2-fold increase in IFN-gamma production by tolerized T cells, relative to naive T cells, was also observed (30 pg/mL versus 15 pg/mL per 100 clonotype+ T cells, respectively, Figure 3B versus 1C). A similar increase in cytokine production, although of a higher magnitude (15-fold increase for IL-2 and 4-fold increase for IFN-gamma, relative to the levels of cytokine produced by naive antigen-specific T cells), was observed when tolerized T cells encountered cognate antigen on DCs treated with LPS + 10 μM STI-571 (Figures 3C-D versus 1B,D, respectively). This increased response of tumor-tolerant T cells compared with that of naive T cells suggests that STI-571–treated APCs are capable of overcoming T-cell unresponsiveness in antigen-experienced cells rather than just enhancing the activation of those naive T cells that may have escaped tolerance induction in vivo.
STI-571 prevents tumor-induced antigen-specific CD4+ T-cell tolerance in vivo
Next, we examined whether in vivo treatment with STI-571 could prevent the development of unresponsiveness of antigen-specific CD4+ T cells following their transfer into mice with established A20 lymphoma cells expressing HAas a model tumor antigen (A20HA). Briefly, A20HA tumor cells were injected intravenously into BALB/c mice (day –10). Half of the tumor-bearing mice as well as a cohort of tumor-free mice received treatment with STI-571 (12.5 mg/kg intraperitoneally per day) for 10 days. On day zero, transgenic naive anti-HA CD4+ T cells were adoptively transferred into untreated or STI-571–treated tumor-bearing mice and their respective tumor-free controls. On day +9 after T-cell transfer, all the mice in each subgroup were immunized subcutaneously with vacc-HA. Immunization of non–tumor-bearing mice resulted in IL-2 production (Figure 4A, No tumor) and differentiation into effector T cells capable of producing IFN-gamma upon in vitro stimulation with HA-peptide (Figure 4B, No tumor). Similar to our findings in Figure 2, the magnitude of this T-cell response, specifically IFN-gamma production, was enhanced in tumor-free mice treated with STI-571 plus vacc-HA (Figure 4B, No tumor + STI).
In vivo treatment of tumor-bearing mice with STI-571 preserves the responsiveness of tumor-specific CD4+ T cells to vaccination. BALB/c mice were injected intravenously with 1 × 106 A20HA tumor cells on day –10. From day–9 to day 0, half of the mice received intraperitoneal injection of STI-571 (12.5 mg/kg per day) or vehicle alone. On day zero, all the mice received 2.5 × 106 anti-HA TCR+transgenic CD4+ T cells intravenously. On day +9, mice were immunized subcutaneously with 1 × 107 pfu vacc-HA. Animals were killed 6 days later (day +15), and T cells were purified from their spleens as indicated in “Materials and methods.” Purified T cells were mixed with fresh splenocytes and HA-peptide as described in Figure 2. Then 48 hours later, supernatants were collected and assayed for IL-2 (A) or IFN-gamma (B) by ELISA. Values are the mean ± SE of triplicate cultures from 3 mice in each group. Data are expressed as the amount of cytokine produced per 100 clonotype-positive T cells/well and are representative of 3 independent experiments with similar results.
In vivo treatment of tumor-bearing mice with STI-571 preserves the responsiveness of tumor-specific CD4+ T cells to vaccination. BALB/c mice were injected intravenously with 1 × 106 A20HA tumor cells on day –10. From day–9 to day 0, half of the mice received intraperitoneal injection of STI-571 (12.5 mg/kg per day) or vehicle alone. On day zero, all the mice received 2.5 × 106 anti-HA TCR+transgenic CD4+ T cells intravenously. On day +9, mice were immunized subcutaneously with 1 × 107 pfu vacc-HA. Animals were killed 6 days later (day +15), and T cells were purified from their spleens as indicated in “Materials and methods.” Purified T cells were mixed with fresh splenocytes and HA-peptide as described in Figure 2. Then 48 hours later, supernatants were collected and assayed for IL-2 (A) or IFN-gamma (B) by ELISA. Values are the mean ± SE of triplicate cultures from 3 mice in each group. Data are expressed as the amount of cytokine produced per 100 clonotype-positive T cells/well and are representative of 3 independent experiments with similar results.
Analysis of HA-specific cytokine release from antigen-specific CD4+ T cells isolated from vaccinated A20HA-bearing mice revealed significant impairment in their capacity to produce IL-2 (Figure 4A, A20HA) as well as the IFN-gamma (Figure 4B, A20HA). However, the antigen-specific CD4+ T-cell tolerance seen in A20HA-bearing mice was prevented when tumor-bearing animals were treated with STI-571. When compared with untreated tumor-bearing mice (A20HA), T cells from A20HA-bearing mice treated with STI-571 produced more IL-2 (Figure 4A, A20HA + STI, P = .002) as well as IFN-gamma (Figure 4B, A20HA + STI, P = .001). This effect does not appear to be the result of a direct cytotoxic effect of STI-571 on lymphoma cells, since the growth of these cells was not affected by in vitro incubation with increased concentrations of STI-571 (data not shown). Overall, in vivo treatment with STI-571 resulted in a degree of IL-2 and IFN-gamma production that was comparable, on a per cell basis, with that observed in immunized mice without tumor.
Analysis of the in vivo effects of imatinib mesylate on APC populations revealed an increase in CD11b+ myeloid cells in tumor-bearing mice treated with vaccine plus STI-571 relative to untreated mice or animals treated with either agent alone (Table 1). No significant differences were observed, however, in the splenic CD11c+ myeloid cell population from untreated or STI-571–treated tumor-bearing mice. Similarly, no changes in the splenic B-cell population occurred in response to treatment with STI-571. Interestingly, in tumor-bearing animals treated with STI-571 we observed a slight increase in the NK1.1 population relative to untreated tumor-bearing mice (1.3% ± 0.2% versus 0.9% ± 0.2%, respectively). This NK-cell expansion was further enhanced when animals were treated with STI-571 along with vacc-HA immunization (1.8% ± 0.1%).
Therefore, at a time when antigen-specific CD4+ T cells from A20HA-bearing mice were functionally impaired, clonotypic T cells from animals treated with STI-571 remained functional and able to respond to a subsequent immunization. This positive in vivo effect of imatinib mesylate was associated with an increase in splenic CD11b+ myeloid cells as well as the NK1.1 population.
STI-571 inhibits LPS-dependent c-kit phosphorylation in APCs
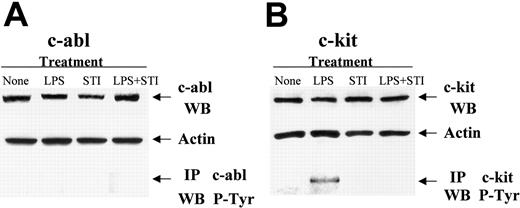
To gain insight into the potential intracellular pathway(s) in APCs being affected by the tyrosine kinase inhibitor STI-571, we performed Western blot studies to determine the expression of molecular targets that are well known to be inhibited by this drug.16 Both c-abl (Figure 5A, upper panel) as well as c-kit (Figure 5B, upper panel) were expressed in either untreated or LPS-treated PEMs. However, no PDGFR expression could be detected in these APCs (data not shown). Flow cytometric studies using an anti-CD117 antibody further confirmed the presence of c-kit on the surface of untreated or LPS-treated PEMs (data not shown). Western blot analysis of c-abl (Figure 5A) and c-kit protein (Figure 5B) revealed no changes in protein expression in PEMs treated with either LPS or LPS + STI-571. Next, phosphorylation of c-abl and c-kit was determined by immunoprecipitation (IP) of whole cell lysates. As shown in Figure 5A (lower panel) no phosphorylation of c-abl was detected in LPS-stimulated PEMs. However, phosphorylation of c-kit was observed in PEMs stimulated with LPS (Figure 5B, bottom panel). This LPS-dependent c-kit phosphorylation was clearly inhibited by STI-571 treatment (Figure 5B, LPS + STI, bottom panel). Taken together, our data suggest that among the known molecular targets of imatinib mesylate, inhibition of c-kit phosphorylation may play a role in the enhanced antigen-presenting function displayed by STI-571–treated APCs.
STI-571 inhibits LPS-dependent phosphorylation of c-kit in APCs. PEMs were treated with either LPS (5 μg/mL), STI-571 (10 μM), LPS + STI-571, or left untreated (None) for 24 hours. Then, whole cell lysates were obtained and Western blot and immunoprecipitation studies were performed as indicated in “Materials and methods.” Western blot (WB) and IP studies were repeated 3 times with similar results.
STI-571 inhibits LPS-dependent phosphorylation of c-kit in APCs. PEMs were treated with either LPS (5 μg/mL), STI-571 (10 μM), LPS + STI-571, or left untreated (None) for 24 hours. Then, whole cell lysates were obtained and Western blot and immunoprecipitation studies were performed as indicated in “Materials and methods.” Western blot (WB) and IP studies were repeated 3 times with similar results.
Phenotypic and functional characteristics of STI-571–treated APCs
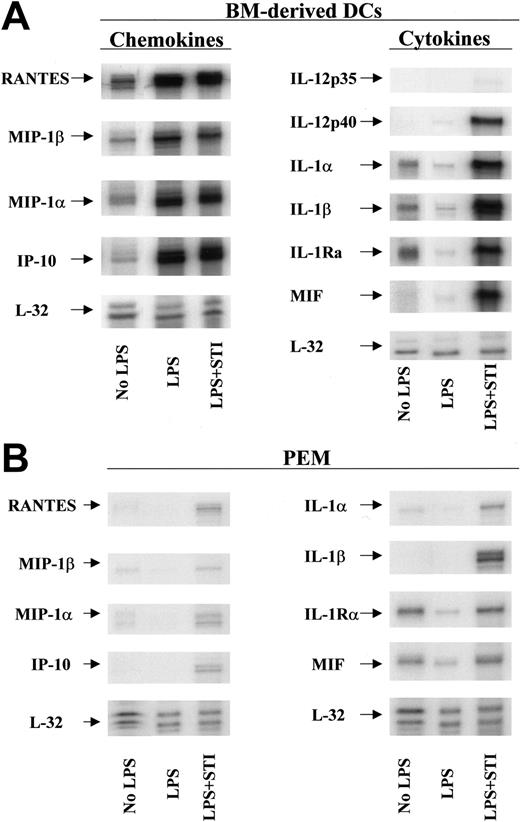
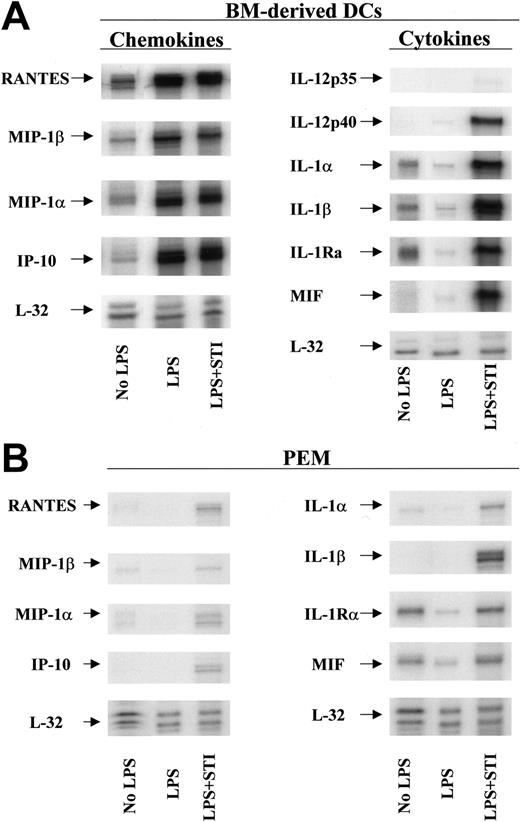
To elucidate the mechanism(s) by which STI-571–treated APCs restore the responsiveness of tolerized antigen-specific T cells, we evaluated next the phenotypic characteristics and the cytokine/chemokine profile of STI-571–treated DCs and PEMs. No significant differences in the expression of MHC class II molecules or B7.1 or B7.2 costimulatory molecules were observed in STI-571–treated APCs compared with LPS-treated APCs (data not shown). Using multitemplate RNA probes we found, however, that in vitro treatment with STI-571 significantly increased the mRNA levels of several proinflammatory molecules in DCs in response to LPS stimulation. First, untreated DCs express detectable levels of the chemokines RANTES, macrophage inflammatory protein 1β (MIP-1β), MIP-1α, interferon-inducible protein-10 (IP-10), and the cytokines IL-1α, IL-1β, and IL-1Ra (Figure 6A, No LPS). Following stimulation with LPS for 24 hours, DCs display an enhanced mRNA expression of RANTES, MIP-1β, MIP-1α, and IP-10 but decreased mRNA levels of IL-1α, IL-1β, and IL-1RA (Figure 6A, LPS). At this time point however, no IL-12p35, IL-12p40, or macrophage migration inhibitory factor (MIF) mRNA was detected in LPS-stimulated DCs. In sharp contrast, stimulation of DCs with LPS in the presence of 10 μM STI-571 resulted in significant expression of IL-12p40 and MIF mRNA (Figure 6A, LPS + STI). Similarly, DCs treated with LPS + STI-571 display enhanced mRNA expression of IL-1α, IL-1β, and IL-1RA compared with untreated or LPS-treated DCs. In addition, treatment with STI-571 also enhances the inflammatory response of PEMs to LPS stimulation (Figure 6B). Although of a lesser magnitude than the inflammatory response in STI-571–treated DCs, treatment of PEMs with LPS + STI-571 resulted in enhanced mRNA levels of IL-1α, IL-1β, MIF, RANTES, MIP-1α, MIP-1β, and IP-10, compared with LPS-treated PEMs.
Inflammatory phenotype of STI-571–treated APCs. Bone marrow–derived DCs (A) or PEMs (B) were treated with either LPS (5 μg/mL) or LPS plus 10μM STI-571 for 24 hours. Then total RNA was isolated and RNA protection assay (RPA) was carried out using chemokine and cytokine multiprobe templates according to the manufacturer's protocol (BD Pharmingen). RPA was repeated 3 times with similar results.
Inflammatory phenotype of STI-571–treated APCs. Bone marrow–derived DCs (A) or PEMs (B) were treated with either LPS (5 μg/mL) or LPS plus 10μM STI-571 for 24 hours. Then total RNA was isolated and RNA protection assay (RPA) was carried out using chemokine and cytokine multiprobe templates according to the manufacturer's protocol (BD Pharmingen). RPA was repeated 3 times with similar results.
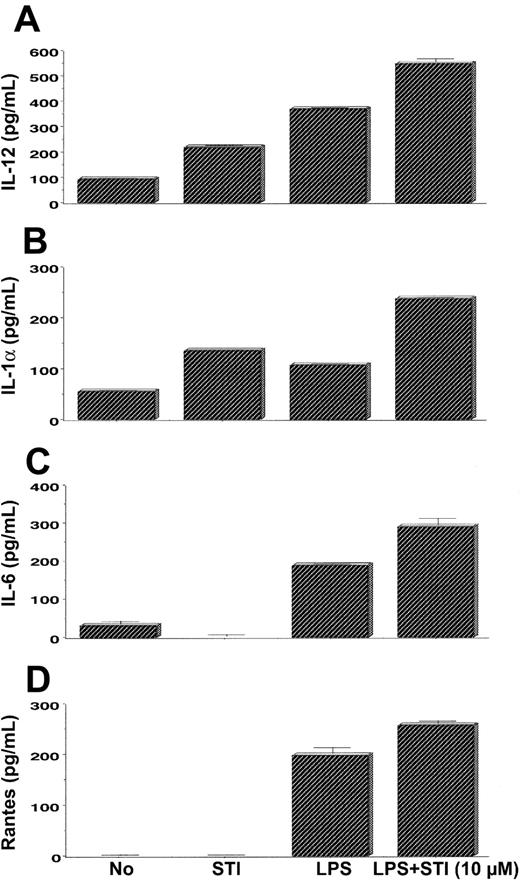
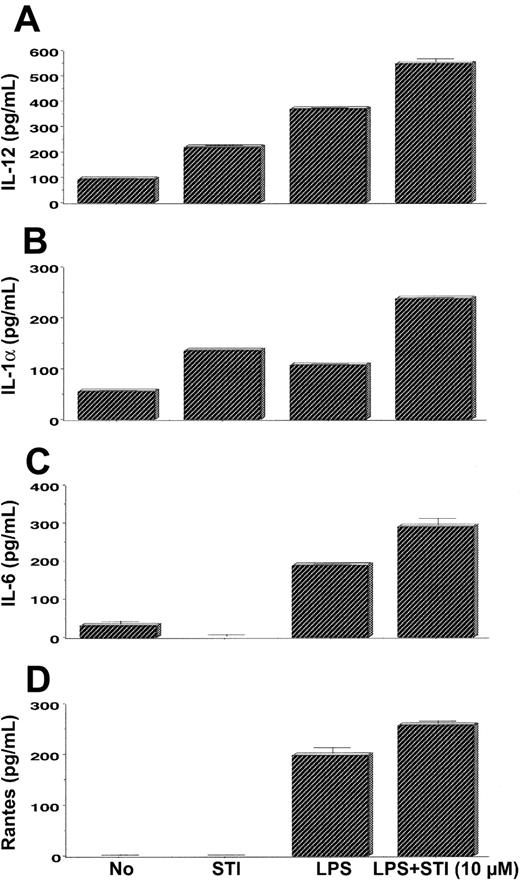
To further confirm that STI-571–treated APCs are capable not only of expressing mRNA of inflammatory mediators but also of secreting them, we measured the levels of several cytokines and chemokines in the supernatants of untreated and treated DCs. As seen in Figure 7A, treatment of DCs with STI-571 (10 μM) resulted in higher levels of IL-12 relative to the levels produced by untreated cells. DCs stimulated with LPS produced even higher levels of IL-12 compared with STI-571–treated DCs. Combination of these 2 agents led to the highest production of IL-12 by these APCs. Similarly, treatment of DCs with STI-571 in combination with LPS also resulted in enhanced production of IL-1α (Figure 7B) and IL-6 (Figure 7C) and slight increase in RANTES (Figure 7D) relative to the production of these inflammatory mediators by DCs treated with LPS alone. No differences in the levels of IL-4, IL-10, IL-18, and TNF-α were found among DCs treated with LPS alone and DCs treated with LPS + STI-571 (data not shown). Therefore, in vitro treatment of DCs or PEMs with imatinib mesylate resulted in enhanced expression of several proinflammatory molecules in response to LPS stimulation.
Production of inflammatory mediators by STI-571–treated APCs. Bone marrow–derived DCs were treated with either LPS (5 μg/mL), STI-571 (10 μM), or LPS (5 μg/mL) + 10 μM STI-571 or left untreated for 6 hours. Then supernatants were collected and assayed for IL-12 (A), IL-1α (B), IL-6 (C), or RANTES (D) by ELISA. Values are the mean ± SE of triplicate cultures. Data are representative of 2 independent experiments with similar results.
Production of inflammatory mediators by STI-571–treated APCs. Bone marrow–derived DCs were treated with either LPS (5 μg/mL), STI-571 (10 μM), or LPS (5 μg/mL) + 10 μM STI-571 or left untreated for 6 hours. Then supernatants were collected and assayed for IL-12 (A), IL-1α (B), IL-6 (C), or RANTES (D) by ELISA. Values are the mean ± SE of triplicate cultures. Data are representative of 2 independent experiments with similar results.
Discussion
In this study, we have identified the tyrosine kinase inhibitor imatinib mesylate as a potential therapeutic strategy to overcome tolerance to tumor antigens mediated by APCs. First, in vitro treatment of dendritic cells or macrophages with STI-571 was capable of restoring the responsiveness of tolerized T cells from tumor-bearing hosts to cognate antigen (Figure 3). Second, treatment with STI-571 prevented the in vivo induction of tolerance of tumor-specific CD4+ T cells (Figure 4). Last, by preserving the responsiveness of tumor antigen–specific CD4+ T cells, in vivo treatment with STI-571 was able to enhance the efficacy of therapeutic vaccination.
Recently, several studies in humans as well as in experimental models have evaluated the in vivo and in vitro effects of imatinib mesylate on the phenotype and function of APCs and T cells. Our results showing a positive immunomodulatory effect of STI-571 are consistent with previous reports demonstrating that treatment with this drug resulted in increased production of IFN-gamma by T cells,17,18 improvement in the antigen-presenting function of DCs,19 and restoration of plasmacytoid dendritic cell (PDC) function in patients with CML who achieved complete cytogenetic or molecular remission after imatinib mesylate treatment.20 However, our data are at odds with other studies showing that treatment with STI-571 impairs DC and T-cell function rather than augments immune responses. For instance, Appel et al have demonstrated that in vitro exposure of mobilized human CD34+ progenitors to imatinib mesylate (1-5 μM) for 10 to 16 days resulted in DCs that express reduced levels of the costimulatory molecules CD80 and CD40 and are unable to induce primary cytotoxic T lymphocyte (CTL) responses.21 Dewar et al have also shown that imatinib mesylate inhibits the in vitro development of the monocyte/macrophage lineage from normal human bone marrow progenitor cells.22 More recently, Dietz et al have found that imatinib mesylate inhibits the in vitro proliferation of primary human T cells in response to allogeneic DCs or phytohemagglutinin (PHA) stimulation. This inhibitory effect of STI-571 was confirmed in an in vivo model of delayed-type hypersensitivity (DTH).23 A similar in vivo inhibitory effect of imatinib mesylate was recently observed by Taieb et al who reported that treatment with this drug impairs FMS-like tyrosine kinase 3 ligand (Flt3L)–mediated DC expansion and abrogates protective antitumor immunity.24
Several differences between the above studies and our results may explain these seemingly conflicting data. For instance, in our in vitro system imatinib mesylate is added for only 24 hours to cell cultures containing already mature bone marrow–derived DCs or macrophages, while in Appel et al's study21 CD34+ progenitors are exposed to imatinib mesylate for 10 to 16 days. Given that DCs now comprise a vast assortment of cells with many subtypes, it is entirely possible that the apparent conflicting data may just represent an effect of imatinib mesylate in DCs' subsets generated under different in vitro conditions. Perhaps the maturation/activation status of the DC subsets at the time of initial exposure to imatinib mesylate and the dose and length of in vitro exposure to this drug could determine the functional capabilities of these APCs and may well explain the differences observed in different studies. Furthermore, antigen presentation by STI-571–treated APCs might have different functional consequences for antigen-specific CTLs versus antigen-specific CD4+ T cells. In Appel et al's study,21 DCs generated from human CD34+ progenitors in the presence of imatinib mesylate failed to elicit CTLs. However, no assessment of CD4+ T-cell responses to cognate antigen was reported. Conversely, our data demonstrating a positive immunomodulatory effect of STI-571 was limited to the evaluation of responses of antigen-specific CD4+ T cells to cognate antigen. Needless to say, we are currently evaluating whether a similar activating effect occurs in antigen-specific CD8+ T cells encountering cognate antigen on STI-571–treated APCs as well as the consequences or requirements of CD4 help on such CD8 treatment conditions.
In spite of the valid concerns raised by those studies demonstrating that imatinib mesylate can impair DC maturation and T-cell function,21-24 our in vivo results together with those from Zeng et al18 indicate that treatment of a tumor-bearing host with STI-571 certainly did not impair antitumor immune responses but instead resulted in enhancement of T-cell responses to vaccination strategies. An important difference between the in vivo studies presented here and those from Dietz et al23 and Taieb et al24 relates to the dose and duration of treatment with imatinib mesylate. While relatively low doses of STI-571 were used in our study (12.5 mg/kg intraperitoneally per day for 10 days), in Dietz et al's study23 demonstrating in vivo inhibition of DTH responses, a higher dose of imatinib mesylate was used (50 mg/kg intraperitoneally per day for 21 days). Even higher doses of this drug (150 mg/kg per gavage/twice a day for 5 days) were used in the study that resulted in inhibition of DC expansion and abrogation of antitumor responses in vivo.24 It is plausible therefore that a dose-dependent effect and the length of treatment with imatinib mesylate may explain—at least in part—the differences observed in different in vivo studies.
Further evidence supporting the potential positive immunomodulatory effects of imatinib mesylate has been provided in a murine model of bcr-abl+ murine leukemia and in 2 recently completed clinical trials for patients with CML in which vaccination strategies were added to imatinib mesylate. First, Zeng et al have shown that the combination of this drug with chaperone-rich cell lysate–loaded DCs resulted in activation of antigen-specific T cells and potent antitumor activity against bcr-abl+ leukemic cells.18 In the clinical arena, Bocchia et al have found that in CML patients with persistent disease despite treatment with STI-571, vaccination with a P-210–derived multipeptide vaccine (CMLVAX100) induces cytogenetic and even molecular improvements in the majority of treated patients. Furthermore, in this cohort of patients, prolonged treatment with STI-571 does not adversely affect T-cell responses since peptide-specific proliferation and IFN-gamma production by CD4+ T cells were clearly detected following multiple immunizations with CMLVAX100.25 In a second study, Li et al treated imatinib mesylate–resistant CML patients with a combination of this drug and an autologous vaccine consisting of heat-shock protein (HSP) obtained from the patient's own malignant cells. This strategy also resulted in improved cytogenetic and molecular responses as well as in the expansion of IFN-gamma–producing CD8+ T cells directed specifically against autologous tumor cells.26
In the immune response to antigens expressed by tumors, host APCs are central in determining the functional outcome of tumor-specific T cells. In the absence of inflammatory signals, TCR engagement with tumor antigen/MHC class II molecules presented by “noninflammatory” APCs (having low levels of MHC, costimulatory molecules, and other adhesion molecules that participate in T-cell priming) induces a “partial activation” state that, by itself, is insufficient to trigger the effector function necessary for achieving tumor rejection. Instead, in the face of persistent antigen and in the absence of additional signals capable of sustaining and/or amplifying this initial response, the default of T-cell responses to tumor antigens is tolerance induction rather than T-cell priming.5,6 In tumor-bearing mice treated with imatinib mesylate, however, this default response toward tolerance induction is significantly altered. As shown here, treatment with STI-571 was able to convert a T-cell encounter with antigen/APC from a tolerizing event into an activating event. Although we have found that in vivo treatment with imatinib mesylate is associated with an increase in CD11b+ myeloid cells and NK1.1 cells (Table 1), the significance of these findings as well as their potential role in determining priming versus tolerance in vivo remains to be elucidated. In any case, the demonstration that imatinib mesylate preserved the responsiveness of tumor-specific CD4+ T cells provides an explanation for the demonstrated ability of STI-571 to augment the efficacy of therapeutic vaccination of tumor-bearing hosts.
Given the accumulating evidence pointing to the maturation/activation status of an APC at the time of antigen presentation as the central determinant of T-cell priming versus tolerance,27,28 our demonstration that STI-571–treated APCs (Figure 6) display a proinflammatory phenotype provided a plausible explanation for their enhanced antigen-presenting capabilities leading to effective activation of T cells and restoration of responsiveness of tolerized T cells. Although the data presented here do not establish a direct causal effect of inhibition of c-kit phosphorylation by STI-571 and enhancement of APC function, our results suggest a potential role for this signaling pathway in limiting the full development of the antigen-presenting capabilities of APCs. As shown in Figures 6, 7, APCs in which LPS-dependent c-kit phosphorylation was inhibited by STI-571 are more prone to produce a variety of inflammatory mediators than APCs treated with LPS alone.
It has become clear in recent years that induction of tolerance to tumor antigens has raised the bar for effective immunotherapy of tumors. Our findings unveiling a previously unknown ability of imatinib mesylate to overcome this barrier have provided the appropriate framework for continued combination of this drug with cancer vaccine formulations. Future confirmatory studies in other experimental systems and eventually in humans might potentially broaden the therapeutic scope of imatinib mesylate as a promising agent in cancer immunotherapy, far beyond its current use as a cytotoxic agent against a limited number of malignancies.
Prepublished online as Blood First Edition Paper, September 28, 2004; DOI 10.1182/blood-2004-01-0027.
Supported by Public Health Service (PHS) grants CA78656, CA87583, and CA100850.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors wish to thank Dr J. Brayer and Dr I. Borrello for helpful discussions and careful review of the manuscript. E. M. Sotomayor is a Junior Faculty of the Lymphoma Research Foundation of America.