Abstract
Essential thrombocythemia (ET) and polycythemia vera (PV) are chronic myeloproliferative disorders that share the involvement of a multipotent progenitor cell and dominance of the transformed clone over normal hematopoiesis. On the other hand, the heterogeneity of these diseases with respect to clonal development from a common progenitor has been well established. To identify useful prognostic indicators, we analyzed telomerase activity (TA), a known marker of neoplastic proliferation, in granulocytes (PMNs) and mononuclear cells (MNCs) from 22 female patients with ET and PV. Clonality status was determined by investigation of X chromosome inactivation patterns (XCIPs). We found a statistically significant positive correlation between high TA and monoclonal pattern of XCIP. Therefore, our data suggest that the use of multiple tumor markers may contribute to a better understanding of the deregulated physiology of these disorders and provide useful prognostic factors.
Introduction
Telomeres are nonencoding regions of DNA capping the ends of chromosomes, essential for genetic stability and cell replication1 ; telomerase is the ribonucleoprotein enzyme complex that maintains telomere integrity. Telomerase activity (TA) is generally undetectable in normal somatic cells, while it is expressed in approximately 85% of the common cancers.2,3 Hematopoietic stem cells express telomerase and contain long telomeres, which become shorter as cells differentiate and mature. While it is acknowledged that TA is elevated in the acute leukemias and aggressive lymphomas,4 exhaustive data on the level and prognostic significance of TA in other common hematopoietic diseases are still lacking.5,6
Chronic myeloproliferative disorders (MPDs) are acquired diseases of the hematopoietic stem cell, characterized by clonal proliferation of one or more cell lineages. Several studies, however, have shown that a sizable proportion of patients with polycythemia vera (PV) and essential thrombocythemia (ET) have polyclonal hematopoiesis.7-9 The significance of this variable clonal expression and its relationships, if any, with other clinical and/or biologic characteristics remain elusive.
To investigate further the relevance and possible correlation(s) of these neoplastic markers, we decided to evaluate TA and the pattern of X chromosome inactivation (XCIP) as marker of clonality in a group of female patients with PV and ET.
Study design
Patients
Heparinized peripheral blood was obtained from 34 women with a diagnosis of PV or ET. Clinical findings of patients are summarized in Table 1. Hematologic diagnosis was according to Polycythemia Vera Study Group (PVSG) criteria,10 and causes of secondary polycythemia (SP) or reactive thrombocytosis (RT) were carefully excluded. In no case was there a family history of MPD. All patients were in chronic phase without immature myeloid cells in the peripheral blood. Granulocytes (PMNs) and mononuclear cells (MNCs) were purified from peripheral blood according to standard methods.11
All samples were obtained after informed consent following the guidelines of the Institutional Review Board of the University of Genova.
Estimation of telomerase activity
TA was determined in PMNs and MNCs with the TRAPeze telomerase detection kit (Intergen, Burlington, MA), based on the original method by Kim et al.2 For each sample, the following controls were included: a heat-treated negative control, a positive control (HL-60 cell line),12 and an internal standard, to monitor polymerase chain reaction (PCR) inhibition and for quantitative analysis of the reaction products. The amount of telomerase product for each reaction was expressed in total product generated units (TPGs). The assay has a linear range of 1 to 300 TPGs, equivalent to the TA from approximately 30 to 10 000 control cells. Thirty healthy age-matched females were included as controls.
XCIP PCR assay
To assess clonality, we took advantage of a highly polymorphic short tandem repeat (STR) within the X-linked human androgen receptor (HUMARA) gene. The HUMARA locus is very useful for X-chromosome inactivation studies because with a PCR-based method, approximately 85% of women are heterozygous, and results correlate well with those obtained by Southern blotting for phosphoglycerate kinase–hypoxanthine phosphoribosyltransferase (PGK-HPRT), and M27β13 or transcriptional polymorphisms assay.7,14,15
Analysis of X-chromosome inactivation was therefore performed according to Karasawa et al16 with some modifications. Primers sequences were as follows: F, 5′TCCAGAATCTGTTCAAGAGCGTGC-3′ and R, 5′GCTGTGAAGGTTGCTGTTCCTCAT-3′. The 5′ end of the forward primer was labeled with 6-carboxyfluoroscein (FAM) fluorescent dye. The PCR products were separated by capillary electrophoresis on an ABI PRISM Genetic Analyzer (Applied Biosystems, Foster City, CA) and analyzed with a GeneScan software (Applied Biosystems).
Results and discussion
Thirty-four female patients with PV or ET were tested for heterozygosity at the HUMARA locus, and 27 (82%) were found informative. Of the 27, 5 were excluded from the study due to excess skewing, defined as greater than or equal to 90% of the cells with the same X-chromosome active.11 Clonality status was therefore determined in PMNs and MNCs by XCIP analysis in 22 patients (Table 1). PMNs expressed a monoclonal XCIP in 9 (69%) of 13 subjects with ET and in 6 (67%) of 9 with PV. MNCs were always polyclonal. In patient 10 with ET (Table 1), the comparison of the undigested PMNs and MNCs after PCR amplification revealed loss of heterozygosity (LOH) at the HUMARA locus in PMNs. Although this finding prevented evaluation of XCIP, the presence of a LOH can be considered as evidence of a monoclonal process17 and will be counted as such.
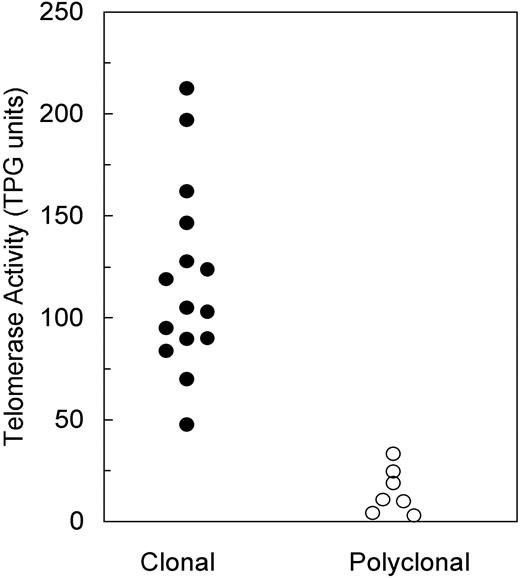
TA was measured in all informative patients and then blindly compared with the results of XCIP analysis. Monoclonal PMNs displayed a TA ranging between 47.6 and 212.5 TPG units (mean, 118.2 ± 45.5 SD), whereas polyclonal PMNs had a TA between 1.0 and 33.3 TPG units (mean, 14.4 ± 11.9 SD) (Figure 1). The difference between TPG values in the 2 groups is highly significant (P < .001, unpaired Student t test). MNCs had a TA similar to that of polyclonal PMNs. TA of controls was 12.2 ± 9.3 TPG units (range, 1.3-28.1).
Distribution of PMN telomerase activity according to clonality expression.
Our intention to analyze and determine the relations between 2 well-known markers of neoplastic proliferation has therefore been fulfilled by the finding of a direct correlation between TA and XCIP.
The stem cell origin and clonal development of MPDs are heterogeneous.7-9,18 One of the leading hypotheses is that the initial step of neoplastic proliferation can occur at different stages during the hemopoietic stem cell maturation process. On the other hand, controversies on the significance of clonality in MPDs are abundant in the literature.14,19,20 Several clinical and laboratory features have been evaluated in the literature, and a correlation between XCIP and vascular complications in ET was recently suggested,21,22 but most factors have shown no correlation with survival, thrombotic complications, or leukemic evolution.22
The results of our study clearly indicate the existence of 2 distinct subgroups of chronic myeloproliferative diseases (CMPDs), one with monoclonal and the other with polyclonal granulopoiesis, each of them characterized by a specific level of TA, respectively, high and normal, but otherwise undistinguishable by conventional clinical parameters. Monoclonally restricted cells are thought to represent the real neoplastic tissue, but in CMPD they have never been found to express unambiguous morphologic or biomolecular markers.23
Several questions remain unanswered, and the foremost concerns the relation, if any, between high or low TA and survival. The positive correlation of a high TA with monoclonal granulopoiesis may represent a powerful prognostic tool in PV and ET, but its significance will only be ascertained by a prospective study of clonality and TA on a larger cohort of patients over a long period of follow-up.
Although ET and PV are diseases with a relatively benign course, death due to progression to myelofibrosis and acute leukemia is not a rare event. Therefore, implications for future efforts to treat the subgroup of patients with monoclonal hematopoiesis and high TA with targeted chemotherapy and/or antitelomerase drugs24 might not be excluded.
Supported by grants no. RBAU013W3J_010 from Fondo per gli Investimenti della Ricerca di Base (FIRB) and no. 3398 from Ministero della Salute.
Prepublished online as Blood First Edition Paper, October 19, 2004; DOI 10.1182/blood-2004-06-2375.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.