Abstract
Activated protein C (APC) treatment is now used for patients with severe sepsis. We investigated its effect in vitro on primary, physiologically relevant cells and demonstrate a novel mechanism of endothelial protein C receptor (EPCR) release that is not inhibited by metalloproteinase inhibitors. Exposure of human umbilical vein endothelial cells or monocytes to APC (6.25-100 nM) results in the release of EPCR-containing microparticles, as demonstrated by confocal microscopy and characterized through flow cytometry, enzyme-linked immunosorbent assay quantitation of isolated microparticles, and Western blotting. The phenomenon is time- and concentration-dependent and requires the APC active site, EPCR, and protease activated receptor 1 (PAR1) on endothelial cells. Neither protein C nor boiled or d-Phe-Pro-Arg-chloromethylketone–blocked APC can induce microparticle formation and antibody blockade of EPCR or PAR1 cleavage and activation abrogates this APC action. Coincubation with hirudin does not alter the APC effect. The released microparticle bound is full-length EPCR (49 kDa) and APC retains factor V–inactivating activity. Although tumor necrosis factor-α (10 ng/mL) can also induce microparticle-associated EPCR release to a similar extent as APC (100 nM), it is only APC-induced microparticles that contain bound APC. This novel observation could provide new insights into the consequences of APC therapy in the septic patient.
Introduction
The protein C (PC) pathway is important in both sepsis and thrombotic disease. The inhibition of blood coagulation by this pathway is triggered when thrombin binds with high affinity (Kd ∼ 0.1 nM) to thrombomodulin, to activate PC that is bound to the endothelial protein C receptor (EPCR).1 The activated protein C (APC) then enzymatically inactivates clotting factors Va and VIIIa to limit further thrombin procoagulant activity.2 Congenital PC deficiency can manifest in widespread microvascular thrombosis and acquired PC deficiency occurs in severe sepsis due to consumption and inactivation by tight complex binding with a number of serine protease inhibitors.3 More recently, the administration of recombinant human APC was shown to significantly reduce the relative rate of mortality by 19.4% in patients with severe sepsis.4
The success of APC in sepsis has been attributed to the combination of both anti-inflammatory and anticoagulant properties. EPCR may provide a pivotal link in this. Structurally, it is a member of the major histocompatibility complex/CD1 superfamily, all of which are involved in inflammation.5 Although first established on endothelial cells, EPCR is also present on monocytes and to a lesser extent on neutrophils and eosinophils.6-8 Its in vivo relevance has been established because blocking its function converts a sublethal baboon model of sepsis into lethality. In these animals, both pronounced microvascular thrombosis and increased neutrophilic infiltration are seen within tissue sections.9
In coagulation, EPCR affects APC bioavailability in 2 ways. First, as mentioned, it promotes PC activation at the cell surface.1 Blocking EPCR presentation of PC to the thrombin-thrombomodulin complex reduces PC activation rates by 80%. In addition to this membrane form, a soluble form of EPCR (sEPCR) has also been identified in human plasma.10 The release of this form is by metalloproteinase-mediated cleavage. This occurs constitutively and is augmented by thrombin and interleukin-1β (IL-1β).11 However, when APC binds to sEPCR, it can no longer inactivate factor Va and this is presumably due to conformational changes caused by proteolytic shedding.12 The consequent decrease in APC activity could have procoagulant effects if circulating concentrations of sEPCR were high, for example, in sepsis and systemic lupus erythematosus.13
EPCR is also required in APC-mediated signaling. One mechanism is as the required coreceptor for the proteolytic action of APC on endothelial cell protease-activated receptor 1 (PAR1).14 This has been shown in both heterologous expression systems and physiologically relevant primary endothelial cells at low, physiologically achievable concentrations of APC (< 10 nM). This potentially confers vascular cell-type specificity in PAR-mediated signaling and therapeutically administered APC may use residual EPCR in signaling to achieve protection from severe sepsis. An alternative mechanism may be through EPCR translocation of APC into the nucleus to affect gene regulation.15 This may, however, vary according to cell type. In this study, we set out to explore the fate of APC in primary physiologic cells by confocal microscopy. We observed that APC induces the formation and release of EPCR-containing microparticles (MPs). This report describes and characterizes this phenomenon.
Materials and methods
Reagents
Human factors Va and Xa, (FVa, FXa), prothrombin, PC, and APC were obtained from Hematological Technologies (Essex Junction, VT). Human APC was also obtained from Sigma Aldrich (Poole, United Kingdom). Monoparin 1000 IU/mL was obtained from CP Pharmaceuticals (Lewes, United Kingdom). The metalloproteinase inhibitor 1,10-phenanthroline was from Sigma Aldrich and Ro31-9790 was a kind gift from Roche (Milan, Italy). d-Phe-Pro-Arg-chloromethyl ketone (PPACK) was from Calbiochem (Nottingham, United Kingdom) and the chromogenic substrates S2366 and S2238 were from Chromogenix Laboratory, (Milan, Italy). RPMI 1640 medium, O-phenylenediamine dihydrochloride (OPD) and annexin V–fluorescein isothiocyanate (FITC) conjugated were from Invitrogen (Paisley, United Kingdom), Dako (Glostrup, Denmark), and BD Pharmingen (BD Biosciences, Oxford, United Kingdom), respectively. In terms of EPCR-specific reagents, sEPCR was kindly provided by Dr H. Nakatake (Chemo-Sero-Therapeutic Research Institute, Saga, Japan) and the anti-EPCR antibodies were MCR-1, RCR-2, RCR-49, and RCR-252 as previously described.16 Antirat immunoglobulin G (IgG, whole molecule) labeled with peroxidase or FITC, isotype control rat IgG (whole molecule), IL-1β, and recombinant human tumor necrosis factor α (TNF-α) were obtained from Sigma Aldrich. CD13-phycoerythrin (PE), CD14-PE, corresponding IgG class control, and goat anti–mouse PE were from BD Biosciences, (San Jose, CA). The PAR1 antibody ATAP2 and antagonist peptide H-Met-Ser-Arg-Pro-Ala-Cys-Pro-Asn-Asp-Lys-Tyr-Glu-OH (T1) were from Santa Cruz Biotechnology (Santa Cruz, CA) and Bachem (St Helens, United Kingdom), respectively.
Cell culture and monocyte preparation
Human umbilical vein endothelial cells (HUVECs) were prepared by the method of Jaffe et al.17 Cells were isolated with trypsin and maintained in Iscove modified Dulbecco medium (IMDM; Life Technologies, Paisley, United Kingdom) with added 20% fetal calf serum (FCS; Labtech International, East Sussex, United Kingdom), 2 mM l-glutamine, 100 μg/mL streptomycin, 100 μg/mL penicillin, and 5 ng/mL epidermal growth factor (Invitrogen and Life Technologies). Only cells from passages 2 or 3 were used.
Monocytes were from peripheral blood of healthy donors, who gave informed consent; blood was collected into heparin anticoagulant, isolated by density-gradient centrifugation on Lymphoprep (Nycomed Pharma, Oslo, Norway), and washed in phosphate-buffered saline (PBS) twice before resuspending in RPMI 1640 with 10% FCS, 2 mM glutamine, 100 μg/mL streptomycin, and 100 μg/mL penicillin. Selection was by plastic adhesion and nonadherent cells were removed through PBS washing. Monocytes were lifted with 0.2% EDTA (ethylenediaminetetraacetic acid)/PBS and stained with CD14-conjugated antibodies for flow cytometric analysis.
APC induction of MP formation
Cells were grown to confluence in 6-well plates (unless otherwise stated) and serum-starved overnight, prior to incubation with PC or APC at varying concentrations (6.25-100 nM) and time durations (up to 24 hours) at 37°C. The APC purity was confirmed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) beforehand. Conditioned medium was collected and cell debris discarded by initial centrifugation at 5000g for 15 minutes at 4°C. The supernatant was then ultracentrifuged at 100 000g for 90 minutes to isolate the MPs. Pelleted MPs were washed in PBS and finally resuspended in PBS and stored at –20°C. MPs were also generated using lipopolysaccharide (LPS; 20 μg/mL; Sigma Aldrich) for 4 hours and isolated as described. In direct comparison experiments to the effect of APC (100 nM for 2 hours), TNF-α at a dose of 10 ng/mL, which has been described to induce MP release,18 was incubated with cells for 2 hours in the presence of Ro31-9790 to prevent sEPCR release.
Laser-scanned confocal microscopy
HUVECs grown to confluence in 8-chamber polystyrene vessel tissue culture-treated glass slides (Becton Dickinson, Bedford, MA) were treated as described (see “Cell culture and monocyte preparation”) and incubated with APC (100 nM) for 2 hours. Baseline controls were treated similarly but without APC. Cells were twice washed in PBS for 5 minutes, fixed with 3.7% formaldehyde in PBS for 20 minutes, washed thrice in PBS, and permeabilized with 0.2% Triton/PBS. Incubation with bovine serum albumin (BSA) then blocked nonspecific binding; staining was with the anti-EPCR antibody RCR-49 (10 μg/mL) at room temperature (RT) for 1 hour in PBS. Incubation with the second layer anti–rat FITC for 30 minutes in the dark occurred after 3 washes in PBS. There were 3 further PBS washes before mounting with Vectashield (Vector Laboratories, Burlingame, CA). The corresponding class control and a second layer control were done simultaneously. Monocytes were seeded onto 8-chamber polystyrene slides, incubated with APC, and stained likewise with the corresponding antibodies, as described. All cells and MPs were analyzed on an Olympus BH-2 microscope with Bio-Rad MicroRadiance (Hercules, CA) confocal scanning system and Laser Sharp 2000 software Bio-Rad. The same experiments were performed with an alternative APC product (see “Reagents”).
Analysis by fluorescence-activated cell sorting
Following PC or APC treatment, cells were lifted with 0.2% EDTA/PBS at 37°C, spun down, and resuspended in HBSS (10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 140 mM NaCl, 4.5 mM KCl, 2.5 mM CaCl2, 1% BSA, 0.1% sodium azide, pH 7.4; 0.2 μm filtered). Monocytes or HUVECs were stained with FITC-conjugated RCR-49 (10 μg/mL) at RT for 30 minutes, with monocytes stained by CD14-PE in HBSS at RT for 15 minutes in the dark. Cells were washed in HBSS, resuspended in FACSflow (Becton Dickinson), and analyzed by fluorescence-activated cell sorting (FACS).
In specific experiments, the role of metalloproteinase inhibition was investigated by incubating cells for 2 hours with either APC (100 nM), in the presence and absence of Ro31-9790 (30 μM) or 1,10-phenanthroline (5 mM), or the respective inhibitor alone. IL-1β (10 ng/mL) was also tested on HUVECs alone and with the metalloproteinase inhibitors at 37°C for 2 hours. Cells were then analyzed by FACS using RCR-49.
The possible influence of thrombin on APC-induced MP formation was investigated similarly with APC (100 nM) in the presence and absence of hirudin (6 μM) for 2 hours at 37°C.19 Investigating the APC active site requirement involved comparing the APC (100 nM) HUVEC effect with zymogen PC (100 nM), heat-inactivated APC (100 nM), prepared by boiling APC for 10 minutes,20 and PPACK-inhibited APC, prepared by preincubating PPACK (500 μg/mL) with APC (100 nM), for 1 hour at 37°C.
To determine the role of EPCR in APC-induced MP formation, the APC-binding site on EPCR was blocked by preincubating 2 × 106 serum-starved endothelial cells with RCR-252 (20 μg/mL), which blocks APC binding to EPCR by 90%16 for 30 minutes at 37°C, in IMDM supplemented with 2% FCS. Similarly, PAR1 activation was blocked by prior incubation with ATAP2 (50 μg/mL) and T1 (50 μM), respectively. After washing off unbound antibodies, cells were incubated with APC (100 nM) for 2 hours. The cells were then subjected to FACS with EPCR staining using RCR-49. MPs were isolated from the media (see “APC induction of MP formation”) and quantified as follows.
Quantitation of MPs
The amount of APC bound to MPs was estimated using an assay based on enzyme-linked immunosorbent assay (ELISA) methods. Coated with RCR-2, the antibody against EPCR that is not affected by APC binding, at 5 μg/mL in 50 mM NaHCO3, pH 9.5 with 2% BSA blocking after coating, isolated MPs were then captured for 1 hour at RT in HBSS/1 mM CaCl2/1 mM MgCl2 (assay buffer). After washing in the same buffer, APC on the captured MPs was detected chromogenically using S2366 with absorbance read at 405 nm on a Spectramax plate reader (Molecular Devices, Stanford, CA) after 30 minutes. Quantitation was from a purified APC-derived standard curve (linear from 0.4 nM to 32.5 nM). The coefficient of variation (CV) for intra-assay and interassay variability with this ELISA method was 8.6% and 12%, respectively. Given the novelty of this ELISA, we sought verification through an established approach using the 1B10 antibody that captures monocyte and endothelial cell-derived MPs and that was used for assaying bound tissue factor (TF) activity.18 Our findings were that the extent of APC bound to MPs was similar, irrespective of 1B10 or RCR-2 capture (data not shown). To study the possibility that APC on MPs could be due to passive binding to MPs already in solution, APC (6.25-100 nM) was added to LPS-generated MPs in assay buffer for 24 hours at 37°C, washed thrice in assay buffer, and APC estimated chromogenically in solution and via RCR-2 capture.
The amount of EPCR in membrane-bound form was measured similarly. Microtiter plates were coated with RCR-2 (5 μg/mL) and blocked with 2% BSA/PBS for 1 hour. MP samples were diluted in assay buffer and captured at RT for 90 minutes. Wells were washed thrice before anti-EPCR RCR-49-biotin (2 μg/mL) in assay buffer for 1 hour, washed thrice, and incubated with ExtrAvidin peroxidase (2 μg/mL; Sigma Aldrich) for 1 hour. After further washes, OPD (1.16 mg/mL) was applied and quenched with 0.5 M SO4H2 before reading absorbance at 492 nm. The obtained values were analyzed against a standard curve of sEPCR (range, 14-450 ng/mL). The intra-assay and interassay CV was 7.5% and 11.8%, respectively.
Flow cytometry was also used to quantify the MPs released in 3 separate experiments from HUVECs or monocytes, respectively, following comparative treatment with APC (100 nM) or TNF-α (10 ng/mL) for 2 hours.18 An internal control of calibrated polypropylene beads (Bangs Laboratories, Fisher, IN) was added and 5000 were counted. MPs were enumerated from the gate corresponding to annexin V–FITC+ events that costained with anti–CD13-PE or anti-EPCR (MCR-1) followed by goat anti–mouse PE.21 CD13 (glycoprotein 150) is expressed in both endothelial cells and monocytes. In all cases, isotypic controls were run at the same time.
Western blotting analysis
HUVECs or MPs were washed in PBS and lysed with 3% CHAPS (3-[(3-cholamidopropyl)dimethylammonio]-1-propane-sulfonic acid)/PBS in ice, followed by sonication and incubation in ice for 30 minutes. sEPCR was diluted in the same buffer to 40 ng/mL. All samples were heated at 95°C for 10 minutes and loaded onto a 5% stacking, 15% SDS/acrylamide-separating gel. Proteins were transferred onto Immobilon-P membrane (Millipore, Billerica, MA), blocked in 5% milk/T-TBS (0.1% Tween 20, 10 mM Tris [tris(hydroxymethyl)aminomethane]–HCl, pH 7.4, 0.1 M NaCl) for 1 hour at RT and incubated with RCR-2 (2 μg/mL) in 3% BSA/T-TBS followed by the peroxidase-conjugated secondary antibody. Detection was with enhanced chemiluminescence (ECL) reagents (Amersham, Little Chalfont, United Kingdom).
mRNA analysis
After incubation with APC (100 nM) from 2 to 24 hours, cells were washed with ice-cold PBS and total RNA extracted using the mini-RNeasy total RNA kit (Qiagen, Sussex, United Kingdom). Then, 1 μg RNA was used for reverse transcription using the first-strand cDNA synthesis kit for reverse transcription–polymerase chain reaction (RT-PCR; AMV; Roche Molecular Biochemicals, Indianapolis, IN). Transcribed cDNA was amplified by PCR using EPCR-specific primers (epcr-1: CAACTTCAGGATGTTGACAA and epcr-2: AATCCAGCCCCTTCTGACG). Primers amplifying β-actin were used as controls. Amplified products were run in 1% agarose gel in 0.5 × tris-borate ethylenediaminetetraacetic acid (TBE) and visualized under ultraviolet light (UV).
Activity of APC on MPs
The ability of the APC in the generated MPs to inactivate FVa was assayed as described by Liaw et al,12 First, MP-bound APC harvested from HUVECS treated with APC (100 nM) and grown in a 25-cm2 flask was quantified chromogenically (see “Quantitation of MPs”). Then, 0.2 nM FVa and 1.6 nM APC-MPs were coincubated in HBS (20 mM HEPES, pH 7.4, 100 mM NaCl), 3 mM CaCl2, and 0.1% gelatin at RT. After 30 minutes, 30 μM phenylmethylsulfonyl fluoride (PMSF) was added to inactivate APC. Residual FVa activity was measured by its ability to generate thrombin in the presence of excess FXa (2 nM), prothrombin (1.4 μM), and 3 mM CaCl2. After 5 minutes, the reaction was stopped with 8 mM EDTA and the thrombin generated determined chromogenically using S2238 at 405 nm absorbance against a standard curve of known α-thrombin concentrations. APC specificity was assessed by inhibition of its FVa-inactivating activity by α1-antitrypsin (α1-AT). For this, the MPs were incubated with α1-AT (1.6 nM APC-MP was incubated with 16 nM α1-AT) at 37°C overnight, prior to the reaction with FVa. A further control was APC in complex with sEPCR, prepared by incubating sEPCR (100 nM) with APC (10 nM) in HBS/3 mM CaCl2/0.6 mM MgCl2/0.1% gelatin at 37°C overnight. As an indicator of APC activity, we used 2 nM APC with 25 nM phosphatidylcholine (PC)/phosphatidylserine (PS) vesicles (70:30) in accordance with the method of Liaw et al.12
Statistical analysis
Data were expressed as the mean and SD of at least 3 independent experiments. In statistical calculations for comparison of groups, the Student paired t test was used; P values of less than .05 were considered statistically significant.
Results
APC induces MP-associated EPCR formation from monocytes and HUVECs
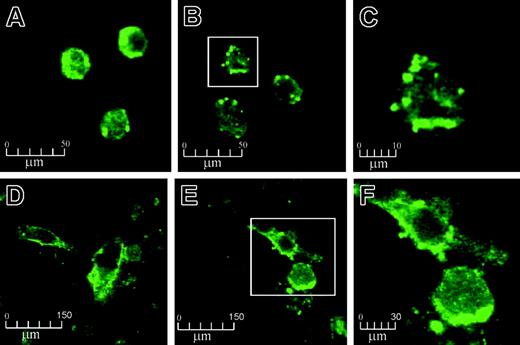
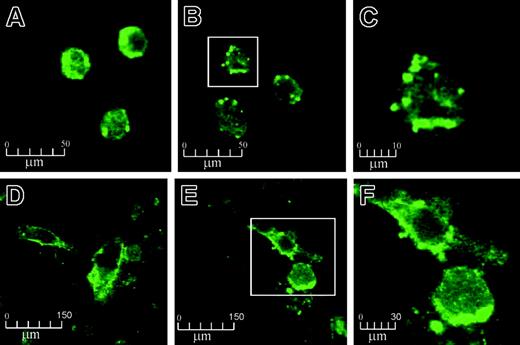
Confocal microscopy of human monocytes and HUVECs, stained against EPCR, revealed thatAPC exposure resulted in the formation and release of MPs from the cell surface (Figure 1). With untreated cells under controlled conditions, EPCR staining was generally distributed over the membrane surfaces (Figure 1A,D). Isotope-matched control was completely negative (data not shown). On APC treatment (100 nM for 2 hours), the EPCR distribution became more clustered with bleb formation and microvesiculation (Figure 1B,E). The MPs were richly stained for EPCR and measured from 0.5 to 3 μm in size (Figures 1C,F). These MPs could also bind annexin V (data not shown). The findings were reproduced with a different source of human APC and the same microscopic changes were still observed, albeit at reduced proportions, when APC concentrations were reduced to 0.5 nM APC (data not shown). No EPCR internalization was visualized in all these experiments.
The effect of APC on EPCR on monocytes and HUVECs by confocal microscopy. Monocytes and HUVECs were incubated with 100 nM APC for 2 hours and stained with EPCR antibody RCR-49, as described in “Materials and methods.” Monocytes and HUVECs are shown at × 630 magnification before treatment (A and D, respectively) and after APC incubation (B and E, respectively). Panels C and F represent further magnifications (× 4) of the boxed cells from panels B and E, respectively.
The effect of APC on EPCR on monocytes and HUVECs by confocal microscopy. Monocytes and HUVECs were incubated with 100 nM APC for 2 hours and stained with EPCR antibody RCR-49, as described in “Materials and methods.” Monocytes and HUVECs are shown at × 630 magnification before treatment (A and D, respectively) and after APC incubation (B and E, respectively). Panels C and F represent further magnifications (× 4) of the boxed cells from panels B and E, respectively.
MP-associated EPCR release is not metalloproteinase-dependent but requires the APC active site
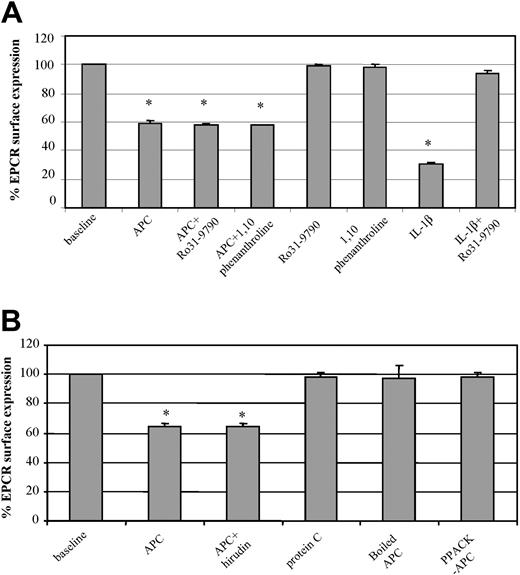
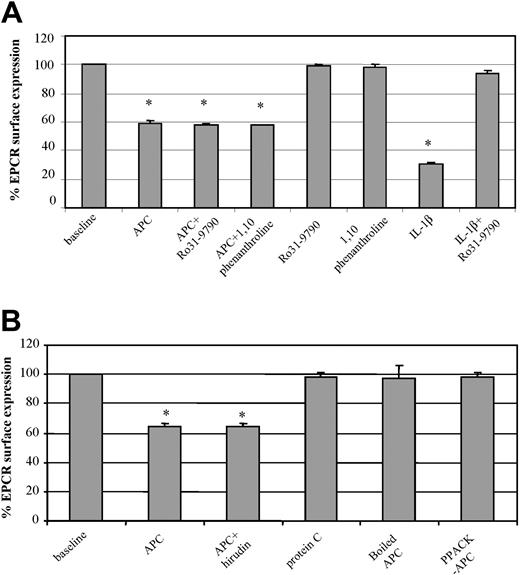
To further characterize the microscopic observation, changes in endothelial expression of EPCR were investigated by FACS. After 2 hours of HUVEC exposure to APC (100 nM), there was a significant 40% drop in EPCR expression (Figure 2A). When the cells were coincubated with metalloproteinase inhibitors Ro31-9790 (30 μg/mL) or 1,10-phenanthroline (5 mM) and APC (100 nM), there was no prevention of this EPCR loss. The respective metalloproteinase inhibitors did not influence EPCR expression but were able to prevent IL-1β induction of surface EPCR loss through metalloproteinase cleavage11 (data with 1,10-phenanthroline not shown). The APC-induced loss of EPCR from the endothelial surface is therefore through mechanisms different from those of sEPCR generation. Similar changes were also observed with human monocytes (data not shown).
Factors involved in APC-induced EPCR release. (A) HUVECs were incubated with and without APC (100 nM for 2 hours) in the presence and absence of metalloproteinase inhibitors, Ro31-9790 (30 μM) or 1,10-phenanthroline (5 mM), labeled with the EPCR antibody RCR-49, and analyzed by flow cytometry. The effects of the respective metalloproteinase inhibitors are included as is that of IL-1β in the absence and presence of Ro31-9790 (30 μM). (B) HUVECs were incubated for 2 hours with APC (100 nM) alone, APC plus hirudin (6 μM), boiled APC (100 nM), PPACK-APC, and PC (100 nM), and then stained for EPCR with analysis by flow cytometry. Results for both panels A and B are expressed as percentage of EPCR surface expression. Values are mean ± SD and the asterisk indicates P < .005. Data are representative of 3 independent experiments with samples performed in duplicate.
Factors involved in APC-induced EPCR release. (A) HUVECs were incubated with and without APC (100 nM for 2 hours) in the presence and absence of metalloproteinase inhibitors, Ro31-9790 (30 μM) or 1,10-phenanthroline (5 mM), labeled with the EPCR antibody RCR-49, and analyzed by flow cytometry. The effects of the respective metalloproteinase inhibitors are included as is that of IL-1β in the absence and presence of Ro31-9790 (30 μM). (B) HUVECs were incubated for 2 hours with APC (100 nM) alone, APC plus hirudin (6 μM), boiled APC (100 nM), PPACK-APC, and PC (100 nM), and then stained for EPCR with analysis by flow cytometry. Results for both panels A and B are expressed as percentage of EPCR surface expression. Values are mean ± SD and the asterisk indicates P < .005. Data are representative of 3 independent experiments with samples performed in duplicate.
We also considered the possible influence of any contaminating thrombin that may be within the APC preparation. The direct thrombin inhibitor, hirudin (6 μM), was used and its coincubation with APC (100 nM) was no different from APC alone in inducing cell surface loss of EPCR (Figure 2B).
To examine the requirement for PC to be in its activated form, we contrasted zymogen PC with APC at equimolar concentrations on HUVECs. Zymogen PC did not induce EPCR loss from the cell surface (Figure 2B) and the absence of MP formation was confirmed on confocal microscopy (data not shown). Heat denaturation of APC also rendered the APC incapable of MP formation and cell surface EPCR release, as did active site inhibition with PPACK (Figure 2B).
APC-induced MP-associated EPCR release is time- and concentration-dependent
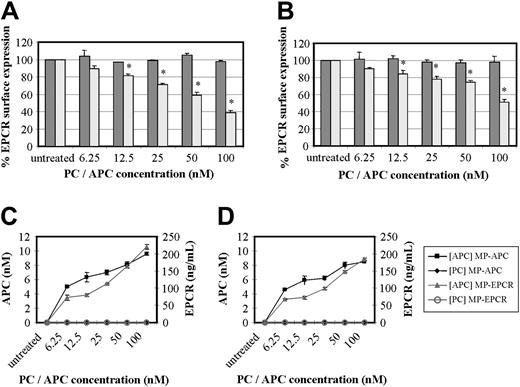
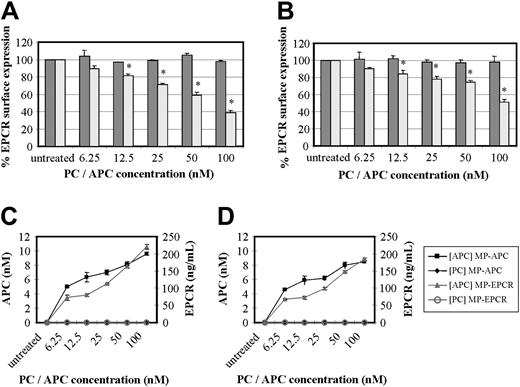
Experiments in which HUVECs and monocytes were incubated with increasing concentrations of PC or APC from 6.25 nM to 100 nM over a period of 24 hours demonstrated a concentration effect for APC only. Cells analyzed by FACS after RCR-49 staining showed an increasing fall in EPCR cell surface expression (Figure 3A-B). At a concentration of 12.5 nM APC, the approximately 20% drop in EPCR was significant (P < .005) in both cell types and this was maximal at 100 nM with a 50% to 60% drop in cell surface EPCR expression. This corresponded with the amount of MPs released (Figure 3C-D). Using 2 ELISA-based approaches, we found MPs containing EPCR and APC in the supernatant of these cell cultures, which were sedimentable. Through capturing the isolated MPs for EPCR, 60 to 70 ng/mL EPCR was detectable at the lowest APC concentration and this reached 180 to 220 ng/mL at 100 nM APC. Secondly, we were able to show that the released MP-associated EPCR had quantifiable APC, at levels of 5 to about 9 nM with respect to the lowest and highest APC exposure concentrations (Figure 3C). This was using a novel EPCR-capture ELISA with APC chromogenic detection, as previously used by others.22 To address if the measurable APC concentrations on MPs were truly bound or just passively acquired from APC in the media, LPS-induced MPs were exposed to APC (6.25-100 nM) in solution. After 24 hours of incubation and subsequent washing, less than 1 nM APC was detected with no concentration effect (data not shown). This indicates that MP-associated EPCRs contain actively bound APC.
Effect of PC and APC on MP-associated EPCR release. (A) HUVECs and (B) monocytes were incubated with concentrations of PC (dark gray bars) or APC (light gray bars) ranging from 6.25 nM to 100 nM for 24 hours and stained with RCR-49 for flow cytometric analysis; data are expressed as percentage of EPCR surface expression and are the mean value of 3 experiments ± SD with the asterisk indicating P < .005. Panels C and D show the corresponding quantitation of MPs isolated from the media after HUVEC and monocyte treatment, respectively, with PC or APC. These were then captured for EPCR and quantified by chromogenic S2366 for the APC bound (nM) and separately for EPCR detected (ng/mL).
Effect of PC and APC on MP-associated EPCR release. (A) HUVECs and (B) monocytes were incubated with concentrations of PC (dark gray bars) or APC (light gray bars) ranging from 6.25 nM to 100 nM for 24 hours and stained with RCR-49 for flow cytometric analysis; data are expressed as percentage of EPCR surface expression and are the mean value of 3 experiments ± SD with the asterisk indicating P < .005. Panels C and D show the corresponding quantitation of MPs isolated from the media after HUVEC and monocyte treatment, respectively, with PC or APC. These were then captured for EPCR and quantified by chromogenic S2366 for the APC bound (nM) and separately for EPCR detected (ng/mL).
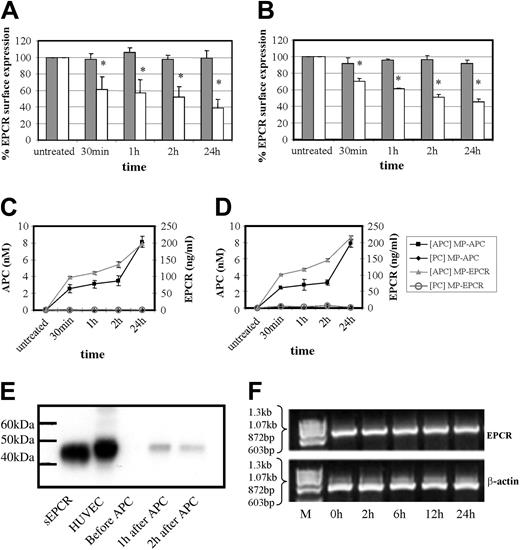
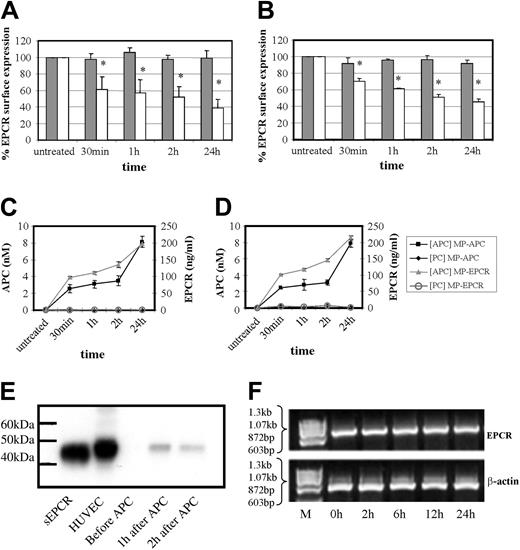
This phenomenon of APC and not PC is also time-dependent as shown in Figure 4A-D. In both cell types, there was a significant drop in cell surface EPCR by approximately 30% to 40% at 30 minutes of APC exposure, increasing to 50% to 60% at 24 hours with the ELISAs detecting EPCR or APC showing reciprocal increases. As shown in Figure 4C-D, MP-associated EPCR levels increased from approximately 100 to 200 ng/mL during 30 minutes to 24 hours of APC incubation with HUVECs and monocytes, respectively. The APC levels on these MPs also increased from approximately 2.5 to 8 nM in the corresponding timeframe for both cell types.
Effect of time on MP-associated EPCR release. Time-dependence experiment of PC (dark gray bars) and APC (100 nM; light gray bars) effect on (A) HUVECs and (B) monocytes by flow cytometric analysis of cellular EPCR expression. Results are expressed as percentage of EPCR surface expression and are the mean value of 3 experiments ± SD with the asterisk indicating P < .005. Panels C and D show the corresponding quantitation of MPs isolated from the media after HUVEC and monocyte treatment, respectively, with PC or APC. These were then captured for EPCR and quantified by chromogenic S2366 for the APC bound (nM) and separately for EPCR detected (ng/mL). (E) HUVECs were incubated with APC (100 nM) for up to 2 hours and MPs were isolated and analyzed by Western blotting, with sEPCR and HUVEC cell lysate as reference standards. (F) HUVECs were incubated with APC (100 nM) for up to 24 hours and EPCR mRNA expression was assessed using RT-PCR with ethidium bromide visualization of PCR-amplified products of EPCR and β-actin mRNA after agarose gel electrophoresis.
Effect of time on MP-associated EPCR release. Time-dependence experiment of PC (dark gray bars) and APC (100 nM; light gray bars) effect on (A) HUVECs and (B) monocytes by flow cytometric analysis of cellular EPCR expression. Results are expressed as percentage of EPCR surface expression and are the mean value of 3 experiments ± SD with the asterisk indicating P < .005. Panels C and D show the corresponding quantitation of MPs isolated from the media after HUVEC and monocyte treatment, respectively, with PC or APC. These were then captured for EPCR and quantified by chromogenic S2366 for the APC bound (nM) and separately for EPCR detected (ng/mL). (E) HUVECs were incubated with APC (100 nM) for up to 2 hours and MPs were isolated and analyzed by Western blotting, with sEPCR and HUVEC cell lysate as reference standards. (F) HUVECs were incubated with APC (100 nM) for up to 24 hours and EPCR mRNA expression was assessed using RT-PCR with ethidium bromide visualization of PCR-amplified products of EPCR and β-actin mRNA after agarose gel electrophoresis.
Western blotting of the MPs, isolated from conditioned medium under nondenaturing conditions, showed that its associated EPCR was the same length (49 kDa) as EPCR from endothelial cell lysates (Figure 4E). The soluble, metalloproteinase-cleaved form of EPCR migrated further in accordance with its lighter molecular weight of 45 kDa. MP-associated EPCR is therefore not a truncated form and is full-length EPCR.
Although the immediate drop in EPCR levels within 30 minutes of APC exposure is unlikely to reflect responses that could be occurring at the EPCR message level, there could still be an influence at later time points. Figure 4F shows that this is not the case and EPCR mRNAlevels did not change at 2 to 24 hours of APC incubation when compared with β-actin controls. The EPCR protein changes from APC exposure therefore reflect cellular surface events.
APC-induced MP release is dependent on EPCR- and PAR1 in both HUVEC and monocytes
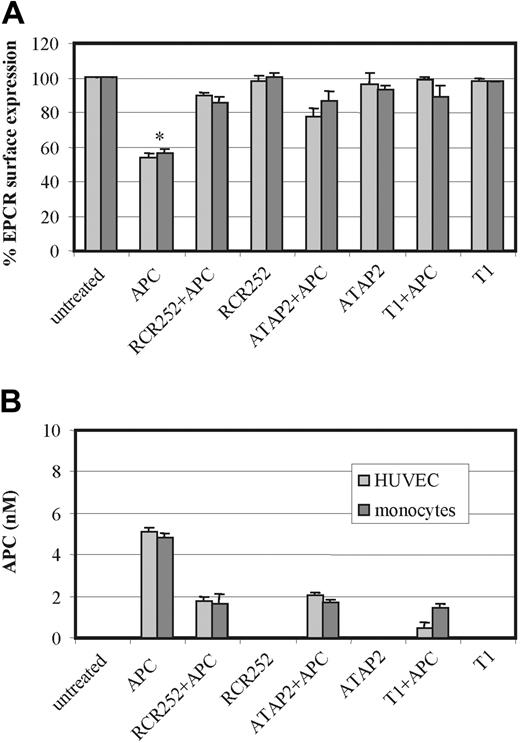
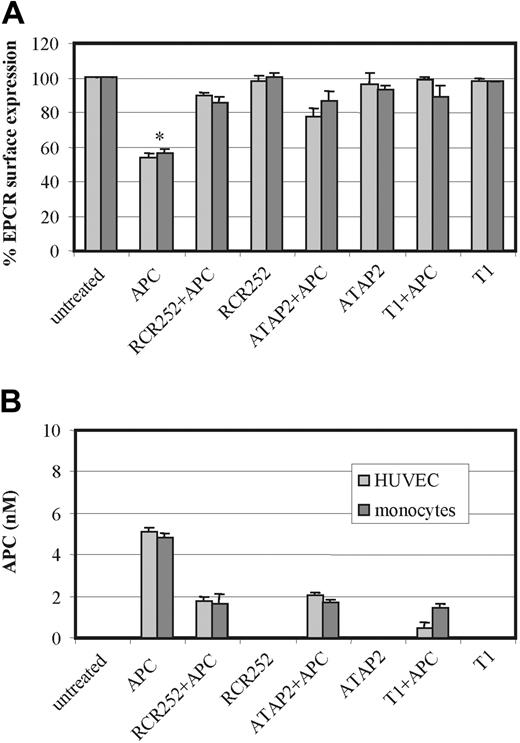
To investigate the respective roles played by EPCR and PAR1 in this APC-induced process, HUVECs and monocytes were preincubated with an EPCR antibody (RCR-252), which blocks APC binding by approximately 90%16 and an antibody that blocks cleavage activation of PAR1 (ATAP2), respectively. Furthermore, cells were preincubated with the PAR1 antagonist peptide T1, which blocks the binding of the tethered ligand, prior to APC incubation. Figure 5 shows that preincubation of both cell types with either antibody or with the peptide abrogated the APC-induced loss of cell surface EPCR in MP form. This would indicate that APC binding to EPCR for cleavage and activation of PAR1 is essential for MP-associated EPCR release.
Role of EPCR and PAR1 on APC-induced MP release. HUVECs (light gray bars) and monocytes (dark gray bars) were incubated with APC 100 nM for 2 hours in the presence and absence of the EPCR-blocking antibody RCR-252, the PAR1 antibody, ATAP2, and the PAR1 antagonist peptide T1. Panel A shows the changes in EPCR surface expression by flow cytometry using RCR49 in HUVECs and monocytes; panel B quantifies the MPs released from HUVECs and monocytes by their APC content through ELISA capture and chromogenic S2366 detection. Results are representative of 3 experiments. Values are mean ± SD; *P < .005.
Role of EPCR and PAR1 on APC-induced MP release. HUVECs (light gray bars) and monocytes (dark gray bars) were incubated with APC 100 nM for 2 hours in the presence and absence of the EPCR-blocking antibody RCR-252, the PAR1 antibody, ATAP2, and the PAR1 antagonist peptide T1. Panel A shows the changes in EPCR surface expression by flow cytometry using RCR49 in HUVECs and monocytes; panel B quantifies the MPs released from HUVECs and monocytes by their APC content through ELISA capture and chromogenic S2366 detection. Results are representative of 3 experiments. Values are mean ± SD; *P < .005.
APC bound to MP-associated EPCR expresses anticoagulant activity
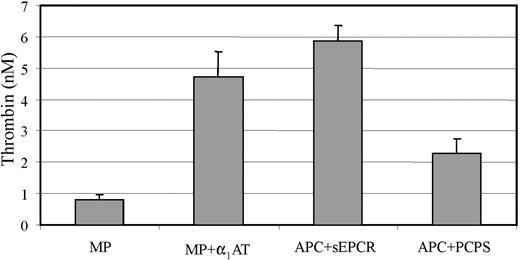
Because APC-induced MPs express full-length EPCR, we postulated that the bound APC could express anticoagulant activity in a similar manner to its activity at the cell membrane level. The experimental reaction involved incubating MPs with FVa and residual amounts after 30 minutes were then assessed for prothrombinase-supporting activity. The results of 3 separate experiments, performed in duplicate (Figure 6), indicate that the bound APC retains anticoagulant activity in retarding thrombin formation. This approximates to the extent achieved by 2 nM APC in the presence of 25 nM PC/PS. This activity was inhibitable with α1-AT, a serine protease inactivator of APC, to increase thrombin generation by approximately 5-fold. Similarly, an equivalent amount of APC but complexed to sEPCR, which is known to inhibit the anticoagulant activity,12 did not reduce thrombin generation. These results indicate that APC bound to MP-associated EPCR can promote anticoagulant activity in solution.
Presence and activity of APC on MP-associated EPCR. HUVECs were incubated with APC (100 nM) and MPs isolated as described in “Materials and methods.” The activity of the APC on the MPs was estimated by a prior FVa inactivation step, followed by a prothrombinase reaction with the extent of thrombin generation reflecting the amount of residual FVa. The isolated MPs were also incubated with α1-AT prior to the functional test to assess for APC specificity. Controls included APC in complex with sEPCR and the activity of 2 nM APC in the presence of 25 nM PC/PS vesicles was also assessed. Results are expressed as generated thrombin (nM) and represent mean (±SD) values of 3 experiments with samples in duplicates; *P < .005.
Presence and activity of APC on MP-associated EPCR. HUVECs were incubated with APC (100 nM) and MPs isolated as described in “Materials and methods.” The activity of the APC on the MPs was estimated by a prior FVa inactivation step, followed by a prothrombinase reaction with the extent of thrombin generation reflecting the amount of residual FVa. The isolated MPs were also incubated with α1-AT prior to the functional test to assess for APC specificity. Controls included APC in complex with sEPCR and the activity of 2 nM APC in the presence of 25 nM PC/PS vesicles was also assessed. Results are expressed as generated thrombin (nM) and represent mean (±SD) values of 3 experiments with samples in duplicates; *P < .005.
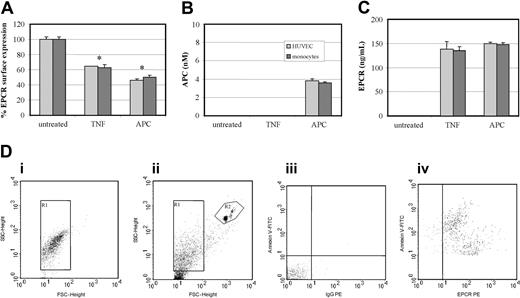
The extent of APC-induced MP release is similar to TNF-α stimulation
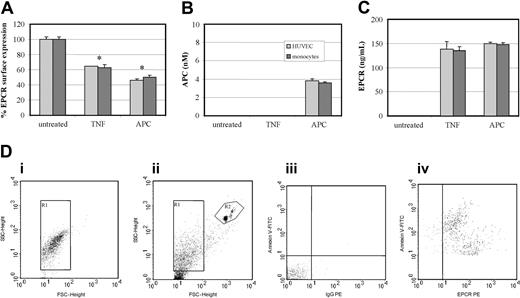
To ascertain the relative capacity of APC to generate MPs, we compared it with TNF-α, a known agonist of MP formation,18 in the presence of Ro31-9790 to prevent metalloproteinase cleavage of cell-surface EPCR.11 At 2 hours, TNF-α induced loss of cell-surface EPCR by 40% in both HUVECs and monocytes (Figure 7A). This was similar to that generated by 100 nM APC for 2 hours, which induced a 50% to 60% loss in EPCR surface expression. These results were confirmed by ELISA-based quantification of APC and EPCR associated to MPs (Figure 7B-C). APC but not TNF-α induced MP-associated EPCR-containing bound APC. The released MPs were also quantified by their ability to bind annexin V, through dual labeling to CD13 or EPCR. An internal control of calibrated polypropylene beads (Bangs Laboratories, Fishers, IN) was added and 5000 were counted. MPs were enumerated from the gate corresponding to annexin V-FITC+ events that costained with CD13-PE or MCR-1 (Figure 7D). Table 1 shows that the number of MPs generated after APC treatment is comparable for monocytes and higher for HUVECs than those generated by TNF-α.
Effect of TNF-α and APC on MP-associated EPCR release. (A) HUVECs (light gray bars) and monocytes (dark gray bars) were incubated with TNF-α (10 ng/mL) or APC (100 nM) or both for 2 hours and analyzed by flow cytometric analysis with the percentage of EPCR surface expression expressed from the mean value of 3 experiments ± SD with the asterisk indicating P < .005. Isolated MPs from the conditioned media were quantified by ELISA through (B) APC detection via chromogenic S2366 (nM) and (C) EPCR content (ng/mL). Results are representative of 3 experiments. Values are mean ± SD; *P < .005. (D) Flow cytometric analysis and quantification of MPs released from cultured monocytes: (i) determination of forward (FSC) and side-scatter (SSC) using 1-μm beads to establish the MP gate (R1); (ii) R2 gate includes the 7-μm beads used for enumeration of MPs; (iii) determination of the limit of negative fluorescence in the presence of EDTA as a negative control for annexin V and the IgG-PE isotypic control; and (iv) MPs dually labeled with annexin V–FITC and anti–EPCR-PE.
Effect of TNF-α and APC on MP-associated EPCR release. (A) HUVECs (light gray bars) and monocytes (dark gray bars) were incubated with TNF-α (10 ng/mL) or APC (100 nM) or both for 2 hours and analyzed by flow cytometric analysis with the percentage of EPCR surface expression expressed from the mean value of 3 experiments ± SD with the asterisk indicating P < .005. Isolated MPs from the conditioned media were quantified by ELISA through (B) APC detection via chromogenic S2366 (nM) and (C) EPCR content (ng/mL). Results are representative of 3 experiments. Values are mean ± SD; *P < .005. (D) Flow cytometric analysis and quantification of MPs released from cultured monocytes: (i) determination of forward (FSC) and side-scatter (SSC) using 1-μm beads to establish the MP gate (R1); (ii) R2 gate includes the 7-μm beads used for enumeration of MPs; (iii) determination of the limit of negative fluorescence in the presence of EDTA as a negative control for annexin V and the IgG-PE isotypic control; and (iv) MPs dually labeled with annexin V–FITC and anti–EPCR-PE.
Discussion
A novel finding in this study is that APC can induce the formation and release of EPCR-containing MPs in a time- and concentration-dependent manner. We confirmed this with a different preparation of human APC and demonstrated the effect to similar extents in both primary physiologic HUVECs and monocytes. This finding led to the following key observations. First, the stimulus is dependent on the active site of APC and is not influenced by any possible contamination by thrombin. Active-site inactivation of APC either by heat denaturation or PPACK blockade will abrogate this action, which is also not observed using zymogen protein C. Second, the microparticle-associated EPCR that is released from the endothelial surface is in its full-length cellular membrane form. This contrasts with the other circulatory form, that is, sEPCR that is metalloproteinase cleaved at the level of the cell membrane and is approximately 4 kDa less in molecular weight than the full-length form. This distinction is demonstrated by the differential electrophoretic migration of the 2 forms with MP-associated EPCR traveling to the same extent as EPCR in endothelial cell lysates (49 kDa). A further difference is in the release of MP-associated EPCR, which is not preventable by the presence of the metalloproteinase inhibitors Ro31-9790 or 1,10-phenanthroline unlike for sEPCR.
A third observation of note is that MP-associated EPCR contains bound APC that is not acquired passively and that expresses anticoagulant activity specifically inhibitable by the APC inhibitor, α1-AT. This serves as a functional distinction from the sEPCR form. As has been previously described and further illustrated in this study, APC bound to sEPCR is no longer capable of FVa inactivation. All these results point to APC binding to full-length EPCR in microparticulate form following release from the cell surface, after exposure to APC.
The mechanism of APC-induced MP formation is dependent on the EPCR-APC-PAR1 axis. Blocking of EPCR or PAR1 cleavage and activation abrogates the effect and the molecular events involved would appear to be similar to those leading on to anti-inflammatory and antiapoptotic properties that have been described in vitro and in vivo.14,23 The consequent signaling mechanisms leading on to APC-induced MP-associated EPCR formation are presently unclear and although the ability of PAR1 to stimulate cellular function has been intensely investigated, each of its pleiotropic effects remains poorly understood.24 PAR1 can couple effectively to Gαi, Gαq, and Gα13 families of G protein α subunits and modify the inner leaflet of the plasma membrane for provision of attachment sites to a host of signaling proteins.25 EPCR is also located within these glycosphingolipid-rich microdomains (rafts) and caveolae that provide a likely pathway for calcium-regulated kinases and phosphatases, guanine nucleotide exchange factors, and mitogen-activated protein kinase cassettes to mediate formation and release of MPs that include EPCR.11,14,26 A possible contrast to MP formation by other agonists, for example, TNF-α, is that the APC effect is much more specific in its restriction to particular cell surface areas, that is, of EPCR-PAR1 coproximity.
Although EPCR internalization of APC into the nucleus could also be involved to affect cellular regulation, we were unable to demonstrate this effect in our system, possibly because of the experimental approach in the timing and availability of probes or our use of primary physiologic cells rather than 293 cells where this effect has been observed.15 EPCR dependence has also been observed in the mechanism by which the PC pathway inhibits directed migration of leukocytes. However, this effect on neutrophils, monocytes, and eosinophils occurs with both zymogen and activated PC in its requirement for the Gla domain rather than the active site of APC.7,8 A further difference is that PAR1 is not involved. Sturn et al7 showed that the presence of an antibody to PAR1 did not affect PC- or APC-induced inhibition of neutrophil chemotaxis. Although monocytes were not similarly investigated for PAR1 involvement in chemotaxis, the molecular mechanism involved appears different from MP formation induced specifically by APC, but not PC, even at varying concentrations and time exposures.
In terms of MP quantitation, there is now a forum within the International Society of Thrombosis and Haemostasis to refine and standardize assay methods by flow cytometry and by capture assay. Capturing MPs can be through either interaction of phosphatidylserine with immobilized annexin V or antigens with immobilized specific antibodies.27,28 The advantage of the latter is to also enable assessment of function and because we are specifically focused on EPCR-containing MPs and the amount and function of its bound APC, this has been our preferred and robustly assessed approach, as demonstrated by the tight inter-CV and intra-CV values. For our purposes, a capture system using immobilized annexin V would have also quantified EPCR-negative MPs and given misleading results. In addition, several studies have now shown that not all, and possibly up to one third of MPs only, express anionic phospholipid in the outer membrane leaflet.21,29
High levels of MPs have usually been discussed in the context of being harmful in pathologically conditions such as sepsis and disseminated intravascular coagulation with isolated MPs ascribed procoagulant function that could further disrupt fine hemostatic balance and control.30,31 The corollary is that a beneficial treatment should lower such MP concentration. Alternatively, functionally opposing MPs, for example, in containing anticoagulant function, could play a beneficial role in overall biologic and homeostatic re-equilibration. As such, the physiologic significance of MP-associated EPCR could be relevant but remains to be explored.
Overall, these findings point to an alternative mechanism of EPCR release from the cell surface that is not metalloproteinase-cleaved but bound to MPs. The retention of membrane conformation in this form means that it can potentially disseminate its function from the cell surface level into the circulation and reach areas of the microvasculature that could derive benefit from its activities.
Prepublished online as Blood First Edition Paper, October 14, 2004; DOI 10.1182/blood-2004-05-1896.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Hai Juan Chen, Bob Harris, and Carol Powell for advice and assistance.