Abstract
We developed a clinical prediction rule for bone marrow involvement (BMI) in Hodgkin lymphoma based on 826 patients and validated it in 654 additional patients. Independent prognostic factors for BMI were x1, B symptoms; x2, stage III/IV prior to bone marrow biopsy; x3, anemia; x4, leukocytes fewer than 6 × 109/L; x5, age 35 years or older; and x6, iliac/inguinal involvement. Each factor was graded as xi = 1, if present, or xi = 0, if absent. A simplified score Zs = 8x1 + 6x2 + 5x3 + 5x4 + 3x5 + 3x6 – 8 was assigned to each patient. The sensitivity, specificity, and positive and negative predictive value of this prediction rule was 97.8%, 51.5%, 10.6%, and 99.8%, respectively. In the validation group, they were 98.1%, 40.3%, 12.7%, and 99.6%. According to Zs value, 3 risk groups for BMI were defined: low risk (Zs < 0, 44% of patients, 0.3% risk), standard risk (Zs, 0-9; 37% of patients; 4.2% risk), and high risk (Zs ≥ 10, 20% of patients, 25.5% risk). Patients with low risk (stage IA/IIA without anemia and leukopenia; stage IA/IIA, younger than 35 years, with either anemia or leukopenia but no inguinal/iliac involvement; and stage IIIA/IVA without any of these 4 risk factors) do not need bone marrow (BM) biopsy. Patients with standard risk should be staged with unilateral biopsy, but patients with high risk may benefit from bilateral biopsy.
Introduction
Bone marrow involvement (BMI) is rare in patients with Hodgkin lymphoma (HL). Its incidence varies between 4% and 14% in the series reported during the past 20 years.1-8 The demonstration of BMI is important because it signifies upstaging to stage IV disease in many patients, which might result to treatment modification. Upstaging may also modify the prognostic classification in terms of the International Prognostic Score (IPS).9 Furthermore, a repeated bone marrow biopsy is mandatory during restaging in patients with initially involved bone marrow. Finally, some studies have suggested that BMI is associated with worse outcome, but this was not confirmed in larger series.9,10
Trephine biopsy, usually unilateral, is the recommended approach to evaluate BMI. According to the Ann Arbor staging criteria for HL,11 which were established in 1971, a bone marrow biopsy is recommended for patients with any of the following: elevated serum alkaline phosphatase, stage III/IV disease, any unexplained cytopenia or radiographic/scintigraphic evidence of osseous involvement. Only a single study has evaluated the validity of these criteria.4 If the presence of at least 1 of these factors were considered as indication for bone marrow biopsy, the sensitivity for the detection of BMI was 100% and the specificity 40%. Furthermore, according to the updated criteria for staging of HL, established during the Cotswolds meeting in 1988,12 a bone marrow biopsy from at least 1 site is recommended for patients with clinical stage III/IV as well as for clinical stage II with adverse features. Despite these formal recommendations many physicians, eg, 74% of hematologists and 40% of clinical oncologists in the United Kingdom,13 perform biopsies in all patients. Therefore, there is no consensus regarding the use of bone marrow biopsy for the initial staging of HL.
Various clinical and laboratory factors have been encountered to predict BMI,1-8 including B symptoms, stage III/IV, peripheral cytopenias, elevated serum lactate dehydrogenase (LDH), splenic involvement, and mixed cellularity/lymphocyte depletion histology. Nevertheless, a clinical prediction rule, which could be used to define groups of patients with virtually no risk of BMI or to characterize groups with high risk of BMI, is lacking from the literature. Since trephine biopsy is a painful and invasive procedure, the identification of a subgroup of patients with HL, who have minimal risk of BMI, would be important. Trephine biopsy might be spared in such patients. Conversely, patients with relatively high risk of BMI might benefit from bilateral trephine biopsy, because the probability of demonstrating BMI can be increased by 16% to 33%, if bilateral biopsy is performed.4,14,15
In the present study we evaluated various conventional demographic, clinical, and laboratory factors to identify independent predictors for BMI and to define subgroups of patients, who might avoid trephine biopsy or benefit from bilateral biopsy.
Patients and methods
Patients and potential prognostic factors
Between 1981 and 2003, 877 consecutive, unselected patients with biopsy-proven HL, aged 14 years or older, were evaluated and received first-line treatment in the Haematology Section of the First Department of Internal Medicine, Medical School, National and Kapodistrian University of Athens, Greece (center 1). The patients were clinically staged according to the Ann Arbor system.11 Staging procedures included a detailed history and complete physical examination; complete blood counts and biochemical profile; chest X-rays; computed tomography of the chest, abdomen, and pelvis; bipedal lymphangiography; and unilateral bone marrow biopsy. Bone marrow biopsy data were available in 869 (99.1%) of the 877 patients, who formed the basis of this report. Approval was obtained from the local institutional review board for these studies.
For the purpose of the present study, patients were analyzed with respect to the following demographic, staging, pathological data, and clinical and laboratory features: age (< versus ≥ 35 years), sex, B symptoms, clinical stage prior to bone marrow biopsy (CSPBM coded as I/II versus III/IV), histologic subtype, presence of bulky mediastinal mass, lung involvement, inguinal and/or iliac involvement, number of involved anatomic sites10 prior to bone marrow biopsy (< versus ≥ 4), anemia (defined as hemoglobin < 130 g/L [13 g/dL] for males and < 115 g/L [11.5 g/dL] for females), leukocyte counts (≥ versus < 6 × 109/L), lymphocytopenia (≥ versus < 1.0 × 109/L), erythrocyte sedimentation rate (ESR; < 50 versus ≥ 50 mm the 1st hour), serum albumin levels (≥ versus < 40 g/L [4 g/dL]), and LDH levels (normal versus elevated). CSPBM was defined as the clinical stage assigned to each patient after history, physical examination, and imaging procedures had been completed but without knowledge of the results of bone marrow biopsy.
Derivation and validation groups
The above-mentioned group of 869 patients of center 1 was evaluated to produce a clinical prediction rule for BMI in HL (derivation group). Once the clinical prediction rule was developed, it was validated in an independent group of 673 patients, who were registered in the databases of 4 centers participating in the Lymphoma Cooperative Group of the Hellenic Society of Hematology, namely (1) Hematology Clinic, Theagenion Cancer Hospital, Thessaloniki (center 2, 265 patients), (2) Third Department of Internal Medicine, National and Kapodistrian University of Athens, Sotiria Hospital, Athens (center 3, 99 patients), (3) Hematology Clinic, Peripheral General Anticancer Hospital-Metaxa-Piraeus (center 4, 122 patients), and (4) Hematology and Lymphoma Clinic, Evangelismos Hospital, Athens (center 5, 187 patients). Complete data regarding bone marrow biopsy and the covariates included in the clinical prediction rule were available in 654 (97.2%) of 673 patients, who formed the validation group of this study.
Statistical methods
The univariate significance of baseline features for the prediction of BMI was estimated by the chi-square test. This analysis was restricted to the derivation group. The accepted level of statistical significance for entry of each covariate into logistic regression analysis was .05. Statistically significant covariates were graded as 0, if the adverse value were absent, or 1, if the adverse value were present.
Logistic regression analysis was used to assess the independent prognostic significance of the covariates, which were significantly associated with BMI in univariate analysis. A forward entry–backward elimination method of selection was used; P values for entry in and removal from the logistic regression model were .05 and .10, respectively. Covariates were handled as categorical (dummy). In logistic regression analysis, the discrimination between patients with and without BMI is based on the predicted probability of being in need of BM biopsy, denoted as prob(BMB), which is calculated according to the formula: Prob(BMB) = 1/(1 + e–Z), where Z = b0 + b1x1 + b2x2 +... + bnxn (equation 1).
In this formula, x1 to xn are the values of various covariates (0 or 1 as already described), b1 to bn are their corresponding coefficients, and b0 is a constant. The values of b0 to bn are calculated by the logistic regression procedure. It is clear from the above formula that each value of prob(BMB) corresponds to a single Z value. Thus, the discrimination of patients with and without BMI was based on the value of Z, which is a linear function of x1 to xn. To further reduce the required calculations, we simplified b0 to bn by rounding to the nearest 0.25 and quadrupling them, so that round numbers were obtained, called simplified coefficients (b0s-bns).16 For example, a calculated coefficient of 1.33 was initially simplified to 1.25 and then quadrupled. The corresponding simplified coefficient was 5 (ie, 1.25 × 4). The simplified Z score, denoted as Zs, is given by the following formula: Zs = b0s + b1sx1 + b2sx2 +... + bnsxn (equation 2).
The sensitivity, specificity, and positive and negative predictive value of the prediction rule were determined as indexes of efficacy.
Results
Development of the prediction rule
Within the derivation group, among 869 patients with available data regarding bone marrow biopsy, 48 (5.5%) had bone marrow involvement. The following covariates were significantly associated with the presence of bone marrow involvement: age 35 years or older (P < .001), B symptoms (P < .001), CSPBM III/IV (P < .001), mixed cellularity or lymphocyte depletion histology (P = .002), number of involved anatomic sites of 4 or more prior to bone marrow biopsy (P < .001), inguinal/iliac involvement (P < .001), anemia (P < .001), leukocytes less than 6 × 109/L (P < .001), lymphocytes less than 1.0 × 109/L (P < .001), albumin less than 40 g/L (4 g/dL; P < .001), and ESR at least 50 mm (P < .001). Sex (P = .13), lung (P = .60), and bulky mediastinal involvement (P = .83) as well as serum LDH levels (P = .24) did not correlate with the presence of BMI. Thus, 11 covariates were examined in a logistic regression model. Among them, 6 remained in the final model as independent predictors of BMI, namely age 35 years or older, the presence of B symptoms and anemia, clinical stage III/IV prior to bone marrow biopsy, a leukocyte count less than 6 × 109/L, and the presence of inguinal and/or iliac involvement. Among 869 patients initially analyzed, 43 had missing data for at least 1 of these 6 covariates, so that 826 patients were evaluable in multivariate analysis. The results of multivariate logistic regression analysis, and the values of the calculated coefficients b0 to b6 and the simplified coefficients b0s to b6s are shown in Table 1. The above-named results led to the determination of a certain Zs score for each patient, given by the equation: Zs = b0s + b1sx1 + b2sx2 + b3sx3 + b4sx4 + b5sx5 + b6sx6 (equation 3).
Table 2 summarizes the sensitivity, specificity, and positive and negative predictive value rates obtained by using different Zs values as cutoff scores. The best discrimination between patients with and without BMI was achieved at a cutoff score Zs = 0 (Table 2). If patients with Zs of 0 or more were considered to have BMI and those with Z less than 0 were considered to have negative bone marrow biopsies, then 45 of 46 patients who actually had BMI would be correctly classified (97.8%; 95% CI, 88.4%-99.6%). Furthermore, 402 of 780 patients (51.5%; 95% CI, 48.0%-55.1%) who had no BMI would be correctly classified. Among 403 patients who were predicted to be bone marrow negative, only 1 was actually bone marrow positive (negative predictive value 402 of 403 or 99.8%; 95% CI, 98.6%-99.96%). At the cutoff of Zs = 0, the positive predictive value was 45 of 423 or 10.6% (95% CI, 8.0%-14.0%).
Validation of the prediction rule
The described rule was also applied to the validation group, consisting of 654 patients from 4 other institutions. The frequency of BMI in this group was 53 of 654 (8.1%). At a cutoff of Zs = 0, 52 of 53 patients (98.1%; 95% CI, 89.8%-99.7%), who actually had BMI, and 242 of 601 patients (40.3%; 95% CI, 36.3%-44.3%), who had no BMI, were correctly classified. Negative predictive value was 242 of 243 (99.6%; 95% CI, 97.6%-99.9%) and positive predictive value was 52 of 411 (12.7%; 95% CI, 9.7%-16.3%). The applicability of the clinical prediction rule was roughly similar to the derivation group for the patients of centers 2, 4, and 5, but it was not satisfactory in terms of specificity for the patients of center 3 (data not shown).
Overall results
As concluded from “Patients and methods,” each value of Z (and Zs) corresponds to discrete values of predicted probability of BMI (equations 1-3; Table 1). The actually observed frequency of BMI within the derivation and validation groups, the whole patient population as a function of Zs, and the corresponding predicted probabilities of BMI are shown in Table 3. The observed probability (frequency) of BMI gradually increased with increasing Zs, being similar to that predicted by the logistic regression model (Table 3).
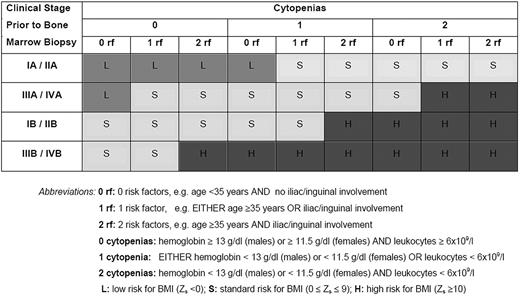
Among 1480 patients studied, 99 had BMI (frequency, 6.7%). A Zs less than 0 was observed in 646 patients (44%), who had only 0.3% probability of BMI, and were classified as low risk (predicted probability < 0.75%). Specifically, this low-risk group included the patients with clinical stage IA/IIA without anemia and leukopenia, those with stage IA/IIA younger than 35 years with either anemia or leucopenia in the absence of inguinal/iliac involvement, and patients with stage IIIA/IVA without any of these 4 risk factors. Patients with standard risk (544 or 37%, with predicted probability 0.75%-10%) had Zs values of 0 to 9 and a 4.2% actual probability of BMI (2.9%-8.4% depending on the exact Zs value). Finally, 290 patients (20%) had Zs of 10 or more, with 25.5% actual probability of BMI (13.6%-32.8%, depending on the exact Zs value), and were considered as high risk (predicted probability > 10%). For the clarity of this presentation the low-, standard-, and high-risk groups, defined as Zs less than 0, 0 to 9, and 10 or more, respectively, are precisely described in terms of clinical and laboratory characteristics in Figure 1.
Clinical and laboratory description of risk groups for bone marrow involvement.
Clinical and laboratory description of risk groups for bone marrow involvement.
Assuming a 25% increase in the yield of positive results by performing bilateral bone marrow biopsies,14 we calculated how many additional cases of BMI could be identified per 1000 bilateral biopsies according to the value of Zs and the corresponding risk group assignment. A bilateral biopsy was not effective in identifying additional patients with BMI in the low- and standard-risk groups (< 1 and 11 additional cases per 1000 additional biopsies, respectively). In contrast, if 1000 patients with high risk underwent bilateral biopsies, 64 additional cases of BMI would be identified in comparison to the use of unilateral biopsy only.
Effect of BM biopsy on staging, prognostic classification, and treatment decisions
The estimation of the effect of unilateral BM biopsy on prognostic classification was restricted to the patients of the derivation group, for whom IPS data were available. The probability of upstaging or upgrade of prognostic classification by unilateral BM biopsy within the derivation group, in relation to the risk group (low, standard, or high) is given in Table 4.
In the low-risk group, only 1 (0.3%) of 387 early-stage patients was upstaged by unilateral BM biopsy. None of the 317 patients with IPS of 2 or less was upgraded to the unfavorable subgroup (IPS of 3 or more) (Table 4).
Although none of the 55 early-stage standard-risk patients was upstaged, 3 (1.8%) of 171 patients with IPS of 2 or less were upgraded to the unfavorable subgroup (IPS of 3 or more), resulting to a potential change in treatment decision (Table 4).
All patients with high risk had advanced disease (Table 4). Treatment strategy could be modified in 6 (11.5%) of 52 patients with IPS of 2 or less, who were upgraded to the unfavorable subgroup with IPS of 3 or more (Table 4).
Within the validation group only 1 (0.4%) of 234 patients with early stage and low risk of BMI were upstaged by unilateral BM biopsy. However, unilateral BM biopsy led to the upstage of 2 (3.6%) of 56 patients with early stage and standard risk of BMI (data not shown as a table).
Incidence of BMI in early-stage patients (IA/IIA)
Overall, among 732 early stage patients (clinical stage IA/IIA) from both the derivation and validation groups, 4 (0.5%) actually had BMI. The probability of BMI was 0.3% (2 of 621) for the patients falling into the low-risk group versus 1.8% (2 of 111) for those falling into the standard-risk group.
Discussion
In the present study we identified 6 simple clinical and laboratory parameters, which were predictive of the probability of BMI in patients with HL. In agreement to previously published data1-8 the frequency of BMI ranged from 5.4% to 11.3% in 5 participating centers.
In accordance to other studies we found that B symptoms,1,4,5 anemia,1,4 relatively low leukocyte counts,5 and advanced clinical stage prior to BM biopsy1,4 were independent predictors of BMI. We also found that age older than 35 years and iliac/inguinal involvement had an independent value for the prediction of BMI. Elevated LDH, histology, and the absence of a large mediastinal mass did not correlate with BMI in our patients, in contrast to some previous reports.1,5
Current practice regarding the use of BM biopsy in the initial staging of HL differs among centers.13 According to the Ann Arbor, Cotswolds, as well as other recently published recommendations, BM biopsy can be spared in selected patients with stage I/II.11,12,17 Despite these recommendations, many centers and practicing physicians perform BM biopsies in all patients with HL.13 Furthermore, the increasing ability to demonstrate BMI by obtaining bilateral or large biopsies may lead to treatment modification in some patients and permit more accurate restaging after chemotherapy, since patients with residual bone marrow disease might benefit from early treatment intensification with high-dose therapy and autologous stem cell support. Thus, a stratification of the risk of BMI based on simple parameters seems useful in everyday clinical practice.
The main goal of this study was to achieve maximal sensitivity and negative predictive value, and minimize the probability of missing cases with BMI. Indeed the clinical prediction rule presented here is characterized by very satisfactory sensitivity and negative predictive value of 97.8% and 99.8%, respectively. These findings were reproducible in the validation group, without significant loss with respect to the efficacy indexes. Thus, this is the first study to present a clinical prediction rule for BMI in patients with HL, which is validated in an independent patient population. Our results appear to apply well to centers specializing in hematologic oncology.
By using the clinical prediction rule described here, 3 groups of patients with low (Zs < 0), standard (0 ≤ Zs ≤ 9), and high (Zs ≥ 10) risk of BMI can be delineated. These are defined in terms of the Zs score, which is based on 6 simple disease parameters. However, calculations of Zs according to equation 3 may not be convenient for many treating physicians. Thus, we consider the schematic representation of the risk groups, as shown in Figure 1, a more practical approach for the clinicians to predict the risk of BMI.
According to our findings, approximately 44% of patients with HL belong to the low-risk group (Zs < 0). This group mostly includes patients with clinical stage (CS) IA and IIA without cytopenias or with 1 cytopenia and no additional risk factors for BMI (age < 35 years and no inguinal/iliac involvement). Patients with clinical stage IIIA/IVA without any of these 4 risk factors are also included in this low-risk group. If a BM biopsy were spared in all these patients, 3 cases of BMI would have been missed per 1000 biopsies avoided. For reasons of simplicity one could modify the definition of the low-risk group to include all patients with clinical stage IA/IIA.8 With this simplified definition, 49% (732 of 1480) of the patients would avoid staging BM biopsy having only 0.5% risk of BMI (5 cases would have been missed per 1000 biopsies avoided). This misclassification rate is low, especially if adriamycin, bleomycin, vinblastine, and dacarbazine (ABVD)–based chemotherapy is planned for all patients with HL. Thus, this simplification facilitates rapid clinical decision making in everyday practice. However, there is a precisely defined subgroup of patients with clinical stage IA/IIA (111 of 732 or 15%) who have a clearly higher risk of BMI of 1.8%. This is the reason for which the model described here provides a more accurate guide to avoid unnecessary bone marrow biopsies.
Approximately 37% of patients with HL belong to the standard-risk group and have a 4.2% frequency of BMI (0 ≤ Zs ≤ 9). Within the derivation group none of the 55 early stage patients of this standard-risk group was upstaged by unilateral BM biopsy. However, within the validation group 2 (3.6%) of 56 patients with early stage and standard risk for BMI could be upstaged by unilateral BM biopsy. Furthermore, 1.8% of the patients who had IPS of 2 or less became prognostically unfavorable (upgrade of IPS from 2 to 3). If a bilateral BM biopsy is performed in patients with standard risk for BMI, approximately 11 additional cases of BMI are expected to be demonstrated per 1000 additional biopsies. On the basis of these data, we propose that patients with standard risk should be evaluated with unilateral BM biopsy.
Finally, approximately 20% of the patients belong to the high-risk group for BMI (Zs ≥ 10). As shown in Figure 1, all high-risk patients have by definition advanced disease prior to BM biopsy. Approximately 11% of such patients will be upstaged to stage IV by unilateral BM biopsy and 11.5% of those who have IPS of 2 or less will become prognostically unfavorable (upgrade of IPS from 2 to 3). In high-risk patients a bilateral BM biopsy may contribute substantial information, because approximately 64 additional cases of BMI are expected to be demonstrated per 1000 additional biopsies. Thus, high-risk patients should probably be evaluated with bilateral BM biopsy.
Except for these general directions for the application of the clinical prediction rule described herein, this rule could also be applied in an individualized fashion. Many patients do not feel comfortable undergoing a BM biopsy. If such patients belong to the low-risk group (Zs < 0), the physician could omit BM biopsy with a low probability of losing significant information. In contrast, as the Zs score increases, the safety of omitting BM biopsy decreases, especially if minimal treatment is planned for nonadvanced disease. The prediction rule could also be used to support the decision for a bilateral biopsy in patients with scores greater than a predefined cutoff, according to the judgment of the treating physician.
In conclusion, in this study we demonstrated that the risk of BMI in patients with HL can be reproducibly stratified on the basis of 6 simple clinical and laboratory parameters. On the basis of our data, patients with HL can be classified as having low, standard, and high risk for BMI. A BM biopsy does not appear necessary in patients with low risk (or alternatively in clinical stages IA/IIA). Patients with standard risk should be staged with unilateral BM biopsy, while patients with high risk may benefit from bilateral BM biopsy. However, decisions made on the basis of this approach should be affected from the referral pattern and the treatment strategies applied in each individual center.
Prepublished online as Blood First Edition Paper, November 9, 2004; DOI 10.1182/blood-2004-01-0379.
Supported in part by a grant provided by “IASIS,” a nonprofit organization raising funds for research in leukemias, lymphomas, and related disorders.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.