Comment on Jordan et al, page 1500
The activity of platelet membrane receptors is closely regulated by stimulation-dependent changes in their extracellular conformation. Accumulating evidence points to a crucial role for enzymatically catalyzed disulfide isomerization in this regulation.
The mammalian protein disulfide isomerase (PDI) family is composed of several structurally related enzymes that can catalyze the formation of disulfide bonds between cysteines, reduce disulfide bonds to free thiols, or exchange the pairing of cysteines in a protein. In addition to their roles as redox catalysts and isomerases, they have chaperone activity. They are residents of the endoplasmic reticulum (ER) and are involved in the processing and maturation of proteins, being part of a quality-control system for the correct folding of nascent proteins. These enzymes are characterized by the presence of one or more domains related to the cytoplasmic protein thioredoxin. Most are composed entirely of repeats of such domains, with at least one domain containing the redox-active CXXC tetra peptide.1
In recent years, a number of PDIs were identified in non-ER locations such as the cell surface, the extracellular space, the cytosol, and the nucleus.2 Their role in these locations is subject to intensive research. The origin of cell surface PDI is not clear and some claim it is secreted and then reattaches to the cell surface, either electrostatically or via binding to other proteins.FIG1
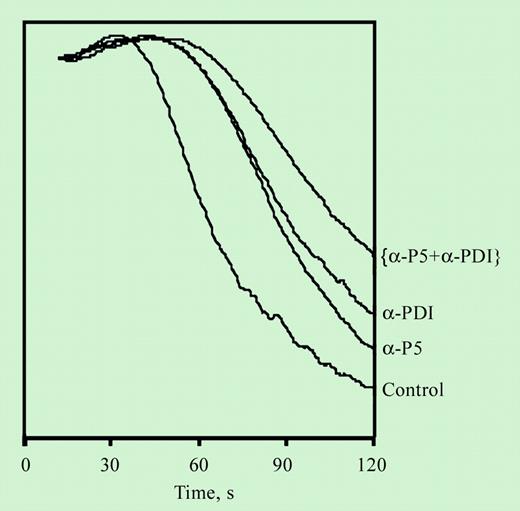
Inhibition of platelet aggregation by an inhibitory antibody for ERP5. See the complete figure in the article beginning on page 1500.
Inhibition of platelet aggregation by an inhibitory antibody for ERP5. See the complete figure in the article beginning on page 1500.
Classical PDI, the most extensively studied member of this family, was detected on the surface of the platelet and was recently shown to regulate integrin-mediated platelet adhesion and aggregation.3,4 Thus, blocking PDI with monoclonal antibodies inhibits adhesion to fibrinogen, fibronectin, and integrin-specific collagen peptide but not to von Willebrand factor (VWF) or glycoprotein VI (GPVI)–specific peptide. The same antibodies inhibit stimulation-dependent platelet aggregation and binding of the stimulation-dependent antibody PAC-1. The mechanism of this regulation is yet to be elucidated; however, involvement of extracellular cysteines of the β subunit of the integrins has been suggested.
Specific inhibition of classical PDI with monoclonal anti-PDI antibodies consistently produces lesser inhibition than direct blocking of free thiols. This observation suggests either incomplete inhibitory effect of the antibodies or the presence of yet another disulfide isomerase on the platelet surface that complements the activity of PDI. The presence of the sequence CXXC on the β subunit of integrins suggests disulfide isomerase activity of the integrin itself. However, the redox activity of αIIbβ3 seems considerably lower than that of PDI.5 In this issue of Blood, Jordan and colleagues show that another member of the PDI family, known as P5, is expressed and active on the platelet surface. Moreover, the activity of the platelet-surface P5, referred to as ERP5 in this publication, complements that of platelet-surface PDI, in that blocking both enzymes had an additive effect on the inhibition of aggregation and fibrinogen binding observed with either enzyme blocked alone. Surface expression of P5 increases with agonist-induced platelet activation, suggesting intracellular localization in the unstimulated platelet with translocation to the surface following stimulation. Moreover, P5 forms a complex with the platelet integrin αIIbβ3. The demonstration of activation-dependent expression of P5 on the platelet surface, its role in fibrinogen-mediated aggregation, and its complexation with the platelet integrin emphasize the significance of the emerging role of surface-associated redox mechanisms in regulating platelet function. ▪