Abstract
Successful hematopoietic cell transplantation (HCT) from an allogeneic donor ideally should produce tolerance to recipient alloantigens while preserving anti-infectious and antitumor immunity. Rapamycin together with costimulation blockade can induce tolerance in organ allograft models by inhibiting G1 → S-phase progression and promoting T-cell apoptosis. In contrast to blocking costimulation through CD28, administration of agonistic CD28-specific antibody 37.51 partially prevents lethal graft-versus-host disease (GVHD) by selective depletion of alloreactive T cells in mice. We hypothesized that combining rapamycin with agonistic CD28 treatment would improve GVHD control by tolerizing a small subset of alloreactive T cells that might escape effects of the CD28-specific antibody. A short course of rapamycin plus agonistic CD28 treatment showed synergism at suboptimal doses, was highly effective in preventing lethal GVHD, and was superior to rapamycin plus CD28 blockade in a major histocompatibility complex class I– and II–mismatched HCT model. The combination treatment reduced the number of proliferating, alloreactive cells in the recipient, promoted donor B- and T-cell reconstitution, and reduced inflammatory cytokine levels. Administration of rapamycin plus agonistic CD28 antibodies offers a promising new therapeutic approach to facilitate tolerance after HCT.
Introduction
Graft-versus-host disease (GVHD) remains a major obstacle to successful hematopoietic cell transplantation (HCT), even though prophylactic and therapeutic administration of immunosuppressive agents has made HCT feasible for treatment of many diseases.1 The use of currently available drugs is hampered by their broad immunosuppressive activity, which predisposes the recipient to infectious complications and relapse of malignant disease. A further disadvantage of current regimens is the need to administer these drugs for an extended period of time until tolerance develops. An ideal regimen would involve short-term treatment that produces tolerance to recipient alloantigens while permitting immune reconstitution and responsiveness to infectious organisms and malignant cells.
GVHD is caused by donor T cells that are activated by host alloantigens. Efficient activation of those T cells requires both antigen recognition through the T-cell receptor (TCR) complex and costimulation provided by antigen-presenting cells (APC).2 Costimulatory signals are provided primarily by interaction of CD28 on T cells with B7 (CD80 and CD86) on APC. Blockade of CD28 costimulation can have immunosuppressive effects in vitro and in vivo.3 Paradoxically, we and others have found that CD28 costimulation by its natural ligands or an agonist antibody promotes depletion of peripheral T cells activated by strong antigens.4,5 Engagement of CD28 on alloactivated T cells with an agonistic antibody (Ab) leads to their depletion and to the prevention of GVHD in mice.4,6
Rapamycin (sirolimus), originally isolated as an antifungal agent, has shown immunosuppressive activity in various experimental and clinical settings.7 In T cells, rapamycin binds to FK-binding protein-12 (FKBP12) and thereby blocks the interleukin-2 (IL-2)–dependent activation of the mammalian target of rapamycin (mTOR), a pathway distinct from calcineurin signaling that is inhibited by cyclosporine and tacrolimus.8 Rapamycin is modestly effective in preventing GVHD in mice and by itself induced an autoimmune-like syndrome.9-11
Blockade of CD28-B7 interactions alone is immunosuppressive in vivo12,13 but does not lead to complete tolerance in stringent transplantation models.14,15 In previous efforts to produce more complete transplantation tolerance, investigators have attempted to block multiple costimulatory events or have added other immunosuppressive agents.14,16-18 Tolerance to skin and cardiac allografts can be achieved by blocking both CD28-B7 and CD40-CD40–ligand interactions.14 Addition of rapamycin to this regimen led to massive apoptosis of alloreactive T cells and long-term tolerance in a very stringent skin allograft model.16 More recently it has been suggested that a CD40-ligand–specific antibody depletes activated alloreactive T cells and acts synergistically with rapamycin to induce tolerance.19
Because the agonistic anti-CD28 monoclonal antibody (mAb) 37.51 induces apoptosis of alloreactive T cells in a murine GVHD model,6 we hypothesized that this treatment could be combined with short-term administration of rapamycin to induce tolerance in a fully myeloablative major histocompatibility (MHC) class I– and II–mismatched HCT model. In this study we show that the combination of anti-CD28 mAb and rapamycin has synergistic activity at suboptimal doses and effectively prevents lethal GVHD.
Materials and methods
Mice
C57BL/6 (B6), B6.SJL-Ly5a Ptprca Pep3b (B6.Ly5.1), B6.129S2-CD28tm1MAK/J (B6 CD28-deficient) and B10.BR mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Mice were housed in microisolator cages at the Fred Hutchinson Cancer Research Center (Seattle, WA). Experimental procedures were reviewed and approved by the Institutional Animal Care and Use Committee.
Transplantation, drug treatment, and T-cell purification
T cell–depleted marrow cells (15 × 106/mouse) plus spleen cells (15-25 × 106/mouse) from B6 donors were transferred into lethally irradiated (1000-1200 cGy) B10.BR (H2k) recipients. Recipients were monitored for survival, body weight, and clinical signs of GVHD. Anti-CD28 mAb 37.51 (kindly provided by Dr J. Allison, University of California at Berkeley, Oakland) or control hamster immunoglobulin G (IgG; IGN Pharmaceuticals, Aurora, OH) were administered intraperitoneally 2 to 3 hours before the transplantation and, in some recipients, every other day afterward until 14 days after the transplantation for a total of 8 doses. Rapamycin (Calbiochem, San Diego, CA) was given intraperitoneally 2 to 3 hours before the transplantation and then daily afterward until 14 days after the transplantation. In some experiments this treatment was continued 3 times weekly until 28 days after the transplantation. Thy1.2+ T cells were purified by positive selection with a magnetic cell separation system (Miltenyi Biotech, Auburn, CA). The purity of T cells used in this study ranged from 95% to 99%. For measurement of proliferative responses in vivo, purified donor T cells were labeled with carboxy-fluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR)20 and transferred via tail vein into previously irradiated (1000 cGy) B10.BR (H2k) recipients (10 × 106/mouse).
Immunofluorescence analysis
Three- or 4-color cell staining was performed to measure the expression of surface molecules according to standard techniques, with the use of a FACScan or a FACScalibur and CellQuest software (Becton Dickinson, San Jose, CA). Abs conjugated with fluoroscein isothiocyanate (FITC), phycoerythrin (PE), Cy-Chrome, allophycocyanin (APC), streptavidin-PE, and streptavidin–Cy-Chrome were purchased from PharMingen (San Diego, CA), and biotin-labeled anti-Ly5.1 Ab was prepared in our laboratory.
Cytokine analysis
Blood samples were obtained on day 14 after transplantation. Cytokine levels in the serum were measured using a cytometric bead array kit according to the manufacturer's instructions (Becton Dickinson, San Jose, CA).
T- and B-cell proliferation assay
Total splenocytes (1 × 105/well) were stimulated in 96-well plates with either Concanavalin A (ConA) at 5 μg/mL or with lipopolysaccharide (LPS) at 10 μg/mL (Sigma-Aldrich, St Louis, MO). 3H-Thymidine incorporation was measured after 3 days of culture.
Statistical analysis
The log-rank test was used to detect statistical differences in survival. Student t test was used to compare the engraftment, expansion, or depletion of donor T cells and the serum cytokine levels in recipients.
Results
Rapamycin has immunosuppressive activity independent of CD28 engagement
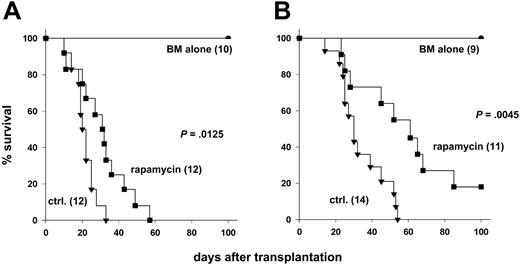
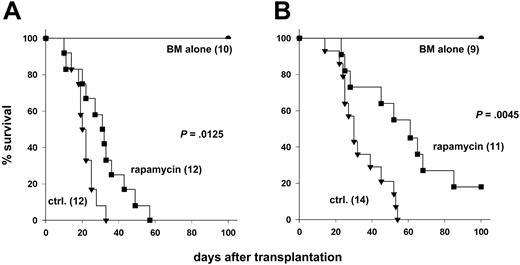
Rapamycin may inhibit multiple T-cell activation pathways, including a cyclosporine-resistant pathway that is activated by CD28.21 To address whether the immunosuppressive activity of rapamycin depends on CD28 costimulation in vivo, we used CD28-deficient (CD28-/-) or wild-type (CD28+/+) donor cells and tested the effects of rapamycin treatment on development of GVHD. Transplantation of CD28-/- T cells significantly delayed GVHD mortality when compared with transplantation of CD28+/+ T cells (P = .002). We tested the immunosuppressive activity of rapamycin administered daily at 1.5 mg/kg for 14 days, a regimen that had been previously shown by others to be effective for preventing GVHD in mice.9 Rapamycin significantly improved survival after transplantation of CD28+/+ T cells (Figure 1A; P = .01) or CD28-/- T cells (Figure 1B; P = .005), compared with controls that did not receive rapamycin. Because we were concerned about the potential toxicity of high-dose rapamycin, we also tested rapamycin at 0.5 mg/kg daily, but this regimen did not alter GVHD lethality mediated by CD28+/+ T cells under the conditions used for these experiments (data not shown). These data demonstrate that the in vivo immunosuppressive effects of rapamycin do not appreciably depend on engagement of CD28.
Rapamycin has immunosuppressive activity independent of CD28 engagement. Lethally irradiated (1000 cGy) B10.BR recipients were given transplants of 15 × 106 T-cell–depleted marrow cells with or without 15 × 106 splenocytes from (A) CD28+/+ or (B) CD28-/- C57BL/6 donors. Recipients were treated intraperitoneally with rapamycin or with control solvent. Numbers of recipients are indicated in parentheses. Data were pooled from 3 (A) and 2 (B) experiments, respectively.
Rapamycin has immunosuppressive activity independent of CD28 engagement. Lethally irradiated (1000 cGy) B10.BR recipients were given transplants of 15 × 106 T-cell–depleted marrow cells with or without 15 × 106 splenocytes from (A) CD28+/+ or (B) CD28-/- C57BL/6 donors. Recipients were treated intraperitoneally with rapamycin or with control solvent. Numbers of recipients are indicated in parentheses. Data were pooled from 3 (A) and 2 (B) experiments, respectively.
Anti-CD28 mAb prevents GVHD lethality in a myeloablative model of T cell–replete marrow transplantation
We have shown previously that CD28 stimulation with an agonistic mAb facilitates peripheral T-cell deletion and partially prevents GVHD in mice.4,6 In further experiments, we investigated whether anti-CD28 mAb is also effective in a very stringent, fully myeloablative GVHD model. Preliminary experiments were carried out to define the lowest effective mAb dose. Lethally irradiated B10.BR recipients were given T-cell–depleted marrow and splenocytes from B6 donors. A single 20-μg dose of anti-CD28 mAb delayed the onset of GVHD and improved survival compared to control Ab (P = .001). A single 100-μg dose protected 60% of recipients from lethal acute GVHD, and survival with this regimen was better than with a single 20-μg dose (P = .02) or with control antibody (P < .001; Figure 2). Results with regimens of 20 μg (Figure 2) or 100 μg (data not shown) administered every other day for 8 days were similar to those with a single 100-μg dose (P = .6 for both). These results show that anti-CD28 mAb can prevent GVHD in a myeloablative, T-cell–replete marrow transplantation model when administered at doses of at least 100 μg per mouse.
Anti-CD28 mAb prevents GVHD lethality in a myeloablative model of T-cell–replete marrow transplantation. Lethally irradiated (1200 cGy) B10.BR recipients were given transplants of 15 × 106 T cell–depleted marrow cells plus 25 × 106 splenocytes from B6 donors. Recipients were treated intraperitoneally with the indicated anti-CD28 mAb (αCD28) dose once on day 0, or every other day × 8, or with control antibody (ctrl.). Numbers of recipients are indicated in parentheses. Data were pooled from 2 experiments.
Anti-CD28 mAb prevents GVHD lethality in a myeloablative model of T-cell–replete marrow transplantation. Lethally irradiated (1200 cGy) B10.BR recipients were given transplants of 15 × 106 T cell–depleted marrow cells plus 25 × 106 splenocytes from B6 donors. Recipients were treated intraperitoneally with the indicated anti-CD28 mAb (αCD28) dose once on day 0, or every other day × 8, or with control antibody (ctrl.). Numbers of recipients are indicated in parentheses. Data were pooled from 2 experiments.
Anti-CD28 mAb and rapamycin are synergistic in preventing lethal GVHD
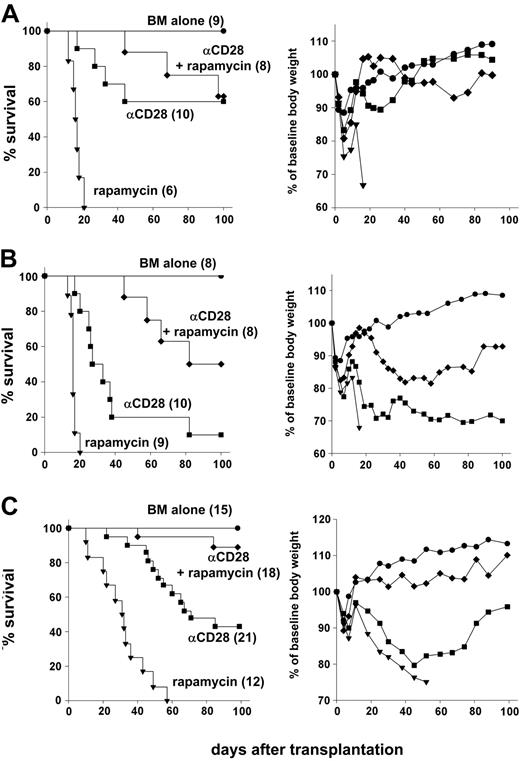
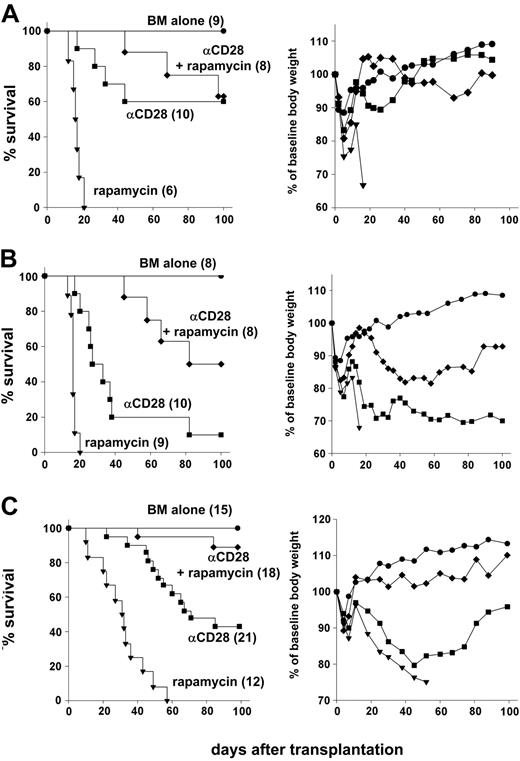
GVHD prevention by anti-CD28 mAb depends on activation-induced T-cell depletion,6 while rapamycin interferes with cell-cycle progression.8 We therefore tested whether rapamycin interferes with the immunosuppressive effects of anti-CD28 mAb, or whether the 2 agents have synergistic activity. When a 28-day course of rapamycin at 1.5 mg/kg was tested together with a single 100-μg dose of anti-CD28 mAb, survivors showed only minimal clinical signs of GVHD and regained normal body weight (Figure 3A). These results indicate that antagonism between the 2 agents was not appreciable.
Anti-CD28 mAb and rapamycin are synergistic in preventing lethal GVHD. (A) Lethally irradiated (1200 cGy) B10.BR recipients were given transplants of 15 × 106 T-cell–depleted marrow cells plus 25 × 106 splenocytes from C57BL/6 donors. Recipients were treated intraperitoneally with either anti-CD28 mAb (αCD28) at 100 μg × 1 day or rapamycin (rapamycin) at 1.5mg/kg × 14 days or both (αCD28 + rapamycin). (B) Lethally irradiated (1200 cGy) B10.BR recipients were given transplants of 15 × 106 T cell–depleted marrow cells plus 25 × 106 splenocytes from C57BL/6 donors. Recipients were treated intraperitoneally with either anti-CD28 mAb at 20 μg × 1 day or rapamycin at 1.5mg/kg × 14 days or both. (C) Lethally irradiated (1000 cGy) B10.BR recipients were given transplants of 15 × 106 T-cell–depleted marrow cells plus 15 × 106 splenocytes from C57BL/6 donors. Recipients were treated intraperitoneally with either αCD28 mAb alone or rapamycin at 1.5mg/kg × 14 days or both. Anti-CD28 mAb was given either at 100 μg × 1 day or 100 μg × 8 days, and data were combined because there was no difference in effect. Left panels show survival, and right panels show average body weight across time compared with baseline (day -1). Numbers of recipients are indicated in parentheses. Data are pooled from 2 (A) and 3 experiments (B-C), respectively.
Anti-CD28 mAb and rapamycin are synergistic in preventing lethal GVHD. (A) Lethally irradiated (1200 cGy) B10.BR recipients were given transplants of 15 × 106 T-cell–depleted marrow cells plus 25 × 106 splenocytes from C57BL/6 donors. Recipients were treated intraperitoneally with either anti-CD28 mAb (αCD28) at 100 μg × 1 day or rapamycin (rapamycin) at 1.5mg/kg × 14 days or both (αCD28 + rapamycin). (B) Lethally irradiated (1200 cGy) B10.BR recipients were given transplants of 15 × 106 T cell–depleted marrow cells plus 25 × 106 splenocytes from C57BL/6 donors. Recipients were treated intraperitoneally with either anti-CD28 mAb at 20 μg × 1 day or rapamycin at 1.5mg/kg × 14 days or both. (C) Lethally irradiated (1000 cGy) B10.BR recipients were given transplants of 15 × 106 T-cell–depleted marrow cells plus 15 × 106 splenocytes from C57BL/6 donors. Recipients were treated intraperitoneally with either αCD28 mAb alone or rapamycin at 1.5mg/kg × 14 days or both. Anti-CD28 mAb was given either at 100 μg × 1 day or 100 μg × 8 days, and data were combined because there was no difference in effect. Left panels show survival, and right panels show average body weight across time compared with baseline (day -1). Numbers of recipients are indicated in parentheses. Data are pooled from 2 (A) and 3 experiments (B-C), respectively.
To test for synergy, we combined treatment with 1.5 mg/kg rapamycin for the first 14 days after transplantation with a single 20-μg dose of anti-CD28 mAb. This regimen led to 50% survival of the recipients, which was significantly better than with anti-CD28 mAb (P = .001) or rapamycin (P < .001) alone (Figure 3B, left panel). These results suggested that rapamycin and anti-CD28 mAb are synergistic in the prevention of GVHD. With this suboptimal dose of anti-CD28 mAb, however, survivors still had clinical signs of GVHD as indicated by failure to regain body weight, compared with recipients of T cell–depleted marrow alone (Figure 3B, right panel). The conditions used for experiments in Figures 2, 3A, and 3B were deliberately selected for high stringency in order to detect dose responses and synergy. In further experiments, we relaxed the model by decreasing the total body irradiation from 1200 to 1000 cGy and by decreasing the number of splenocytes in the grafts from 25 × 106 to 15 × 106 per recipient. When treated with anti-CD28 mAb and rapamycin, 89% of the recipients survived for more than 100 days, which was longer than with either agent alone (P < .001). Survivors treated with the combined regimen were largely free of clinical GVHD as indicated by the pattern of body weight gain and immune reconstitution (see Figure 3C, right panel, and Table 2).
In summary, these experiments demonstrate synergy between rapamycin and anti-CD28 mAb at suboptimal doses of either reagent. At higher doses the effect was too large to appreciate synergy or additive effects, but the results excluded an inhibitory effect of rapamycin on anti-CD28 mAb-mediated signals. These data further show that a combination of anti-CD28 mAb with rapamycin is effective in preventing lethal GVHD in a clinically relevant, fully myeloablative, MHC class I– and II–mismatched HCT model.
Rapamycin does not interfere with anti-CD28 mAb-mediated depletion of alloreactive T cells
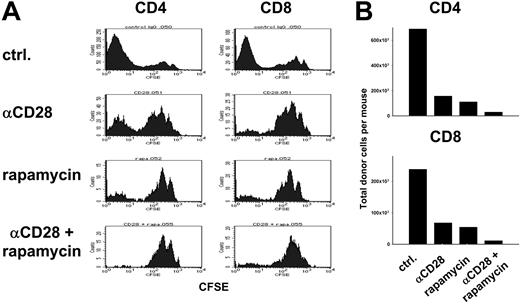
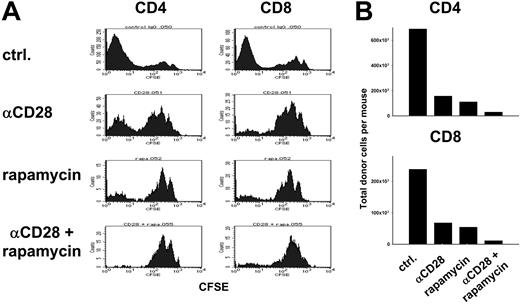
Because anti-CD28 mAb-induced apoptosis requires T-cell activation6 while rapamycin interferes with T-cell proliferation by inhibiting G1 → S phase progression,22 we were concerned that rapamycin might inhibit T-cell apoptosis induced by anti-CD28 mAb. To test this possibility, CFSE-labeled B6 T cells were injected into lethally irradiated B10.BR recipients, and cell division and expansion of donor CD4+ and CD8+ cells in vivo were assessed 4 days later (Figure 4). Treatment with anti-CD28 mAb depleted activated donor cells that proliferated rapidly in response to recipient alloantigens (Figure 4A), consistent with previously published results.6 Treatment with either anti-CD28 or rapamycin decreased the number of CFSE-dull donor cells on day 4, consistent with inhibition of proliferation or T-cell depletion (Figure 4A). With the combination of both anti-CD28 mAb and rapamycin, the total numbers of donor CD4+ cells and CD8+ cells in the recipients were lower than with anti-CD28 mAb or rapamycin alone (Figure 4B). Taking our previously published data into account,6 these results indicate that rapamycin does not interfere with the depletion of rapidly proliferating T cells by anti-CD28 mAb.
Rapamycin and anti-CD28 mAb limit expansion of alloreactive T cells. Lethally irradiated (1200 cGy) B10.BR recipients were given transplants of 10 × 106 CFSE-labeled T cells from B6 donors. Recipients were treated intraperitoneally with either anti-CD28 mAb at 20 μg × 1 day (αCD28), or rapamycin at 1.5 mg/kg × 4 days (rapamycin) or both combined (αCD28 + rapamycin). Splenocytes were harvested on day 4 after transplantation and stained for H2-Kb, CD4, and CD8. (A) CFSE profile is shown separately for donor type (H2-Kb) CD4+ and CD8+ cells. (B) Absolute numbers of donor CD4+ and CD8+ cells are shown for each group. Samples were pooled from 3 to 4 recipients per group. Results are representative of 2 replicate experiments.
Rapamycin and anti-CD28 mAb limit expansion of alloreactive T cells. Lethally irradiated (1200 cGy) B10.BR recipients were given transplants of 10 × 106 CFSE-labeled T cells from B6 donors. Recipients were treated intraperitoneally with either anti-CD28 mAb at 20 μg × 1 day (αCD28), or rapamycin at 1.5 mg/kg × 4 days (rapamycin) or both combined (αCD28 + rapamycin). Splenocytes were harvested on day 4 after transplantation and stained for H2-Kb, CD4, and CD8. (A) CFSE profile is shown separately for donor type (H2-Kb) CD4+ and CD8+ cells. (B) Absolute numbers of donor CD4+ and CD8+ cells are shown for each group. Samples were pooled from 3 to 4 recipients per group. Results are representative of 2 replicate experiments.
Combination of anti-CD28 mAb and rapamycin inhibits donor CD4+ cell expansion, improves early B-cell reconstitution, and reduces inflammatory cytokine levels
To determine how rapamycin enhances the ability of anti-CD28 mAb to prevent GVHD, we analyzed the numbers of donor spleen and marrow-derived cells in the spleens of recipients 14 days after transplantation (Table 1). The combination of anti-CD28 mAb treatment and rapamycin led to a significant reduction in the number of donor spleen–derived CD4+ cells, which contribute to GVHD in this model. The combined treatment also induced a significant increase in the number of donor marrow–derived CD4+ cells. Because the donor marrow was T cell–depleted before transplantation, these cells represent the progeny of precursors that differentiated in the recipient thymus. Treatment with rapamycin alone also resulted in significantly higher numbers of marrow-derived CD4+ cells, but treatment with anti-CD28 mAb alone did not have these effects. These results might indicate that the addition of rapamycin enhances the reconstitution of tolerant donor marrow–derived T cells. Compared with either rapamycin or anti-CD28 mAb alone, the combination increased the number of donor marrow–derived B cells in the spleens of recipients on day 14 after transplantation. B-cell reconstitution is a surrogate marker for immune tolerance in this GVHD model.
To assess long-term functional capacity of donor cells we followed recipients until day 120 and assessed T- and B-cell functions in vitro. Mice that received marrow plus spleen cells without rapamycin or anti-CD28 mAb all died before day 120. Mice that received rapamycin alone also died before day 120. Recipients surviving after treatment with anti-CD28 mAb alone had reduced numbers of T- and B-cells in the spleen, and proliferative responses after stimulation with ConA and LPS, respectively, were decreased compared with those with cells from marrow recipients (Table 2). Total numbers of T- and B-cells were also decreased in recipients surviving after treatment with both rapamycin anti-CD28 mAb, but proliferative responses were comparable to those with cells from marrow recipients.
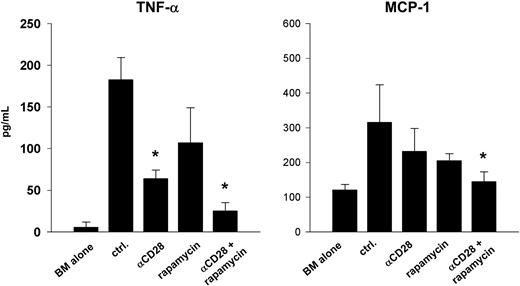
We also tested the effect of combined treatment on the secretion of inflammatory cytokines, previously shown by others to play an important role in GVHD pathophysiology.20,23 On day 14, recipients treated with both agents had significantly reduced serum levels of tumor necrosis factor-α (TNF-α) compared with control treatment, rapamycin alone, or anti-CD28 mAb alone. Macrophage chemoattractant protein 1 (MCP-1) levels were also reduced significantly when compared with control treatment (Figure 5). Levels of IL-6, IL-10, IL-12, and interferon-α were either not different between groups or below the threshold of detection in the assay (data not shown). Taken together, these results demonstrate that a combination of rapamycin and anti-CD28 mAb prevents GVHD by inhibiting the expansion of mature, alloreactive donor CD4+ cells, decreasing secretion of inflammatory cytokines, and by promoting T- and B-cell reconstitution.
Rapamycin and anti-CD28 mAb reduce serum levels of inflammatory cytokines. Lethally irradiated (1000 cGy) B10.BR recipients were given transplants of 15 × 106 T-cell–depleted marrow cells from B6 donors plus 15 × 106 splenocytes from B6.Ly5.1 donors or marrow alone. Recipients of marrow and splenocytes were treated intraperitoneally with either control solvent (ctrl.), anti-CD28 mAb at 100 μg × 1 day (αCD28), rapamycin at 1.5 mg/kg × 14 days (rapamycin) or the combination of both (αCD28 + rapamycin). Serum cytokine levels were drawn on day 14. Asterisks denote significant differences compared with control treatment. Groups contained 3 to 4 recipients. Data are displayed as means ± SD and are representative of 2 replicate experiments.
Rapamycin and anti-CD28 mAb reduce serum levels of inflammatory cytokines. Lethally irradiated (1000 cGy) B10.BR recipients were given transplants of 15 × 106 T-cell–depleted marrow cells from B6 donors plus 15 × 106 splenocytes from B6.Ly5.1 donors or marrow alone. Recipients of marrow and splenocytes were treated intraperitoneally with either control solvent (ctrl.), anti-CD28 mAb at 100 μg × 1 day (αCD28), rapamycin at 1.5 mg/kg × 14 days (rapamycin) or the combination of both (αCD28 + rapamycin). Serum cytokine levels were drawn on day 14. Asterisks denote significant differences compared with control treatment. Groups contained 3 to 4 recipients. Data are displayed as means ± SD and are representative of 2 replicate experiments.
Discussion
In this study we found that the immunosuppressive activity of rapamycin is independent of CD28 engagement and that rapamycin plus an agonistic anti-CD28 mAb is superior to rapamycin plus CD28 blockade for preventing lethal acute GVHD in mice. Rapamycin and anti-CD28 mAb treatment showed synergism at suboptimal doses, and therapeutic doses were highly effective in preventing lethal GVHD in a myeloablative model of MHC class I– and II–mismatched HCT. The combination treatment reduced the number of proliferating, alloreactive cells in the recipient, promoted B- and T-cell reconstitution, and reduced inflammatory cytokine levels. These results indicate that agonistic antibodies specific for CD28 combined with rapamycin might offer a promising, new therapeutic approach to target cells that recognize transplant alloantigens.
The main effect of anti-CD28 mAb treatment is depletion of alloactivated, dividing T cells, while T cells undergoing homeostatic repopulation are spared.6 Rapamycin inhibits the proliferation of activated T cells by blocking G1 to S phase progression,8 and increases activation-induced apoptosis.16,24-27 These two effects of rapamycin are not contradictory because activation-induced apoptosis occurs in late G1 phase.28 Our results show that rapamycin does not completely block T-cell division (Figure 4), and data presented on Table 1 indicate that alloreactive T cells remain susceptible to depletion by anti-CD28 mAb in the presence of rapamycin. A similar effect of rapamycin has also been reported for anti–CD40-ligand treatment, where rapamycin allows the nuclear factor κB (NF-κB)–dependent activation of T cells.29
Depletion of alloreactive T cells by anti-CD28 mAb was not complete. In this study we have shown that, when added to anti-CD28 mAb, rapamycin further depleted total T-cell numbers and decreased the fraction of proliferating, alloreactive T cells. These data cannot distinguish whether rapamycin enhances T-cell depletion or inhibits replication; both mechanisms may be operative. We favor the model that, while permitting depletion of activated T cells by anti-CD28 mAb, rapamycin was responsible for further depleting residual alloreactive T cells, because the numbers of donor spleen-derived CD4+ T cells were further reduced by the addition of rapamycin to anti-CD28 mAb, while the numbers of donor marrow–derived CD4+ T cells were increased (Table 1).
The molecular mechanisms by which rapamycin permits T-cell depletion and enhances GVHD protection by anti-CD28 mAb remain to be investigated. Rapamycin does not interact with the Ca++-dependent inositol 3′ phosphate pathway initiated by T-cell–receptor binding and CD28 costimulation that ultimately leads to transcription and secretion of IL-2 and other T cell growth factors.30 Recently, Thompson and colleagues31,32 found that CD28-signals increase the rate of glycolysis and protein metabolism, which is mediated through two signaling pathways, the phosphati-dylinositol 3-kinase/Akt/mTOR pathway and the serine/threonine kinase pim-2 pathway.33 While the activity of mTOR is blocked by rapamycin,8 the activation of pim-2 is resistant to rapamycin.31 Therefore, we surmise that in the presence of rapamycin, engagement of CD28 with its agonistic Ab could signal through the proviral integration site 2 of murine leukemia (pim-2)–dependent pathway, but whether pim-2 activation leads to T-cell apoptosis remains to be determined. Depletion of most alloreactive T-cell clones may be necessary to allow regulatory T cells to overcome and control the remaining alloreactive cells.19,34,35 The effects of rapamycin and agonistic CD28 signaling on regulatory T cells remain to be evaluated in future studies.
We tested whether rapamycin could induce tolerance on alloreactive T cells and prevent GVHD lethality when CD28 is absent on donor T cells. Although treatment with rapamycin significantly delayed GVHD lethality after transplantation of CD28-/- as well as CD28+/+ donor T cells, tolerance was not induced, since most recipients eventually died (Figure 1). These results showed that blockade of CD28 in combination with rapamycin is not sufficient to prevent GVHD. Because treatment with an agonistic anti-CD28 mAb plus rapamycin prevented GVHD lethality and produced tolerance in most CD28+/+ T-cell recipients (Figure 3), these data support the concept that agonistic anti-CD28 mAbs might be more potent than inhibitors of CD28 in preventing GVHD and facilitating immunologic tolerance after T cell–replete marrow transplantation.
Because HCT is used most often to treat malignant disease, future studies will have to address whether graft-versus-malignancy effects can be preserved when the combination of rapamycin and anti-CD28 is used to prevent GVHD. Interaction between CD28 and B-7 is an important part of the immune response against malignant cells after HCT.36 We therefore believe that short-term agonistic engagement of CD28 on T cells could be advantageous compared with strategies using costimulatory blockade to prevent GVHD. Furthermore, rapamycin itself has antitumor activity, potentially enhancing graft-versus-tumor effects after transplantation.37
Our findings provide a rationale for the development of an improved GVHD prevention regimen for patients undergoing HCT because short-term immunosuppressive therapy with the combination of rapamycin and agonistic CD28 signaling allows for tolerance induction. Unlike established T cell–depleting agents such as antithymocyte globulin or alemtuzumab (Campath-1H; Berlex, Richmond, CA), agonistic anti-CD28 mAb treatment selectively removes alloactivated T cells, thereby leaving intact immunity against infections and malignant cells. For initial clinical studies, anti-CD28 mAb treatment will have to be combined with one or more routinely used immunosuppressive medications. Our current results provide support for the combination of rapamycin with agonistic anti-CD28 mAbs, but other immunosuppressive medications remain to be tested for synergistic activity with agonistic anti-CD28 mAbs.
Prepublished online as Blood First Edition Paper, September 30, 2004; DOI 10.1182/blood-2004-08-3305.
Supported by National Institutes of Health Grants CA 84132 (X.-Z.Y.), CA 18029, and AI 51693 (C.A.), and by a Dr.-Mildred-Scheel fellowship from Deutsche Krebshilfe (German Cancer Aid) (M.H.A.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Sasha Mayer, Margaret Castor, and Kelli McIntyre technical assistance.