Abstract
Neuropilin-1 (NRP-1) is a type 1 membrane protein that binds the axon guidance factors belonging to the class-3 semaforin family. In endothelial cells, NRP-1 serves as a co-receptor for vascular endothelial growth factor (VEGF) and regulates VEGF receptor 2 (VEGFR-2)–dependent angiogenesis. Although gene-targeting studies documenting embryonic lethality in NRP-1 null mice have demonstrated a critical role for NRP-1 in vascular development, the activities of NRP-1 in mature endothelial cells have been incompletely defined. Using RNA interference-mediated silencing of NRP-1 or VEGFR-2 in primary human endothelial cells, we confirm that NRP-1 modulates VEGFR-2 signaling-dependent mitogenic functions of VEGF. Importantly, we now show that NRP-1 regulates endothelial cell adhesion to extracellular matrix proteins independently of VEGFR-2. Based on its dual role as an enhancer of VEGF activity and a mediator of endothelial cell adhesiveness described here, NRP-1 emerges as a promising molecular target for the development of antiangiogenic drugs.
Introduction
Vascular endothelial growth factor (VEGF, also named vascular permeability factor, VPF) is a key regulator of physiologic and pathologic angiogenesis, and a molecular target for effective therapeutic intervention.1-6 The biologic activities of VEGF are mediated, for the most part, by 2 receptor tyrosine kinases, Flt-1 (VEGFR-1) and KDR (VEGFR-2).7,8 VEGFR-1 and VEGFR-2 are essential for fetal angiogenesis, and mouse embryos null for either receptor die in utero between days 8.5 and 9.5.9,10 Compelling evidence indicates that VEGFR-2, and not VEGFR-1, is the major mediator of VEGF-induced proliferation and migration of endothelial cells, despite both receptors having high affinity binding to VEGF.11-14 Chimeric fusion receptors containing the intracellular domain of VEGFR-1 or VEGFR-2 and the extracellular domain of other receptors provided evidence that VEGFR-1 can serve as a competitive inhibitor of VEGFR-2 mitogenic signals.13,14 Gene targeting studies have shown that whereas hematopoietic and vascular development are severely impaired in VEGFR-2 null mice,10 overgrowth of endothelial cells and disorganized blood vessels are the cause of death in VEGFR-1 null embryos,9 providing additional evidence for VEGFR-1's role as a negative regulator of VEGF function. Besides these canonical VEGF receptors, the angiogenic roles of VEGF are modulated by the co-receptor molecules neuropilin NRP-1 and NRP-2.15,16
NRP-1 and NRP-2, originally identified as receptors for class-3 semaphorins mediating neuronal guidance,17,18 were subsequently found to bind several isoforms of VEGF and to form complexes with VEGFR-1 and VEGFR-2.19-22 When coexpressed with VEGFR-2, NRP-1 enhanced the binding of VEGF to VEGFR-2 and enhanced VEGF-mediated chemotaxis.16 When complexed with VEGFR-1, NRP-1 prevents the binding of VEGF to NRP-1, providing evidence for a common surface for interaction of NRP-1 with either VEGFR-1 or VEGF.23 Consistent with NRP-1 playing an important role in regulating VEGF availability and function, NRP-1–deficient mice die at the embryo stage with defects in the heart, vasculature, and nervous system.24,25 Transgenic mice overexpressing NRP-1 also exhibited abnormalities in the cardiovascular and nervous systems, contributing to embryonic lethality.26,27 NRP-1 knockin mice expressing NRP-1 mutants that bind either VEGF or class 3 semaphorins demonstrated that VEGF/NRP-1 signaling is required for vascular development, whereas semaphorin-3A/NRP-1 signaling is required for neuronal, but not vascular, development.28 In addition, endothelial cell–specific NRP-1 null mice displayed marked vascular abnormalities, confirming that NRP-1 expression in endothelial cells is essential for normal vascular development.28
NRP-1 is a single spanning transmembrane protein with only a short intracytoplasmic tail of 40 amino acids. Deletion studies indicated that semaphorin function in neuronal cells is not dependent upon the intracellular domain of NRP-1 but requires plexin signaling, with NRP-1 serving as a high-affinity binding co-receptor for class 3 semaphorins.29,30 However, NRP-1 supported VEGF autocrine function and cell migration in tumor cells that lack expression of VEGFR-1 and VEGFR-2,16,31,32 raising the possibility that NRP-1 may either interact with other signaling receptors or independently promote cell signaling. Recently, endothelial cells transduced with a chimeric fusion receptor composed of the extracellular domain of the epidermal growth factor receptor and the intracellular domain of NRP-1 could migrate in response to the specific ligand.33
Targeted deletion of NRP-1 in endothelial cells provides strong evidence for a nonredundant role of NRP-1 in vascular development in the mouse, but it is currently unclear whether NRP-1 continues to play a unique role in endothelial cells postnatally. Taking advantage of RNA silencing techniques known as RNA interference (RNAi), we have selectively silenced NRP-1 and VEGFR-2 gene expression in human endothelial cells to dissect NRP-1 function in these cells. We show that NRP-1 is a critical regulator of endothelial cell adhesion to extracellular matrix proteins.
Materials and methods
Cells and cell cultures
Human umbilical vein endothelial cells (HUVECs) were propagated through passage 5 in HUVEC medium consisting of M199 medium (Gibco-BRL, Grand Island, NY) supplemented with 20% newborn calf serum (Gibco-BRL), 5% human AB serum (Sigma Chemical, St Louis, MO), 1.6 mM l-glutamine (Gibco-BRL), 25 ng/mL porcine heparin (Sigma), 50 ng/mL ascorbate (Fisher Scientific, Fair Lawn, NJ), and 15 μg/mL endothelial cell growth supplement (Sigma).
siRNA transfection
HUVEC cells at 70% confluency were transfected in OPTIMEM medium (Invitrogen, Carlsbad, CA) with the indicated siRNA duplexes using Lipofectamine 2000 (Invitrogen). After 4 hours the transfection medium was removed, the cells were washed twice with phosphate-buffered saline (PBS), and then maintained in complete HUVEC medium for 48 to 72 hours before performing the experiments. The sequences for the nonsilencing control and VEGFR-2 siRNA were synthesized by Qiagen (Valencia, CA). Target sequences were control, AAT TCT CCG AAC GTG TCA CGT; and VEGFR-2, AAG CGG CTA CCA GTC CGG ATA. NRP-1 siRNAs sequences were synthesized by Dharmacon (Lafayette, CO). The sequences for NRP-1 silencing have been reported previously.32
RNA preparation and reverse transcription–polymerase chain reaction
Total RNA was extracted using Absolutely RNA Microprep kit (Stratagene, La Jolla, CA). Quantitative reverse transcription–polymerase chain reaction (RT-PCR) was performed using OneStep RT-PCR kit in combination with SYBR Green PCR kit (Qiagen). The reaction was carried out in an Abi Prism 7900HT sequence detection system (Applied Biosystems, Foster City, CA). Primer sequences were NRP-1, sense, 5′-CAA GGC GAA GTC TTT TGA GG; antisense, 5′-CAC CTG TGA GCT GGA AGT CA; VEGFR-1 sense, 5′-GCA CCT TGG TTG TGG CTG AC; antisense, 5′-CGT GCT GCT GCT TCC TGG TCC; VEGFR-2 sense, 5′-GGA AAT CAT TAT TCT AGT AGG CAC GAC G; antisense, 5′-CCT GTG GAT ACA CTT TCG CGA TG; CXCR4 sense, 5′-GGC AAA CTG GTA CTT TGG GA; antisense, 5′-GAC GCC AAC ATA GAC CAC CT; GAPDH sense, 5′-GCC ACC CAG AAG ACT GTG GAT GGC; antisense, 5′-CAT GAT GGC CAT GAG GTC CAC CAC. In each case, the presence of a single specific band was confirmed by separation of the amplified samples on 1% agarose gels containing ethidium bromide.
Flow cytometry
The cells were washed with ice-cold PBS, detached with 2 mM EDTA (ethylenediaminetetraacetic acid), washed with PBS containing 1% bovine serum albumin (BSA), and incubated (5 × 105/mL in 50 μL PBS/1% BSA) with antibodies. For CXCR4 staining, we used a phycoerythrin-conjugated mouse monoclonal anti-CXCR4 antibody (Collaborative; BD PharMingen, San Diego, CA); for VEGFR-2 staining we used a mouse monoclonal anti–VEGFR-2 antibody (ab9530; Abcam, Cambridge, MA) followed by Alexa 594–labeled goat antimouse antibody (Molecular Probes, Eugene, OR); for α5β1 integrin staining we used a mouse monoclonal anti-integrin α5β1 antibody (MAB 1969; Chemicon International, Temecula, CA) followed by Alexa 488–labeled goat antimouse antibody (Molecular Probes); for β1 integrin staining we used an allophycocyanin (APC)–conjugated mouse anti–human CD29 antibody (303007; Biolegend; San Diego; CA); and for α3 integrin detection the cells were permeabilized with Cytofix/Citoperm (BD) and stained with a polyclonal rabbit anti-α3 antibody (AB 1920; Chemicon International) followed by Alexa 488–labeled goat antirabbit antibody (Molecular Probes, Eugene, OR). Data collected in a FACS Calibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ) were analyzed using CELLQuest software (Becton Dickinson).
ERK-1/2 phosphorylation and immunoblotting
HUVEC cells were detached with 2 mM EDTA, washed twice with PBS, and suspended in 199 medium containing heparin 25 μg/mL (Sigma). The cells were stimulated with 50 ng/mL VEGF-A (R&D Systems, Minneapolis, MN) for 10 or 15 minutes or with 500 ng/mL SDF-1α (R&D Systems) for 15 minutes and lysed in 1% sodium dodecyl sulfate (SDS), 50 mM Tris[tris(hydroxymethyl)aminomethane]-HCl [pH = 7.4], 150 mM NaCl, 1 mM phenylmethylsulfonyl fluoride, 2 mg/mL aprotinin, 2 mg/mL leupeptin, 1 mg/mL pepstatin, 20 mM NaF, and 0.1 mM NaVO4. After boiling, cellular debris was removed by centrifugation (500 g, 10 minutes). Protein samples were resolved on 10% to 20% Tricine gels (Novex, San Diego, CA), wet-transferred to nitrocellulose, and immunoblotted with anti–phospho-ERK (Cell Signaling Technology, Beverly, MA) and anti-ERK (Sigma) antibodies. Detection was performed by chemoluminescence using enhanced chemiluminescence (ECL) (Amersham Pharmacia Biotech, Piscataway, NJ). For NRP-1 protein detection, HUVEC cells were lysed in 50 mM Tris-HCl [pH = 8], 150 mM NaCl, 0.5% NP-40, 1 mM phenylmethylsulfonyl fluoride, 2 mg/mL aprotinin, 2 mg/mL leupeptin, 1 mg/mL pepstatin, 20 mM NaF, and 0.1 mM NaVO4 and immunoblotted with anti–NRP-1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) or anti–β-actin (Santa Cruz).
Cell proliferation assay
Cells were plated (4000 cells/well, 96 well plates, in 0.2 mL M199 supplemented with 18% heat inactivated FBS) with or without 1 to 100 ng/mL VEGF. DNA synthesis was measured by 3H thymidine deoxyribose uptake (0.5 μCi [5.0 × 104 Bq]/well, 6.7 Ci [247.9 GBq]/mmol; New England Nuclear, Boston, MA) during the last 18 hours of 72-hour culture. Results are expressed as mean cpm/culture.
In vitro matrigel assay
The in vitro matrigel assay was performed as described34 by plating cells (60-90 000 cells) onto 24-well plates coated with 200 to 250 μL matrigel (a crude extract of the Englebreth-Holm-Swarm tumor, Collaborative; BD PharMingen, San Diego, CA). After 18 hours of incubation, cells were photographed (using a Retiga 1300 digital camera; Qimaging, Burnaby, BC, Canada) under phase-contrast microscopy (Olympus 1 × 51 microscope, with a 10 ×/0.25 Ph L lens; Olympus Optical, Melville, NY), and images obtained with IPLab for Windows software (Scanalytics, Fairfax, VA) were imported into Adobe Photoshop (Adobe Systems, San Jose, CA). Tube formation was measured by counting the number of vascular joints in 4 nonoverlapping fields (each field defined as the area visualized by a 10 × magnification lens).
Confocal microscopy
For CXCR4 staining, HUVECs were fixed by the addition of a 3-fold volume of 3.7% paraformaldehyde for 10 minutes at room temperature, extensively washed, and cell membranes permeabilized (2 minute) on ice in a solution containing HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) (20 mM), sucrose (300 mM), NaCl (50 mM), MgCl2 (3 mM), and Triton-X 100 (0.1%). Thereafter, HUVEC cells were blocked with PBS 1% BSA 20 minutes at room temperature, washed, and stained with the primary anti-CXCR4 monoclonal antibody 12G5 (Becton Dickinson) 1 hour at room temperature. After washing with PBS 1% BSA, Alexa 594–conjugated goat anti–mouse IgG (Molecular Probes) was added to visualize CXCR4. Intracytoplasmatic F-actin immunofluorescence was performed on HUVEC grown on plates coated with gelatin and fixed with 1% paraformaldehyde, and permeabilized with 0.1% saponin PBS, pH 7.5 for 10 minutes at room temperature. F-actin was detected by staining with an Alexa 594–conjugated antiphalloidin antibody (Molecular Probes) for 30 minutes at room temperature. DNA was counterstained with DAPI (4,6 diamidino-2-phenylindole). Images were obtained with an LSM 510 Meta confocal microscopy system (Carl Zeiss, Thornwood, NY), equipped with a 63 × lens (1.4 aperture) and imported into Adobe Photoshop.
Migration assay
Migration assays were performed using gelatin-coated polycarbonate filters (pore size 8 μm) of transwells separating the upper and lower chamber of 24-well plates (Costar, Cambridge, MA). Chemotaxis medium (RPMI with 0.5% BSA and 10 mM HEPES) alone or supplemented with VEGF-A 100 ng/mL or SDF-1 100 ng/mL was added to the bottom chamber. HUVEC cells (0.5 × 106/well) were placed in the upper chamber. The plates were incubated at 37°C overnight. Viable cells in the lower chamber were collected and counted.
Endothelial cell attachment
Cell adhesion was evaluated as previously described.35 Briefly, 96-well neutral polystrene plates (Immulon I, Dynex Technologies) were coated at 4°C overnight with 5 μg/mL murine EHS-laminin (Sigma), bovine fibronectin (Sigma), and 3% BSA (Sigma) in Dulbecco PBS (DPBS; Gibco-BRL) or 0.2% gelatin in water (Sigma), 100 μL/well. Wells were washed in DPBS and blocked with PBS containing 3% BSA at 37°C for 1 hour. After washing, the siRNA-transfected cells (3 × 104 cells/well) were added and allowed to adhere in a humidified incubator (37°C for 7 hours). Nonadherent cells were removed by washing 3 times with DPBS. Adherent cells were stained with 0.05% crystal violet in 20% ethanol. After rinsing, plates were allowed to dry. After addition of methanol (100 μL/well), absorbance was measured at 595 nm.
CXCR4 expression and internalization
Analysis of surface CXCR4 with or without SDF-1/CXCL12 treatment was performed as previously described.36 Briefly, HUVECs were incubated at 37°C with or without addition of SDF-1 (1000 ng/mL, PeproTech (Rocky Hill, NJ). After 30 minutes, the samples were cooled in ice, and surface CXCR4 expression was evaluated by flow cytometry as described earlier.
Results
Effective silencing of VEGFR-2 and NRP-1 expression in primary human endothelial cells
Since conventional application of RNAi techniques to HUVEC led to poor transfection efficiencies and high cell mortality, a series of optimization experiments were undertaken. By modifying the culture conditions and reducing the time of cell exposure to siRNA duplexes and the concentration of the lipofectin-based transfection reagent, we consistently achieved transfection rates above 70% with a control plasmid expressing enhanced green fluorescent protein (EGFP) (data not shown). Similar transfection efficiencies were achieved with a fluorescein-conjugated control (nonsilencing) siRNA (Figure 1A). This high transfection efficiency of siRNA duplexes permits the conduct of RNAi experiments in early passage HUVECs, without a requirement for clonal selection-expansion steps and associated phenotypic changes through multiple cell passages.37,38
Reduced expression of VEGFR-2 and NRP-1 by short-interfering RNAs. (A) Transfection of siRNA duplexes into HUVECs evaluated by fluorescence microscopy. The image shows a cell culture of HUVEC 6 hours after transfection with a fluorescein-conjugated (nonsilencing) control siRNA duplex. The image was collected using an Olympus 1 × 51 microscope (20 ×/0.40 Ph1 lens; Olympus Optical) with a Retiga 1300 digital camera (Qimaging) and IPLab acquisition software (Scanalytics). (B) Specific silencing of VEGFR-2 and NRP-1 by RNAi. Relative mRNA levels of VEGFR-1, VEGFR-2, and NRP-1 (normalized to glyceraldehydes-3-phosphate dehydrogenase [GAPDH] levels) in transfected HUVEC cultures. Black bars represent control siRNA; red bars, VEGFR-2 siRNA; and cream bars, NRP-1 siRNA. Data shown reflect the means ± SD from 3 RNA preparations obtained 48 hours after transfection of siRNA duplexes. (C) Western blot analysis of NRP-1 and actin levels in control siRNA and NRP-1 siRNA-transfected cells. Cell lysates were obtained 48 hours after transfection. (D) Surface levels of VEGFR-2 detected by flow cytometry in control siRNA (open black curve) and VEGFR-2 siRNA-transfected cells (open green curve). Control (IgG1) is represented by the filled red curve. Cells were obtained 48 hours after transfection.
Reduced expression of VEGFR-2 and NRP-1 by short-interfering RNAs. (A) Transfection of siRNA duplexes into HUVECs evaluated by fluorescence microscopy. The image shows a cell culture of HUVEC 6 hours after transfection with a fluorescein-conjugated (nonsilencing) control siRNA duplex. The image was collected using an Olympus 1 × 51 microscope (20 ×/0.40 Ph1 lens; Olympus Optical) with a Retiga 1300 digital camera (Qimaging) and IPLab acquisition software (Scanalytics). (B) Specific silencing of VEGFR-2 and NRP-1 by RNAi. Relative mRNA levels of VEGFR-1, VEGFR-2, and NRP-1 (normalized to glyceraldehydes-3-phosphate dehydrogenase [GAPDH] levels) in transfected HUVEC cultures. Black bars represent control siRNA; red bars, VEGFR-2 siRNA; and cream bars, NRP-1 siRNA. Data shown reflect the means ± SD from 3 RNA preparations obtained 48 hours after transfection of siRNA duplexes. (C) Western blot analysis of NRP-1 and actin levels in control siRNA and NRP-1 siRNA-transfected cells. Cell lysates were obtained 48 hours after transfection. (D) Surface levels of VEGFR-2 detected by flow cytometry in control siRNA (open black curve) and VEGFR-2 siRNA-transfected cells (open green curve). Control (IgG1) is represented by the filled red curve. Cells were obtained 48 hours after transfection.
For NRP-1 silencing we used oligonucleotide sequences available from the literature.32 For VEGFR-2 silencing we designed and tested various candidate siRNA sequences and selected the one with the highest silencing activity. As shown in Figure 1B, 48 hours after transfection, VEGFR-2 and NRP-1 siRNA duplexes triggered a marked decrease in the mRNA levels for the target proteins in HUVEC, with minimal effect on the expression of VEGFR-1 and glyceraldehyde phosphate dehydrogenase (GAPDH). Consistently, NRP-1 and VEGFR-2 protein levels were reduced in these cells as evaluated by immunoblotting or FACS analysis with specific antibodies, respectively (Figure 1C,D). Similar reduction levels were detected as late as 96 hours after HUVEC transfection (data not shown). Thus, we achieved specific and effective silencing of VEGFR-2 and NRP-1 expression in primary human endothelial cells.
Distinct requirements for VEGFR-2 and NRP-1 in VEGF-induced proliferation
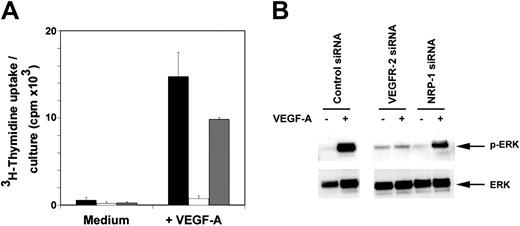
Current data from mouse models and overexpression strategies support the view that VEGF-mediated mitogenic signaling in endothelial cells uses VEGFR-2 as the signaling receptor with NRP-1 functioning as a co-receptor.16,28 To validate RNAi-mediated silencing of VEGFR-2 and NRP-1 in our model system and directly evaluate the discrete functions of VEGFR-2 and NRP-1, we examined the effect of VEGFR-2 or NRP-1 down-regulation on VEGF-induced endothelial cell proliferation (Figure 2A). Whereas HUVEC cells transfected with the VEGFR-2 siRNA essentially failed to respond to VEGF stimulation, NRP-1–depleted cells consistently showed a modest decrease in proliferation compared with control transfectants. Similar results were obtained with HUVEC 48 and 72 hours after transfection, at VEGF concentrations ranging between 1 and 100 ng/mL, and at different time points (24-72 hours) after addition of VEGF (data not shown). These results confirm the essential role of VEGFR-2 in mediating VEGF-induced mitogenic responses and provide evidence for a contribution of NRP-1 to VEGF-induced proliferation of endothelial cells.
Analysis of the requirements for VEGFR-2 and NRP-1 in VEGF-induced mitogenesis and signaling. (A) VEGF-induced proliferation in VEGFR-2 and NRP-1 silenced HUVECs. SiRNA-transfected cells (4000 cells/well) obtained 48 to 72 hours after transfection were cultured for 72 hours in culture medium supplemented with 18% heat-inactivated FBS and 25 ng/mL heparin with or without VEGF-A (25 ng/mL). ▪ indicates control siRNA; □, VEGFR-2 siRNA; and ▦, NRP-1 siRNA. Results represent the mean ± SD of 5 experiments, each performed in triplicate. (B) ERK activation in HUVECs after transfection of control, VEGFR-2, or NRP-1 siRNA evaluated by Western blotting with specific antibodies to phosphorylated (p) and total ERK. Cells obtained 48 hours after transfection were starved for 16 hours and then exposed for 10 minutes to 50 ng/mL VEGF-A.
Analysis of the requirements for VEGFR-2 and NRP-1 in VEGF-induced mitogenesis and signaling. (A) VEGF-induced proliferation in VEGFR-2 and NRP-1 silenced HUVECs. SiRNA-transfected cells (4000 cells/well) obtained 48 to 72 hours after transfection were cultured for 72 hours in culture medium supplemented with 18% heat-inactivated FBS and 25 ng/mL heparin with or without VEGF-A (25 ng/mL). ▪ indicates control siRNA; □, VEGFR-2 siRNA; and ▦, NRP-1 siRNA. Results represent the mean ± SD of 5 experiments, each performed in triplicate. (B) ERK activation in HUVECs after transfection of control, VEGFR-2, or NRP-1 siRNA evaluated by Western blotting with specific antibodies to phosphorylated (p) and total ERK. Cells obtained 48 hours after transfection were starved for 16 hours and then exposed for 10 minutes to 50 ng/mL VEGF-A.
We examined whether NRP-1 contributes to VEGF-induced mitogenic responses by modulating VEGFR-2 signaling. In HUVECs, mitogenic responses to VEGF require activation of the mitogen-activated protein kinase (MAPK) (ERK1/2) signaling cascade with maximal activation occurring after 10 minutes of VEGF exposure.14,39 Phosphorylation of ERK-1/2 can therefore be used as a measure of VEGF mitogenic signaling in HUVEC. We readily detected ERK-1/2 phosphorylation 10 minutes after VEGF addition in HUVEC transfected with control siRNA (Figure 2B). However, under the same conditions, we detected virtually no pERK1/2 in HUVECs transfected with VEGFR-2 siRNA, a result that is consistent with the cells' failure to significantly proliferate in response to VEGF. HUVEC transfected with NRP-1 siRNA displayed an intermediate level of pERK1/2 phosphorylation. Together, these results confirm the essential role of VEGFR-2 and ERK1/2 phosphorylation as mediators of VEGF-induced proliferation in HUVEC cells. In addition, the observation that NRP-1 silencing reduces levels of VEGF-induced pERK1/2 phosphorylation in HUVEC without affecting VEGFR-2 expression levels (Figure 1B) supports the notion that NRP-1 functions as an enhancer of VEGF/VEGFR-2–induced mitogenic responses in human endothelial cells.
VEGFR-2 and NRP-1 regulate migration and tube formation in HUVECs
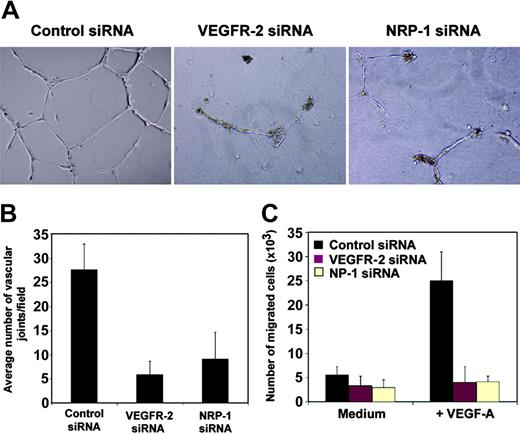
A hallmark of endothelial cells is their ability to undergo morphogenic changes into vascular structures.40 In vitro studies of angiogenesis have provided evidence that primary endothelial cells form a network of tubular structures when incubated on extracellular matrices (ECMs) such as collagen, fibrin, or matrigel, which represent a mixture of collagen IV, laminin, heparan sulfate proteoglycans, and entactin-nidogen.40-42 With HUVECs, matrigel-mediated tube formation takes place over 24 hours of incubation and does not require cell division.34,42 We analyzed the role of NRP-1 and VEGFR-2 in this nonmitogenic function of endothelial cells. Within 24 hours of incubation on matrigel-coated surfaces, control siRNA-transfected HUVECs formed characteristic tubelike structures assembled in branching reticular networks (Figure 3A). However, the transfection of HUVECs with VEGFR-2 or NRP-1 siRNA consistently prevented formation of tubular structures under the same conditions. Instead, HUVECs appeared mostly isolated or in clumps of rounded cells without cellular protrusions, with only occasional short nonconnected cords being identified throughout the matrigel surface (Figure 3A). Quantitative analysis showed a significant decrease in tube formation by VEGFR-2 and NRP-1–depleted cells compared with control transfected cells (P < .01), but no significant difference (P > .05) between VEGFR-2 and NRP-1–depleted cells (Figure 3B). These results provide evidence that VEGFR-2 and NRP-1 are critical mediators of the assembly of human endothelial cells into tubular structures on ECM.
Contribution of VEGFR-2 and NRP-1 to endothelial cell tube formation and migration. (A) HUVECs (60 × 104) obtained 48 hours after transfection were suspended in HUVEC medium, plated on matrigel-coated 24-well plates, and incubated for 24 hours. Images reflect representative fields visualized by phasecontrast microscopy (original magnification, 10 ×). Results were reproduced in 5 different experiments. (B) Quantitative analysis of matrigel-induced tube formation in control and NRP-1–defective HUVECs. Tube formation under the conditions described in “VEGFR-2 and NRP-1 regulate migration and tube formation in HUVECs” was measured as a function of the number of vascular joints per visual field (10 × magnification) and expressed as an average of 4 nonoverlapping fields. The results represent the average (± SD) from 4 independent experiments. (C) Contribution of VEGFR-2 and NRP-1 to VEGF-induced endothelial cell migration. HUVEC transfected 24 hours earlier with control (black bars), VEGFR-2 (red bars), or NRP-1 siRNA (cream bars) were placed in the upper chamber of transwells precoated with gelatin; VEGF (100 ng/mL) was placed in the lower chamber. The results reflect the mean (± SD) number of migrated cells from 3 independent experiments.
Contribution of VEGFR-2 and NRP-1 to endothelial cell tube formation and migration. (A) HUVECs (60 × 104) obtained 48 hours after transfection were suspended in HUVEC medium, plated on matrigel-coated 24-well plates, and incubated for 24 hours. Images reflect representative fields visualized by phasecontrast microscopy (original magnification, 10 ×). Results were reproduced in 5 different experiments. (B) Quantitative analysis of matrigel-induced tube formation in control and NRP-1–defective HUVECs. Tube formation under the conditions described in “VEGFR-2 and NRP-1 regulate migration and tube formation in HUVECs” was measured as a function of the number of vascular joints per visual field (10 × magnification) and expressed as an average of 4 nonoverlapping fields. The results represent the average (± SD) from 4 independent experiments. (C) Contribution of VEGFR-2 and NRP-1 to VEGF-induced endothelial cell migration. HUVEC transfected 24 hours earlier with control (black bars), VEGFR-2 (red bars), or NRP-1 siRNA (cream bars) were placed in the upper chamber of transwells precoated with gelatin; VEGF (100 ng/mL) was placed in the lower chamber. The results reflect the mean (± SD) number of migrated cells from 3 independent experiments.
Endothelial cell tube morphogenesis is a complex process involving a variety of steps including cell attachment and spread, protrusive activity, migration, and cavitation.40,43-45 Since VEGF is a critical endogenous mediator of endothelial cell protrusive activity that allows endothelial cells to form spindle-shaped processes in vivo46 and tube formation in vitro,47 acting as an attractant for VEGFR-2–expressing endothelial cells,48 we evaluated VEGF-induced endothelial cell migration in siRNA-treated endothelial cells. We found that VEGFR-2 and NRP-1 siRNA-transfected HUVECs, unlike control cells, consistently failed to migrate across gelatin-coated polycarbonate filters in response to VEGF, even at the highest concentrations used (100 ng/mL; Figure 3C). These results demonstrate that VEGFR-2 and NRP-1, individually, are essential mediators of human endothelial cell migration to VEGF and provide an explanation for disrupted ECM-dependent tube formation by endothelial cells defective of VEGFR-2 or NRP-1.
NRP-1 regulates endothelial cell attachment independently of VEGFR-2
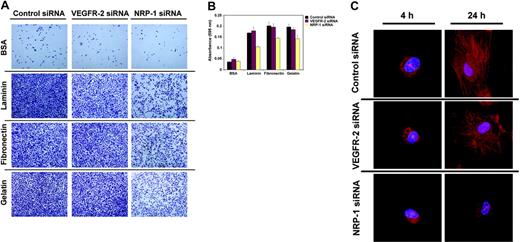
The failure of NRP-1–defective HUVECs to migrate in response to VEGF is inconsistent with NRP-1 simply playing an enhancing role for VEGF/VEGFR-2–induced responses, which would be expected to reduce, but not abolish, migratory responses to VEGF. Since endothelial cell movement in response to attractive cues has been shown to be critically dependent upon physical interaction with the ECM,49 we surveyed the attachment capacity of endothelial cells in which VEGFR-2 or NRP-1 was silenced. Endothelial cells fail to attach to noncharged polystyrene surfaces, unless these surfaces are appropriately coated with ECM-mimicking compounds such as BSA, fibronectin, EHS-laminin, and gelatin.35 Using this model system, we found that whereas reduced expression of VEGFR-2 has a minimal effect on the attachment of HUVECs, reduced expression of NRP-1 expression consistently leads to a significant reduction (P < .01 laminin and fibronectin; P < .05 gelatin) in the adhesion capacity of the endothelial cells to all extracellular matrix surfaces tested (Figure 4A,B).
Contribution of NRP-1 and VEGFR-2 to endothelial cell attachment. (A) Endothelial cell attachment to plates coated with BSA, laminin, fibronectin, and gelatin after 7 hours' incubation. HUVECs were transfected with control, VEGFR-2, or NRP-1 siRNA 48 hours earlier. Representative images from inverted microscopy (Olympus 1X51 microscope with 20 ×/0.40 Ph1 lens; Olympus Optical) display the fraction of cells that attached. Images were obtained with a Religa 1300 digital camera (Qimaging) using IPLab acquisition software (Scanalytics). (B) HUVEC attachment to wells measured by absorbance at 595 nM after staining the attached cells with crystal-violet. HUVECs were transfected with control, VEGFR-2, or NRP-1 siRNA 48 hours earlier and allowed to attach to coated wells for 7 hours. Representative experiment of 3 performed. (C) F-actin expression and distribution in HUVEC transfected with control, VEGFR-2, and NRP-1 siRNA, and subsequently allowed to attach for 4 or 24 hours on gelatin-coated dishes. Transfection was carried out 48 hours prior to attachment. Representative confocal images from 1 of 3 experiments performed.
Contribution of NRP-1 and VEGFR-2 to endothelial cell attachment. (A) Endothelial cell attachment to plates coated with BSA, laminin, fibronectin, and gelatin after 7 hours' incubation. HUVECs were transfected with control, VEGFR-2, or NRP-1 siRNA 48 hours earlier. Representative images from inverted microscopy (Olympus 1X51 microscope with 20 ×/0.40 Ph1 lens; Olympus Optical) display the fraction of cells that attached. Images were obtained with a Religa 1300 digital camera (Qimaging) using IPLab acquisition software (Scanalytics). (B) HUVEC attachment to wells measured by absorbance at 595 nM after staining the attached cells with crystal-violet. HUVECs were transfected with control, VEGFR-2, or NRP-1 siRNA 48 hours earlier and allowed to attach to coated wells for 7 hours. Representative experiment of 3 performed. (C) F-actin expression and distribution in HUVEC transfected with control, VEGFR-2, and NRP-1 siRNA, and subsequently allowed to attach for 4 or 24 hours on gelatin-coated dishes. Transfection was carried out 48 hours prior to attachment. Representative confocal images from 1 of 3 experiments performed.
Upon endothelial cell attachment to the ECM, actin microfilaments organize into the so-called “stress fibers” reflective of actin polymerization, which mediate various protein-protein interactions critical to cytoskeleton organization.50 To test whether defective attachment in NRP-1 siRNA-transfected HUVECs is linked to defective cytoskeleton reorganization upon cell adhesion, we evaluated the intracellular distribution of filamentous actin (F-actin) during attachment. Confocal microscopy revealed characteristic time-dependent F-actin changes in HUVECs plated on gelatin (Figure 4C, representative images). By 4 hours after plating, most control siRNA-transfected cells had acquired focally distinguishable F-actin staining along the periphery and displayed membrane blebs extending from those positive areas. By 24 hours, a vigorous network of intensely staining fibers was detectable throughout the cytoplasm of virtually all cells. VEGFR-2–defective cells were not different from control cells in the patterns of F-actin distribution over the 24-hour period of observation. In particular, at the 24-hour time-point virtually all cells stained intensely and diffusely for F-actin. By contrast, and consistent with the impaired attachment observed in these cells, NRP-1–defective cells displayed a marked reduction in F-actin organization. Even after 24 hours' incubation onto gelatin, most cells displayed only occasional F-actin staining and displayed focal blebs extending from these positive areas. Only after 48 hours did NRP-1–defective cells display F-actin staining that was similar in distribution and intensity to that of control cells (data not shown). Thus, NRP-1 is a critical early regulator of the endothelial cell attachment machinery. This function of NRP-1 appears independent of VEGFR-2 co-operation.
Neuropilin-1 regulates CXCR4 expression in endothelial cells
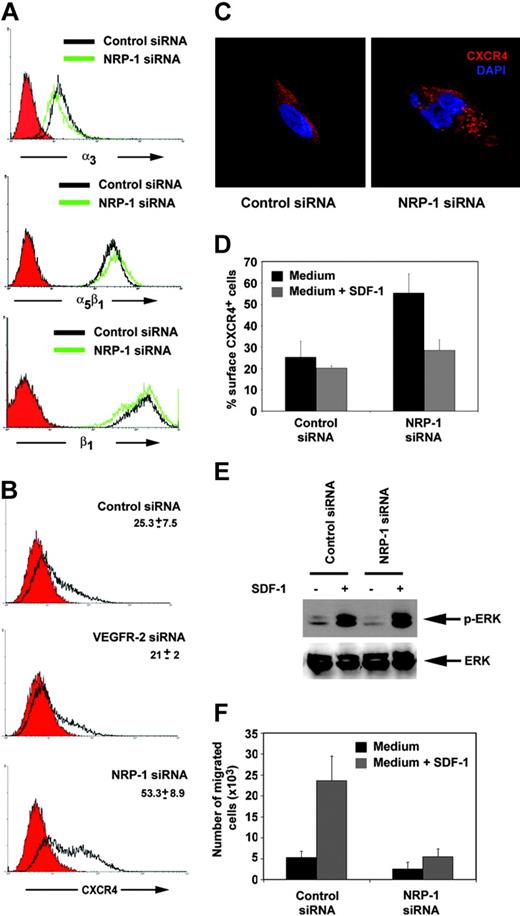
Among adhesion receptor families, integrins are widely recognized as the principal cell surface receptors by which cells attach to ECM and mediate mechanical and chemical signals,51-53 and previous studies have established the contribution of integrins to angiogenesis.54,55 Since we found that NRP-1–depleted HUVECs are defective in attachment to fibronectin and laminin, we analyzed their expression of α3β1 and α5β1 integrins, which mediate specific cell binding to laminin and fibronectin, respectively (Figure 5A). We found that expression levels of these integrins are similar in control and NRP-1–defective HUVECs, proving evidence that α3β1 and α5β1 integrin expression levels alone do not explain defective attachment in NRP-1–defective cells. Also, levels of expression of the adhesion molecules αvβ5 or L1-CAM were similar in NRP-1–defective and control cells (data not shown). By contrast, expression of the chemokine receptor CXCR4, which regulates endothelial cell morphogenesis and integrin adhesiveness to specific ligands,56 was significantly higher in NRP-1–defective cells compared with control cells (Figure 5B). Quantitative RT-PCR showed higher levels of CXCR4 mRNA in NRP-1–defective cells compared with the controls (data not shown), providing evidence for increased CXCR4 transcription. By confocal microscopy, CXCR4 is generally uniformly distributed in the cytoplasm of the permeabilized endothelial cell and displays a finely punctuated staining (Figure 5C). This staining pattern was similarly detected in VEGFR-2 siRNA-transfected cells (data not shown). However, in NRP-1 siRNA-transfected HUVECs, CXCR4 presented in the form of intracytoplasmic aggregates much larger in size than those detected in the control, suggesting greater abundance and/or abnormal distribution of the receptor in these cells (Figure 5C).
CXCR4 expression and function in NRP-1–defective endothelial cells. (A) Expression of the integrins α3, α5β1, and β1 in HUVECs transfected 48 hours earlier with control (open black curve) or NRP-1 siRNA (open green curve) detected by flow cytometry. (B) Flow cytometric analysis of surface CXCR4 expression in HUVECs transfected 48 hours earlier with control, VEGFR-2, or NRP-1 siRNA. Filled red curves represent IgG2a (control); open black curves, CXCR4. (C) Representative confocal images showing the different distribution of intracellular CXCR4 in HUVECs transfected with NRP-1 or control siRNA. The cells were immunostained for CXCR4 (red) and counterstained with DAPI (blue). (D) CXCR4 internalization in NRP-1–silenced cells. HUVECs transfected with control or NRP-1 siRNA 48 hours earlier were incubated for 30 minutes at 37°C with the CXCR4 ligand SDF-1α (▦; 500 ng/mL) and then immunostained for surface CXCR4. ▪ indicates control medium only. Results reflect the mean (± SD) of 3 experiments performed. (E) SDF-1–induced ERK activation in HUVECs transfected with control or NRP-1 siRNA evaluated by Western blotting with specific antibodies to phosphorylated and total ERK. Cells obtained 48 hours after transfection were starved for 16 hours and then exposed for 15 minutes to 500 ng/mL SDF-1α. (F) Endothelial cell migration in response to SDF-1. HUVECs transfected 48 hours earlier with control or NRP-1 siRNA were placed in the upper chamber of transwells precoated with gelatin; SDF-1 (▦; 100 ng/mL) was placed in the lower chamber. ▪ indicates control medium only. The results reflect the mean (± SD) number of migrated cells from 4 independent experiments.
CXCR4 expression and function in NRP-1–defective endothelial cells. (A) Expression of the integrins α3, α5β1, and β1 in HUVECs transfected 48 hours earlier with control (open black curve) or NRP-1 siRNA (open green curve) detected by flow cytometry. (B) Flow cytometric analysis of surface CXCR4 expression in HUVECs transfected 48 hours earlier with control, VEGFR-2, or NRP-1 siRNA. Filled red curves represent IgG2a (control); open black curves, CXCR4. (C) Representative confocal images showing the different distribution of intracellular CXCR4 in HUVECs transfected with NRP-1 or control siRNA. The cells were immunostained for CXCR4 (red) and counterstained with DAPI (blue). (D) CXCR4 internalization in NRP-1–silenced cells. HUVECs transfected with control or NRP-1 siRNA 48 hours earlier were incubated for 30 minutes at 37°C with the CXCR4 ligand SDF-1α (▦; 500 ng/mL) and then immunostained for surface CXCR4. ▪ indicates control medium only. Results reflect the mean (± SD) of 3 experiments performed. (E) SDF-1–induced ERK activation in HUVECs transfected with control or NRP-1 siRNA evaluated by Western blotting with specific antibodies to phosphorylated and total ERK. Cells obtained 48 hours after transfection were starved for 16 hours and then exposed for 15 minutes to 500 ng/mL SDF-1α. (F) Endothelial cell migration in response to SDF-1. HUVECs transfected 48 hours earlier with control or NRP-1 siRNA were placed in the upper chamber of transwells precoated with gelatin; SDF-1 (▦; 100 ng/mL) was placed in the lower chamber. ▪ indicates control medium only. The results reflect the mean (± SD) number of migrated cells from 4 independent experiments.
We examined CXCR4 function in NRP-1–defective cells and tested for ligand-induced CXCR4 internalization and signaling. Exposure to SDF-1 induces desensitization and endocytosis-mediated internalization of CXCR4, which can eventually be recycled to the cell surface.57 Control, VEGFR-2, and NRP-1 siRNA-transfected cells were incubated for 30 minutes in medium alone or with 1 μg/mL SDF-1 and changes in CXCR4 cell surface expression examined. As shown in Figure 5D, NRP-1 siRNA-transfected cells could internalize CXCR4 in presence of SDF-1, indicating that this receptor function is preserved. We also observed that addition of SDF-1 triggered a normal calcium-flux in NRP-1–silenced HUVECs (data not shown).
In endothelial cells, CXCR4 signaling activates the MAPK (ERK1/2) signaling cascade. Phosphorylation of ERK-1/2 can therefore be used as a measure of SDF-1–induced CXCR4 signaling. ERK-1/2 phosphorylation was similarly detected in NRP-1 and control-transfected HUVEC cells 15 minutes after addition of SDF-1 (Figure 5E), suggesting that CXCR4 signaling is preserved in NRP-1–silenced cells.
We also measured the capacity of HUVECs transfected with NRP-1 siRNA to migrate in response to SDF-1, and compared the results to results for control transfectants. As shown in Figure 5F, NRP-1–depleted cells had a significantly reduced capacity to migrate in response to SDF-1 in comparison to control cells. Since we had determined that ERK-1/2 is normally phosphorylated in these cells after SDF-1 stimulation, we conclude that defective SDF-1–induced chemotaxis in NRP-1–defective cells is not likely attributable to defective CXCR4 function, but rather to defects in any one of the complex set of coordinated processes that regulate cell movement in response to directional cues.58
Discussion
Considerable experimental evidence has shown that NRP-1 plays an essential role in the development of the vascular system.24,59 In the mouse, selected deletion of NRP-1 in the developing endothelial cells causes marked vascular abnormalities, which likely account for embryonic lethality in these mice.28 NRP-1 functions as a co-receptor for members of the VEGF family and enhances VEGFR-2 activity in the presence of VEGF.16,21,22 Since inactivation of a single VEGF allele results in embryonic lethality in mice,60,61 the role of NRP-1 in vascular development has been linked to an essential amplification of VEGF-dependent angiogenesis.1,28,59 Unlike NRP-1's documented role during development, it has remained thus far unclear whether NRP-1 continues to play a critical role in angiogenesis postnatally.
In this study, we have selectively deleted NRP-1 expression in primary human endothelial cells and uncovered a unique role of NRP-1 as a regulator of endothelial cell attachment to extracellular matrix proteins. Cell attachment to extracellular matrix is essential to endothelial cell survival, growth, movement, and angiogenesis.62 Unlike endothelial cell precursors, which can circulate in the blood,63 mature endothelial cells die by apoptosis or “anoikis” when contact is lost, providing extracellular matrix adhesion an active control of cell and vascular integrity.64,65 In addition, since VEGFR-2–defective endothelial cells display normal attachment, the endothelial cell adhesive function of NRP-1 appears independent of VEGF/VEGFR-2 signaling.
Previous studies with tumor cells lacking VEGFR-2 expression have suggested that NRP-1 may transduce VEGF growth signals either alone or in concert with other receptors.31 Recently, the intracellular domain of NRP-1 fused with the extracellular domain of the epidermal growth factor was found to mediate ligand-dependent endothelial cell migration.66 In neuronal cells, NRP-1 forms complexes with semaphorin3A, a secreted protein that regulates axon guidance, and with a signal-transducing subunit from class-A plexins.17,18 NRP-1 is necessary for semaphorin3A-induced axon guidance but does not mediate semaphorin3A signaling, which derives from the intracellular domain of plexin-A1 or plexin-A2.22,29 NRP-1 also can form stable complexes with the adhesion molecule L1-CAM in neuronal cells and convert semaphorin signals from repulsive into attractive.67,68
In addition to its role as a modulator of VEGF-A and semaphorin signaling due to its specific interaction with VEGFR-2, plexin-B1, and L1-CAM, there is evidence that NRP-1 can function as an adhesion molecule itself when overexpressed in a mouse fibroblast cell line.69,70 Mapping studies have identified the b1 and b2 domains of the extracellular domain of NRP-1 as essential for cell adhesion and shown that class-3 semaphorins, plexinA family members, and VEGF do not function as the adhesion ligands for NRP-1.69,70 Although such NRP-1 ligand (or ligands) appear to be expressed by a wide range of cells,70 they have yet to be identified. In addition, the functional consequences of the adhesive properties of NRP-1 have not been previously investigated in any cell that naturally expresses NRP-1.
The results presented here document that NRP-1 is a critical mediator of primary endothelial cell attachment to extracellular matrix. It is important to note that while early attachment was severely compromised, most endothelial cells defective of NRP-1 eventually attached, providing evidence for an early adhesive role of NRP-1 and confirming the complexities and redundancy of the cell attachment processes, which are still incompletely understood. Additional studies will be required to establish whether NRP-1 itself can function as an adhesion molecule in endothelial cells or whether it acts indirectly. We found that surface expression levels of the adhesion molecule L1-CAM, which can associate with NRP-1 in neuronal cells,67,68 and expression levels of the integrins αvβ3, α3β1, and α5β1, which are critical regulators of cell adhesion to multiple substrates,51-53 are not significantly altered in NRP-1–defective endothelial cells. Interestingly, expression of the chemokine receptor CXCR4, which regulates endothelial cell tube formation by specifically interacting with cell surface SDF-134 and can enhance integrin-mediated cell adhesion,56,71 was consistently enhanced, perhaps reflective of a compensatory mechanism in NRP-1–silenced cells.
Based on results from VEGFR-2 null mice, VEGF mutants, and receptor inactivation studies, VEGFR-2 is recognized as the major mediator of VEGF-induced survival, mitogenic, and angiogenic effects of VEGF.1 By effectively disrupting VEGFR-2 expression, we confirm here the essential role of VEGFR-2 and the Raf-MEC-MAP signaling pathway as mediators of VEGF responses in primary human endothelial cells. We also provide evidence confirming a critical role of endogenous VEGF in endothelial cell morphogenic processes.47
Gene silencing by RNAi-based technology overcomes some of the previous difficulties at achieving gene knockout in primary human endothelial cells, which can only undergo a limited number of cell divisions in culture without phenotypic change, loss of telomerase activity, or evidence of senescence.37,72-74 Unlike other gene-silencing approaches that require clonal selection and multiple passaging, RNAi is shown here to represent a powerful tool for angiogenesis research. Application of this method has uncovered a previously unrecognized function of NRP-1 as a critical regulator of endothelial cell attachment to extracellular matrix.
There is considerable interest in the development of anti-angiogenic therapies for the treatment of cancer and other diseases. Neutralizing antibodies directed at VEGF have shown efficacy at delaying disease progression in several malignancies, but disease progression has generally occurred, suggesting either incomplete VEGF neutralization or the existence of angiogenic pathways that are independent of VEGF. Hence, other anti-angiogenic agents that target integrins,75,76 VEGF, and PDGF signaling77,78 are in various stage of development.79 Recently, NRP-1 was found to reduce experimental leukemia progression in mouse, presumably by reducing bone marrow neovascularization.80 Based on its dual function as a modulator of VEGF activity and mediator of endothelial cell adhesion identified in this study, NRP-1 represents an attractive target for therapeutic development.
Prepublished online as Blood First Edition Paper, November 2, 2004; DOI 10.1182/blood-2004-07-2598.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Ms L. Sierra and Drs Lei Yao, David Roberts, and Andre Nussenzweig.

![Figure 1. Reduced expression of VEGFR-2 and NRP-1 by short-interfering RNAs. (A) Transfection of siRNA duplexes into HUVECs evaluated by fluorescence microscopy. The image shows a cell culture of HUVEC 6 hours after transfection with a fluorescein-conjugated (nonsilencing) control siRNA duplex. The image was collected using an Olympus 1 × 51 microscope (20 ×/0.40 Ph1 lens; Olympus Optical) with a Retiga 1300 digital camera (Qimaging) and IPLab acquisition software (Scanalytics). (B) Specific silencing of VEGFR-2 and NRP-1 by RNAi. Relative mRNA levels of VEGFR-1, VEGFR-2, and NRP-1 (normalized to glyceraldehydes-3-phosphate dehydrogenase [GAPDH] levels) in transfected HUVEC cultures. Black bars represent control siRNA; red bars, VEGFR-2 siRNA; and cream bars, NRP-1 siRNA. Data shown reflect the means ± SD from 3 RNA preparations obtained 48 hours after transfection of siRNA duplexes. (C) Western blot analysis of NRP-1 and actin levels in control siRNA and NRP-1 siRNA-transfected cells. Cell lysates were obtained 48 hours after transfection. (D) Surface levels of VEGFR-2 detected by flow cytometry in control siRNA (open black curve) and VEGFR-2 siRNA-transfected cells (open green curve). Control (IgG1) is represented by the filled red curve. Cells were obtained 48 hours after transfection.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/5/10.1182_blood-2004-07-2598/6/m_zh80050574800001.jpeg?Expires=1767733364&Signature=2LdWqq1vPODCHoHaXblnQzlAK1WYTtfIJ~hX5e7TzXEdiTcHh9Tj7xsP3CndjzDBG~eONx9VUMOk20KCY8cBpiKP-l4GmPg-NcvxSVRWWYk8lK84gTHVM02P3Rpg-eVy2KmhHKaLAZPnYuc3kqFEag~M8Z8HLT~OgUPDwaaTH51VYDrA0DXmZDTieUPT4YBSQvvnn8V0cqO6gTAgWFFpGc-~JWTbvV2MaC1nAAUchXUYS6vw9p5wxMNR6UwjdzVD5HOTby0u6TT3EkqGFNrYbkS47RKx0k0DnP-I8vArgnQmbajBig2EoiMAcxNwL2BBVkn~JZRClUQJ066YitKUOw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)