Abstract
Immune function has been restored in 9 of 10 children with X-linked severe combined immunodeficiency by γc gene transfer in CD34+ cells. The distribution of both T-cell receptor (TCR) Vβ family usage and TCR Vβ complementarity-determining region 3 (CDR3) length revealed a broadly diversified T-cell repertoire. Retroviral integration site analysis in T cells demonstrated a high number of distinct insertion sites, indicating polyclonality of genetically corrected cell clones, in all patients. Detection of γc transgene expression on patients' mature myeloid cells has prompted us to investigate the nature of the most immature transduced hematopoietic precursor cells. Insertion sites shared by T and B lymphocytes as well as highly purified granulocytes and monocytes demonstrate the correction of common multipotent progenitor cells. Moreover, our data show that differentiated leukocytes share the same exact insertion sites with CD34+ cells that we obtained 8 months later and that were able to generate long-term culture-initiating cells (LTC-ICs). This finding demonstrates the initial transduction of very primitive multipotent progenitor cells with self-renewal capacity. These results provide a first evidence in the setting of a clinical trial that CD34+ cells maintain both lymphomyeloid potential as well as self-renewal capacity after ex vivo manipulation.
Introduction
The successful transfer and expression of new genetic sequences in hematopoietic stem and progenitor cells may improve the management of inherited blood diseases and eventually might replace conventional allogeneic hematopoietic stem cell (HSC) transplantation in some indications.1,2 One of the major problems limiting this approach is the absence of a clear understanding of the nature of the HSC pool in humans allowing the targeting of only the stem cells responsible for long-term engraftment. Xenotransplantation of human hematopoietic cells including cells with in vitro long-term culture-initiating cell (LTC-IC) capacity into preimmune sheep3,4 and more commonly into immunodeficient mice5 provided powerful assay systems to characterize the more immature hematopoietic progenitor cell and its growth requirements. CD34+ vascular endothelial growth factor receptor-2–positive (VEGFR2+)6 CD38– and lineage-negative (Lin–) cell population is the phenotype of the more immature progenitor cell with the highest potential to generate LTC-IC activity7 as well as nonobese diabetic/severe combined immunodeficiency (NOD-SCID) and fetal sheep repopulating ability. However, the demonstration in the setting of clinical trials of the clonal differentiation capacity and self-renewal potential of CD34+ stem cells is still lacking, conversely to that recently shown in single-cell murine HSC transplantation experiments.8,9 An alternative approach to the transplantation of a homogeneous pure fraction of human HSCs, which is currently not feasible in a clinical setting, is to mark the heterogeneous CD34+ cell population with retroviral vectors. Retroviral transduction is indeed the only clinically available means of marking individual progenitors. Each integration site is a distinct clonal marker of the cell progeny that arises after transplantation. This clonal marker can be detected at the molecular level as the fusion sequence between genomic DNA and the randomly inserted 5′ and 3′ long terminal repeat (LTR) of the vector. Linear amplification-mediated polymerase chain reaction (LAM-PCR) is a powerful tool to track clonal contributions even in highly complex samples.10 A limitation of retrovirus vector modification of stem cells is that viral integration requires cycling of target cells, which could induce commitment and loss of HSC characteristics during ex vivo culture. Nevertheless, the first positive results obtained by retroviral-mediated gene transfer protocols targeting CD34+ cells to treat severe combined immunodeficiency (SCID) offer the opportunity to address key questions regarding the differentiation capacity of these marked cells and their nature.
The first informative stem cell gene therapy protocol for a primary immunodeficiency associated with a selective advantage provided to corrected cells was performed to treat adenosine deaminase deficiency SCID (ADA-SCID).11 Cord blood CD34+ cells from 3 newborn patients were ex vivo transduced with a Moloney-derived retrovirus. A marked difference was reported in the frequency of transgene-containing cells between T lymphocytes (1% to 10%) and the myeloid lineage (0.01% to 0.1%), validating the concept of selective advantage without, however, generating a competent polyclonal immune system. In both patients who received cells with the highest gene transfer rate, the activity of gene-corrected cells was oligoclonal. In one of them, a single provirus integration site was found predominant at a stable level for 8 years.12 This cell progenitor was able to generate a restricted pattern of T-cell receptor (TCR) rearrangements. This same signature has also been detected in a monocytic cell sample 4 and 8 years after transplantation of the gene-corrected cells, suggesting that a common lymphoid and myeloid progenitor had been transduced. The polyethylene glycol–ADA (PEG-ADA) substitutive treatment administered simultaneously to the gene-modified hematopoietic cells as well as the nonoptimal ex vivo transduction protocol are likely responsible for the limited efficacy observed in this trial.
Success in the X-linked SCID (SCID-X1) gene therapy protocol has benefited from several scientific advances reported since the pioneering earlier trials, including the cloning of 2 early acting cytokines, FLT-3 ligand (FLT-3L) and megakaryocyte growth and development factor (MGDF),13-16 and the availability of a recombinant fibronectin fragment17 that can partially supplant stroma function.18 Several reports have emphasized the ability of these growth factor receptor–mediated signals to partially alter HSC self-renewal decisions,19-21 particularly when stem cell factor (SCF) and FLT-3L are used at higher concentration than interleukin-3 (IL-3).
Success of this protocol is also based on the selective advantage provided to T-cell precursors by the expression of the γc transgene.22 In this setting, it is remarkable that the correction of the T-cell immunodeficiency is accompanied by a low but stably persistent level over time (of up to 5 years) of transduced neutrophils.23 These findings led us to address the question of whether this myeloid gene marking indicates the existence of transduced cells capable of giving rise to both T cells and myeloid cells. By using the LAM-PCR tool, it was possible to assess the clonal diversity of integration sites within the T-cell compartment and the origin of transduced leukocytes.
Materials and methods
Isolation of mature circulating cells and bone marrow CD34+ cells from treated patients
Ten consecutive patients without HLA-identical donors were enrolled in the SCID-X1 gene therapy trial between March 1999 and April 2002. The protocol was approved by the Agence Française de Sécurité Sanitaire des Produits de Santé (AFSSAPS) and the local ethics committee, and written informed consent was obtained from the parents. Nine of these 10 patients restored a normal immunologic phenotype. The case of the patient who failed to develop any T cells was detailed elsewhere.23 Two patients developed a monoclonal lymphoproliferation that has been described in detail elsewhere.24
Peripheral blood cells were obtained at 3-month intervals in preservativefree heparin. Peripheral blood mononuclear cells and granulocyte fractions were separated by density centrifugation on lymphopep (Nycomed, Oslo, Norway), and then the different subpopulations were sorted by flow cytometry using fluorescence-labeled monoclonal antibodies against CD3 (T cells), CD14 (monocytic cells), CD15 (granulocytes), or CD19 (B cells). To detect any contamination of the selected CD15+ sample by CD3+ cells, the sorted cells were analyzed by immunofluorescence (more than 50 000 events). In the absence of any CD3+ cells detectable by fluorescence-activated cell sorting (FACS), a CD3 delta reverse transcriptase (RT)–PCR was performed (forward primer 5′-TGCAATACCAGCATCACATGGGTAGAGGGAAGGGT-3′; reverse primer 5′-CTTGTTCCGAGCCCAGTTTCCTCCAAGGTGGCTGT-3′). This RT-PCR was shown to detect a CD3 contamination as low as 0.01% (data not shown). It was found that all used CD15+ samples were negative for CD3 delta mRNA signal. From surplus cells of a bone marrow aspirate per patient required by the protocol to rule out replication-competent retroviruses (RCRs), isolation of bone marrow CD34+ cells was performed by an immunomagnetic procedure (Miltenyi Biotec, Bergisch Gladbach, Germany). Two immunomagnetic cycles increased the purity of CD34+ population to 99%. The samples were cryopreserved in liquid nitrogen until utilization, or genomic DNA was extracted from cells using proteinase K digestion, phenol-chloroform extraction, and ethanol precipitation. The pellet was resuspended in trisethylenediaminetetraacetic acid (Tris-EDTA) 10:1 buffer and stored at –20°C until use.
Immunoscope analysis
Complementary DNA prepared from the peripheral blood mononuclear cells (PBMCs) was amplified with each of 24 TCR variable β (TCRBV) family-specific primers together with a TCR β chain (TCRBC) primer and a minor groove binder (MGB)–Taqman probe for TCRBC.25,26 Real-time quantitative PCR was carried out on an ABI5700 system (Applied Biosystems, Foster City, CA). PCR products were then subjected to runoff reactions by using a nested fluorescent primer specific to the Cβ segment. The fluorescent products were separated and analyzed on a 373A sequencer. The size and intensity of each band were analyzed with the Immunoscope software. On the y-axis the fluorescent intensity is plotted in arbitrary units while the x-axis represents the different lengths of complementarity-determining region 3 (CDR3) in amino acids. The Gaussian distribution of the different CDR3 lengths is characteristic of normal Vβ repertoire.
LAM-PCR and tracking PCR
An integration site restriction fragment length display was obtained by linear amplification-mediated PCR (LAM-PCR) consisting of repeated primer extension, second strand synthesis, restriction digest, cassette ligation, and exponential amplification as described previously.10 LAM-PCR was performed on DNA directly isolated from FACS-sorted cell samples (CD3: 2 to 200 ng; CD14 and CD15: 10 to 100 ng; LTC-ICs: 0.001 to 1 ng). The final products were separated either on 2% agarose or on Spreadex gels, excised, and sequenced. Proviral integration site sequences were aligned to the human genome using the National Center for Biotechnology Information (NCBI) BlastN (http://www.ncbi.nlm.nih.gov/blast/); the University of California, Santa Cruz, BLAT search tools (http://genome.ucsc.edu/); and the Ensembl database (http://www.ensembl.org/).
After the integration site in myeloid cells had been sequenced, new tested PCR primers were designed to specifically amplify each unique genomic-proviral junction by exponential PCR. Tracking PCR was performed on DNA directly isolated from FACS-sorted leukocytes (CD3: 1 to 200 ng; CD19: 10 to 20 ng) using the unique genomic flanking primers in combination with the LTR primers previously described.10 The genomic flanking primer sequences were as followed: clone 3682: 1. PCR: 5′-CAGGTACCAAAACTGGTTTC-3′, 2. PCR: 5′-AACTGGTTTCTTTGTGGGTC-3′; clone 3686: 1.PCR: 5′-CCTCTGTCAATGAAAGGTTT-3′, 2.PCR: 5′-TTCTCGGCATTGGCTTTAAG-3′; clone 5521: 1.PCR: 5′-CCTCTTTCCACACGGTTCTT-3′, 2.PCR: 5′-ACACGGTTCTTTCAGGCCAA-3′; clone 5522: 1.PCR: 5′-ACACAGGAAACAGCTATGAC-3′, 2.PCR: 5′-CAGCTATGACCATGATTACG-3′; clone 5523: 1.PCR: 5′-CTTGCTCAAGGTCAGGTGAT-3′, 2.PCR: 5′-TTAGTAAGAGGCTGACCTCG-3′; clone 1529: 1.PCR: 5′-ACCAAAAATGTTCCCAGTCT-3′, 2.PCR: 5′-TCACTCTCCACTGAGCATCA-3′; clone 1770: 1.PCR: 5′-GATGAAGTAGCTTAGTGGAGG-3′, 2.PCR: 5′-AGTGGAGGTGAGCAGATGCG-3′; clone 7318: 1.PCR: 5′-GTCTTGAACTCCTGGTTTCA-3′, 2.PCR: 5′-CCATCTAGGCCTGCTAAAGT-3′; clone 7324: 1.PCR: 5′-AAGAACTGCATAGGAGGTCT-3′, 2.PCR: 5′-GTCTTCATGCAGTTGAGTGT-3′.
For sequencing, either single bands have been isolated and purified or the PCR product was purified and shotgun cloned into a TOPO TA vector (Invitrogen, Carlsbad, CA).
Quantification of T-cell receptor excision circles
The real-time quantitative polymerase chain reaction Taqman assay (Applera France Courtaboeuf, France) for signal joint (sj) T-cell receptor excision circles (TRECs) was performed in 25 μL containing 10 μL DNA prepared from patients' PBMCs (equivalent to 1 × 105 to 5 × 105 cells) and 1 × universal master mix (Applera France), 200 nM of each primer, and Taqman-specific probes. The conditions of PCR were 50°C for 2 minutes, 95°C for 10 minutes, followed by 50 cycles of amplification (95°C for 15 seconds, 60°C for 1 minute). For each sample, the input DNA was normalized with the human albumin (Alb) sequence measurement and run in duplicate. For the quantification in absolute copy number, we used an sj TREC and albumin standard curve (10 to 106 copies) generated from pTopo TREC/Alb plasmid containing the 376 bp sj TREC sequence (from Pgth 310, provided by Dr J. P. de Villartay, INSERM U429) cloned into the pTopo Alb (Genethon III, Evry, France) construction. As a control, DNA extracted from PBMCs of healthy children was included in each run. DNA from human fibroblasts was used as negative controls. The data were analyzed by the ABIPRISM 7700 with the sequence detector system software (Applied Biosystems) and exported in Microsoft Excel sheet. Two standard curves (CT = f [log copy number]) were obtained, making possible the determination of the absolute copy number of sj TRECs and Alb by using the regression method. For each sample, the ratio copy number of sj TRECs per copy number of Alb per 105 was calculated and gives the number of sj TRECs in 105 PBMCs. The primers and probes sequences were as follows: sj TREC forward: 5′-AGAACGGTGAATGAAGAGCAGACA-3′; sj TREC reverse: 5′-CACATCCCTTTCAACCATGCTGACA-3′; sj TREC probe: 5′FAM-TGCCCACTCCTGTGCACGGTG-TAMRA-3′; Alb forward: 5′-GCTGTCATCTCTTGTGGGCTG T-3′; Alb reverse: 5′-ACTCATGGGAGCTGCTGGTTC-3′; Alb probe: 5′VIC-CCTGTCATGCCCACACAAATCTCTCC-TAMRA-3′.
Immunofluorescence analysis
The γc was assessed on sorted granulocytes by using the phycoerythrin-labeled monoclonal antibody CD132 (BD Biosciences Pharmingen, San Diego, CA) and fluoroisothiocyanate-labeled CD15 (BD Biosciences, Le Pont de Claix, France). T-cell phenotype was assessed by using monoclonal antibodies against CD3+, CD45RO, and CD45RA antigens, as previously described.27
Long-term culture-initiating cell (LTC-IC) assay by limiting dilution analysis (LDA) and provirus integration in LTC-ICs
CD34+ cells isolated after 2 immunomagnetic procedures were assessed for 6-week long-term culture on preestablished irradiated MS-5 stromal feeder in limiting dilution as described elsewhere.28,29 Briefly, adherent feeder layers were established in minicultures (flat-bottom 96-well culture). From 10 000 to 150 CD34+ cells were seeded per well into 24 replicate wells for each cell dilution culture and maintained at 37°C on irradiated MS-5 with weekly one-half medium exchanges (Myelocult H5100; Stem Cell Technologies, Vancouver, BC, Canada). After 6 weeks culture-adherent and nonadherent cells from each individual well were plated in methycellulose culture and scored as positive (1 or more colony-forming units [CFUs]) or negative (no colony-forming cells [CFCs]) 14 days later. The LTC-IC frequency is given by the reciprocal of the concentration of test cells that gives 37% negative wells. Individual granulocyte macrophage CFU (CFU-GM) colonies were picked on day 14, pooled, and lysed. DNA was analyzed by PCR to determine the percentage of γc retrovirus-positive LTC-ICs.
For γc amplification, 2 primers were used, one mapping into the retrovirus sequence and the other into the γc chain sequence, respectively: PM-Reverse: 5′-GACCACTGATATCCTGTCTTCAAC-3′; γc: F5′-CCAGCCTACCAACCTCACT-3′.
DNA was amplified by PCR using 30 cycles at an annealing temperature of 60°C. PCR was performed in a 25-μL reaction mixture, and each amplified product was loaded on a 1% agarose gel and submitted to size separation electrophoresis. The PCR products were transferred and the blots hybridized with a 32P-labeled fragment of the total coding region of γc.
Results
A polyclonal T-cell population contributes to a fully diversified T-cell repertoire
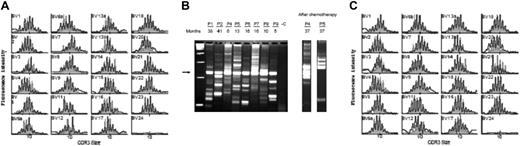
The broad diversity of the patients' peripheral T-cell repertoire has been assessed by immunoscope analysis. A polyclonal αβ T-cell repertoire indistinguishable from age-matched controls was consistently found in all 9 patients who developed normal peripheral T-cell counts after genetic correction as shown by TCR Vβ usage and normal distribution of CDR3 length within every Vβ family23,24 (Figure 1A and not shown). To assess the number of precursor cells that have reconstituted this broadly diversified repertoire, we performed in vivo integration site analysis by LAM-PCR that allows the individual clonal progeny of each transduced cell to be distinguished by its unique proviral-genomic fusion sequence. Integration site analysis by LAM-PCR of FACS-sorted CD3+ cells revealed a polyclonal lymphopoietic repopulation in all patients (Figure 1B). We could previously show that each cell carries only one insertion, so that the number of insertion sites detected is directly proportional to the number of gene-modified T-cell clones in the sampled material.24 The whole T-cell population has remained consistently polyclonal for up to 5 years of follow-up after gene therapy (Figure 1C) with the exception of 2 patients (P4, P5) who developed a monoclonal lymphoproliferation 2.5 years after treatment.24 Interestingly, even after highly aggressive induction chemotherapy in these 2 patients, a polyclonal gene-corrected lymphopoiesis returned initially (Figure 1B). To assess whether continued T-cell maturation was limited by the availability of gene-corrected T-cell precursors and the functional status of the thymic epithelium, we quantified the subgroup of circulating naive and TREC-positive cells at various times after transplantation (Figure 2). The number of circulating naive T cells and TREC-positive T cells were found comparable to that of age-matched children (mean value of naive T cells for healthy children younger than 1 year is 66% ± 10% and for children older than 1 year is 60% ± 5%).30,31 These data strongly suggest that thymopoiesis remains active more than 4 years after gene therapy.
Polyclonality of genetically modified T-cell populations. (A,C) Immunoscope study of Vβ T lymphocytes from P8 and P2, 11 months and 45 months after gene therapy, respectively, and age-matched controls (gray-shaded outline). Vβ usage (black) is similar to normal controls (gray). (B) In vivo 5′LTR integration site analysis of P1, P2, P4, P5, P6, P7, P8, and P9 T-cell clones in 8 patients. Ten to 200 ng of DNA from sorted T cells (CD3+) was analyzed by using LAM-PCR at different time points (5 to 41 months) after gene therapy. The electrophoresed amplification product displays a restriction length polymorphism of integration sites, with each band indicating the presence of a different insertion locus in the assayed material. Presence of a polyclonal population of gene-modified CD3 cells was detected in each patient. Numbers denote months after transplantation. The arrow indicates the internal vector 3′LTR amplification product. A total of 1.0 μg nontransduced human leukocyte DNA was used as a negative control (-C); first lane, 100-bp ladder. After chemotherapy: integration site analysis after chemotherapy in P4 and P5. LAM-PCR was performed on 100 ng of DNA isolated from peripheral blood leukocytes 3 months after induction chemotherapy. Clonality analysis reveals the recurrence of multiple clones contributing to lymphopoiesis.
Polyclonality of genetically modified T-cell populations. (A,C) Immunoscope study of Vβ T lymphocytes from P8 and P2, 11 months and 45 months after gene therapy, respectively, and age-matched controls (gray-shaded outline). Vβ usage (black) is similar to normal controls (gray). (B) In vivo 5′LTR integration site analysis of P1, P2, P4, P5, P6, P7, P8, and P9 T-cell clones in 8 patients. Ten to 200 ng of DNA from sorted T cells (CD3+) was analyzed by using LAM-PCR at different time points (5 to 41 months) after gene therapy. The electrophoresed amplification product displays a restriction length polymorphism of integration sites, with each band indicating the presence of a different insertion locus in the assayed material. Presence of a polyclonal population of gene-modified CD3 cells was detected in each patient. Numbers denote months after transplantation. The arrow indicates the internal vector 3′LTR amplification product. A total of 1.0 μg nontransduced human leukocyte DNA was used as a negative control (-C); first lane, 100-bp ladder. After chemotherapy: integration site analysis after chemotherapy in P4 and P5. LAM-PCR was performed on 100 ng of DNA isolated from peripheral blood leukocytes 3 months after induction chemotherapy. Clonality analysis reveals the recurrence of multiple clones contributing to lymphopoiesis.
Evaluation of recent thymic emigrants over time. (A) Longitudinal kinetics of TREC quantification in peripheral blood mononuclear cells of treated patients (P1, P2, P4 to P9). Kinetics of P4 and P5 are shown up to the time of the clonal lymphoproliferation.24 Day 0 corresponds to the date of the treatment. (B) Phenotypic quantification of naive CD45RA (▪) and memory CD45RO (▦) CD4+ T cells at 12 months (left panel) and at last follow-up for P1 (M [months] + 47), P2 (M + 53), P4 (M + 30), P5 (M + 30), P7 (M + 24), P8 (M + 22), P9 (M + 12) (right panel). In age-matched healthy children, the mean value of naive T cells is 66% ± 10% for children younger than 1 year of age and 60% ± 5% for children 1 year and older.
Evaluation of recent thymic emigrants over time. (A) Longitudinal kinetics of TREC quantification in peripheral blood mononuclear cells of treated patients (P1, P2, P4 to P9). Kinetics of P4 and P5 are shown up to the time of the clonal lymphoproliferation.24 Day 0 corresponds to the date of the treatment. (B) Phenotypic quantification of naive CD45RA (▪) and memory CD45RO (▦) CD4+ T cells at 12 months (left panel) and at last follow-up for P1 (M [months] + 47), P2 (M + 53), P4 (M + 30), P5 (M + 30), P7 (M + 24), P8 (M + 22), P9 (M + 12) (right panel). In age-matched healthy children, the mean value of naive T cells is 66% ± 10% for children younger than 1 year of age and 60% ± 5% for children 1 year and older.
Transduction of a common lymphomyeloid precursor cell
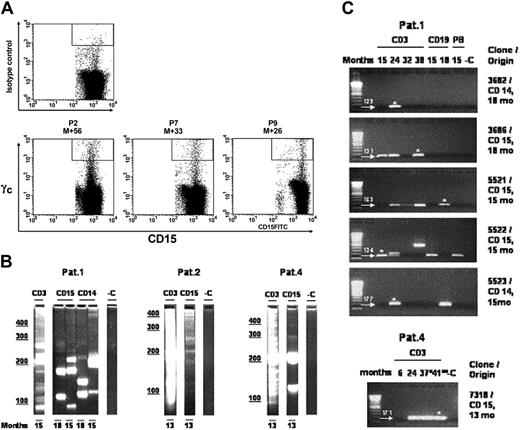
A low but stable percentage of granulocytes (0.1% to 0.2%) was found to continuously express the γc transgene over time in all studied patients (Figure 3A). This stable expression was further confirmed by quantitative PCR showing a similar frequency of transduced monocytes (CD14+) and granulocytes (CD15+).23 We have identified and sequenced vector insertion sites of individual FACS-sorted CD14+ and CD15+ cells by integration site analysis. Based on the genomic part of the sequence information of the LAM-PCR amplicons, we then designed new primers for each individual characterized integration site that allowed, in combination with vector-specific primers, a fast and efficient tracking of individual clones in lymphoid cells. Table 1 summarizes the results of the “myeloid” integration site analysis and the “lymphoid” tracking analysis. At multiple time points, insertion sites originally detected in myeloid cells were detectable in the highly purified T- or B-cell population, proving the existence of transduced lymphomyeloid precursor cells in patients enrolled in this trial.
Multipotent transduced progenitors contribute to hematopoiesis. (A) Long-term γc expression on circulating CD15+ granulocytes. The proportions of transduced granulocytes are 0.19% for P2, 0.12% for P7, and 0.3% for P9 at 56, 33, and 26 months (M indicates months) after gene therapy, respectively. An isotype control ofγc is also shown (upper panel). (B) LAM-PCR analysis of CD14 and CD15 FACS-sorted myeloid cells. DNA samples directly isolated from sorted peripheral blood leukocytes (CD3: 2 to 20 ng; myeloid cells: 10 to 100 ng) were analyzed at different time points after treatment. Compared with the polyclonal pattern of insertion sites in the CD3 samples, oligoclonal insertion was observed in the myeloid cell populations. Individual myeloid LAM-PCR amplicons were sequenced to obtain the insertion site fusion sequences. The genomic component of all fusion sequences was aligned to the human genome (Table 1). Numbers denote months after reinfusion; CD3, T cells; CD14, monocytes; CD15, granulocytes; -C, water control. (C) Follow-up tracking of CD14- and CD15-derived myeloid insertion sites in T cells. The sequence information of 7 individual retroviral integration sites was used to design genomic flanking primers that were unique for each individual site. Follow-up tracking was performed on 1 to 200 ng of purified T cells at different time points after genetic correction and reinfusion. In highly purified T cells, 6 of the 7 myeloid insertion sites (P1: clones 5521, 5522, 5523, 3682, 3686; P4: clone 7318) could be retrieved at different time points, thus demonstrating that multipotent progenitor cells were genetically corrected and engrafted in this clinical trial. Note that the 2 additional tracking PCR amplicons found with tracking primers for clone 5522 (P1; fourth panel from top) 24 and 38 months after gene transfer have been sequenced and correspond to individual unique integration sites different from clone 5522, detected due to partial genome homology of the flanking primers used. Arrows with numbers denote position and size of specific PCR amplicons in base pairs. White asterisks on gels (C) indicate sequenced; (Pat. 4) 37° indicates after chemotherapy; and 41°° indicates after allotransplantation; months, time after gene therapy; CD3, T cells; CD19, B cells; PB, peripheral blood mononuclear cells; CD14, granulomonocytic cells; CD15, granulocytes; -C, 1.0 μg nontransduced human leukocyte DNA was used as a negative control; first lane, 100-bp ladder.
Multipotent transduced progenitors contribute to hematopoiesis. (A) Long-term γc expression on circulating CD15+ granulocytes. The proportions of transduced granulocytes are 0.19% for P2, 0.12% for P7, and 0.3% for P9 at 56, 33, and 26 months (M indicates months) after gene therapy, respectively. An isotype control ofγc is also shown (upper panel). (B) LAM-PCR analysis of CD14 and CD15 FACS-sorted myeloid cells. DNA samples directly isolated from sorted peripheral blood leukocytes (CD3: 2 to 20 ng; myeloid cells: 10 to 100 ng) were analyzed at different time points after treatment. Compared with the polyclonal pattern of insertion sites in the CD3 samples, oligoclonal insertion was observed in the myeloid cell populations. Individual myeloid LAM-PCR amplicons were sequenced to obtain the insertion site fusion sequences. The genomic component of all fusion sequences was aligned to the human genome (Table 1). Numbers denote months after reinfusion; CD3, T cells; CD14, monocytes; CD15, granulocytes; -C, water control. (C) Follow-up tracking of CD14- and CD15-derived myeloid insertion sites in T cells. The sequence information of 7 individual retroviral integration sites was used to design genomic flanking primers that were unique for each individual site. Follow-up tracking was performed on 1 to 200 ng of purified T cells at different time points after genetic correction and reinfusion. In highly purified T cells, 6 of the 7 myeloid insertion sites (P1: clones 5521, 5522, 5523, 3682, 3686; P4: clone 7318) could be retrieved at different time points, thus demonstrating that multipotent progenitor cells were genetically corrected and engrafted in this clinical trial. Note that the 2 additional tracking PCR amplicons found with tracking primers for clone 5522 (P1; fourth panel from top) 24 and 38 months after gene transfer have been sequenced and correspond to individual unique integration sites different from clone 5522, detected due to partial genome homology of the flanking primers used. Arrows with numbers denote position and size of specific PCR amplicons in base pairs. White asterisks on gels (C) indicate sequenced; (Pat. 4) 37° indicates after chemotherapy; and 41°° indicates after allotransplantation; months, time after gene therapy; CD3, T cells; CD19, B cells; PB, peripheral blood mononuclear cells; CD14, granulomonocytic cells; CD15, granulocytes; -C, 1.0 μg nontransduced human leukocyte DNA was used as a negative control; first lane, 100-bp ladder.
T-cell contamination of sorted myeloid cell samples, as a source of this positive signal, can be excluded on the basis of 3 additional findings. First, RT-PCR for the detection of CD3 delta RNA with a sensitivity of 0.01% was found negative in the analyzed purified myeloid samples (data not shown). Second, we can exclude that these clones are contaminations of a predominant clone in the T-cell population because, by high throughput sequencing of vector insertions into the genome performed in an independent study, we have never retrieved one of the “myeloid” integration sites in the CD3+ samples by LAM-PCR (sequenced amplicons total more than 1000; unpublished data, 2005). Third, identical insertion sites from CD15+ cells were repeatedly detected in independent samples, an observation that is highly unlikely to be the result of a random cell contamination event originating from a very polyclonal T-cell pool in which the studied clone accounts only for a minority of cells. Taken together, these data are consistent with the presence of gene-modified lymphomyeloid precursor cells.
Engraftment of multipotent gene-corrected precursor cells with self-renewal capacity
To analyze multipotency and self-renewal capacity of individually transduced cells, we initiated a first set of experiments by sequencing insertion sites found in mature myeloid CD14/CD15 cells (6 from P1: clones 7463, 5521, 5522, 5523, 3682, 3686; 1 from P4: clone 7318; Figure 3B; Table 1). PCR tracking analysis was then performed in highly purified T cells of the respective patients. As shown in Figure 3C, 6 “myeloid” insertion sites could be detected in T cells of P1 (clones 5521, 5522, 5523, 3682, 3686) and P4 (clone 7318) at different time points spanning a 3-year period. In addition, 3 of these insertion sites (P1: clones 5521, 5522, 5523) were also found in CD19+ B-cell populations (Figure 3C).
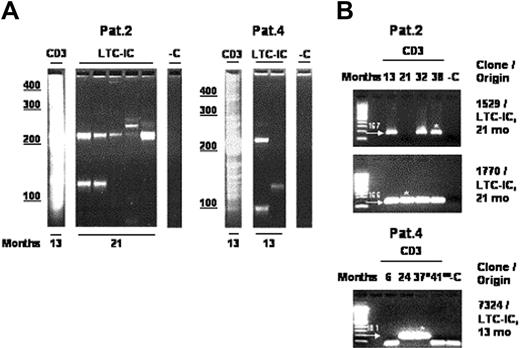
Then, CD34+ cells from marrow samples of P2 and P4, obtained 21 and 13 months after gene therapy respectively, were analyzed by the LTC-IC assay in limiting dilution analysis. Isolated CD34+ cells were cultured for 6 weeks on MS-5 stromal cells and then transferred into a semisolid medium to determine whether colony-forming cells (CFCs) were present and transduced. One percent to 5% of these LTC-ICs (the frequencies of which were 1 per 1000 and 1 per 500 plated CD34+ cells for patient 2 and patient 4, respectively) carried the γc transgene.23 To further demonstrate the multipotency of the gene-modified CD34+ cells, all LTC-IC–derived insertion sites (Figure 4A; Table 1) were sequenced. The individual clones were traced by PCR tracking analysis in highly purified T cells of the respective patients at different time points. In total, 3 distinct LTC-IC–derived insertion sites (P2: clones 1529 and 1770; P4: clone 7324) could be detected by PCR tracking analysis in highly purified T-cell populations (Figure 4B). Of note, the LTC-IC–derived insertion sites 1529 and 1770 in patient 2 were detected in highly purified T-cell populations harvested 8 months prior to the marrow aspiration, providing molecular evidence that a transduced clone had spun off progenitor cells that matured into detectable T cells more than half a year prior to generating a CD34+ cell capable of LTC-IC production. These results differ from those reported in allogeneic hematopoietic stem cell transplantation where, in the absence of myeloablative therapy, no engraftment of donor stem cells has been observed.32
Multipotent transduced progenitors capable of self-renewal contribute to hematopoiesis. (A) LAM-PCR analysis of LTC-IC–derived colonies. From 0.01 to 1 ng of DNA directly isolated from LTC-IC–derived colonies was analyzed, sequenced, and aligned to the human genome. Numbers denote months after reinfusion; CD3, T cells; LTC-IC, long-term culture-initiating cell–derived colonies; -C, water control. (B) Follow-up tracking of LTC-IC–derived myeloid insertion sites in T cells. Genomic flanking primers were designed for 6 individual LTC-IC–derived retroviral integration sites (Table 1). Three LTC-IC–derived insertion sites (P2: clones 1529 and 1770; P4: clone 7324) could be identified in highly purified T cells (1 to 200 ng) over time at time points of up to 8 months prior to the bone marrow aspiration from which the LTC-IC assays were performed, demonstrating that these clones generated mature lymphocytes a long time before generating LTC-ICs and indicating that human CD34+ cells with self-renewal capacity have been transduced and have long-term activity in this clinical study. Note that the additional bands in size below 100 bp detected in PCR tracking specific for clone 7324 have been sequenced and correspond to primer multimers. Arrows with numbers denote position and size of specific PCR amplicons in base pairs. Months indicates months after gene therapy; *, sequenced; °, after chemotherapy; °°, after allotransplantation; CD3, T cells; LTC-IC, long-term culture-initiating cells; -C, 1.0 μg nontransduced human leukocyte DNA was used as a negative control; first lane, 100-bp ladder.
Multipotent transduced progenitors capable of self-renewal contribute to hematopoiesis. (A) LAM-PCR analysis of LTC-IC–derived colonies. From 0.01 to 1 ng of DNA directly isolated from LTC-IC–derived colonies was analyzed, sequenced, and aligned to the human genome. Numbers denote months after reinfusion; CD3, T cells; LTC-IC, long-term culture-initiating cell–derived colonies; -C, water control. (B) Follow-up tracking of LTC-IC–derived myeloid insertion sites in T cells. Genomic flanking primers were designed for 6 individual LTC-IC–derived retroviral integration sites (Table 1). Three LTC-IC–derived insertion sites (P2: clones 1529 and 1770; P4: clone 7324) could be identified in highly purified T cells (1 to 200 ng) over time at time points of up to 8 months prior to the bone marrow aspiration from which the LTC-IC assays were performed, demonstrating that these clones generated mature lymphocytes a long time before generating LTC-ICs and indicating that human CD34+ cells with self-renewal capacity have been transduced and have long-term activity in this clinical study. Note that the additional bands in size below 100 bp detected in PCR tracking specific for clone 7324 have been sequenced and correspond to primer multimers. Arrows with numbers denote position and size of specific PCR amplicons in base pairs. Months indicates months after gene therapy; *, sequenced; °, after chemotherapy; °°, after allotransplantation; CD3, T cells; LTC-IC, long-term culture-initiating cells; -C, 1.0 μg nontransduced human leukocyte DNA was used as a negative control; first lane, 100-bp ladder.
Discussion
In this report, we show that successful gene therapy of γc-deficient SCID-X1 is associated with the sustained development of a highly diversified T-cell repertoire that is originating from a limited set of transduced clones in the magnitude of 100. Our data also demonstrate that distinct integration sites of the provirus are shared between T and B lymphocytes on the one hand and myeloid cells in the form of granulocytes, monocytes, and/or colony-forming cells derived from long-term culture-initiating cells (LTC-ICs) on the other hand. These results indicate that multipotent progenitors have been transduced and engrafted and would also be compatible with the transduction of hematopoietic stem cells.
Immunoscope analysis of the TCRβ chain shows a polyclonal pattern in all tested patients that cannot be distinguished from normal controls. Together with the robust immunity these patients have developed up to now, this observation strongly suggests that the T-cell repertoire is highly diversified, although a more formal proof would be obtained by saturation sequencing of clones using distinct TCR Vβ-Jβ combinations.33 These results indicate that a substantial rate of cell proliferation occurred between the stage at which T-cell precursors were transduced and the final T-cell maturation as defined by successful TCR rearrangements. It is remarkable that transduced clones that are in an order of magnitude of 100 are sufficient to maintain at least the circulating fraction of a fully diversified T-cell compartment. This result is in full agreement with our previous observation that following a spontaneous reverse mutation of the γc gene in a single T-cell precursor of a SCID-X1 patient,22 approximately 1 × 103 TCRβ chains could be identified in the periphery within the memory T-cell population, representing around 1% of the control TCR diversity.22,34 These data support the concept that the successful gene therapy in these patients is based on the selective advantage conferred by the transgene expression to a polyclonal but finite number of progenitors. The observation that multiple mature T-cell clones can derive from each gene-corrected cell is also in agreement with the previous finding that following ex vivo ADA gene transfer into cord blood CD34+ cells, a single clone, as identified by its provirus integration site, could give rise to a somewhat diversified T-cell repertoire that could be detected over years.12 The sustained detection of naive T cells over a 5-year period not only shows that the thymus is fully functioning in these patients but that gene-corrected T-cell precursor cells, likely of CD34+ phenotype, continuously sustain production of the progenitors required for thymopoiesis. In contrast, previously published data describing the kinetics and the quality of immunologic reconstitution after allogeneic bone marrow transplantation performed without any conditioning regimen showed a decline in thymopoiesis over time. This difference may be due in part to the absence of allogeneic CD34+ engraftment after nonconditioned allogeneic transplantation or, alternatively, the limited capacity of thymus from SCID patients to support thymopoiesis over time.35-37
The reconstitution of a complete T-cell repertoire led us to address the question of the differentiation stage at which hematopoietic precursor cells have been transduced, given the heterogeneity of the selected CD34+ cell population used for gene transfer. The presence of a low but consistent fraction of transduced granulocytes in the peripheral blood and transduced CD34+ cells in the bone marrow for more than 5 years indicates that at least common myeloid progenitors (CMPs),38,39 in addition to lymphocyte progenitors, were transduced. The tracking of the transduction signature, made possible by the precise identification of integration sites, unequivocally showed that hematopoietic cells were transduced. These cells retained a multipotent progenitor cell potential. Molecular evidence of multipotency was detected in the form of integration sites common to T lymphocytes, B lymphocytes, monocytes, and granulocytes in the analyzed samples. Evidence for the detection of multipotent human hematopoietic progenitor cells has been observed in the past. In vitro, prior studies have shown human progenitor cell multipotentiality, demonstrating the ability of a single cord blood–derived CD34+ cell to generate T, B, and natural killer (NK) lymphocytes and granulocytes/monocytes by combining fetal thymic organ cultures and coculture on competent murine stromal cell feeders.40 Xenotransplantation assays further showed that a single-marked Lin–CD34+ clone cell can generate both multilineage B and myeloid progeny.41 Our previous observation of a continued contribution of gene-corrected progenitor cells to normal hematopoiesis of ADA-SCID patients after transplantation with gene-modified CD34+ cord blood cells over more than 8 years suggests that at least progenitor cells with lineage commitment must have self-renewed to produce peripheral progeny for such extended times.12
To investigate self-renewal potential in gene-corrected hematopoiesis, long-term culture-initiating cells were grown from bone marrow aspirates that were available in 2 patients. Insertion site sequence information from these immature progenitor cells could be traced back to highly purified T cells sampled at earlier time points after transplantation. The fact that differentiated leukocytes were circulating in the peripheral blood more than 8 months prior to the harvest of very immature hematopoietic cells shown to share the same insertion site indicates a self-renewal capacity of the primitive multipotent progenitor cell. Taking further into account that thymopoiesis has been stable over more than 5 years as shown by the persistent detection of TREC-positive T cells after treatment and that γc-positive granulocytes are stably detectable over time, it is thus very likely that hematopoietic cells with self-renewal capacity have been efficiently transduced. Several conclusions can be drawn from these observations.
Although no estimation of the number of transduced multipotential hematopoietic cells with self-renewal capacity can be made from our work, the results raise hope that correction of the immunodeficiency in SCID-X1 patients enrolled in this trial could be sustained for a long time. This assertion obviously warrants careful long-term evaluation. Proof of the transduction of very immature hematopoietic cells with a classical retroviral vector is somewhat surprising given the well-established concept that retrovirus-derived vectors do only integrate into cycling cells whereas immature progenitor and stem cells are usually noncycling.42 It could well be that cytokine treatment with MGDF, SCF, and FLT-3L as used in our protocol has allowed some stem cells to enter into cycling without differentiation. One also cannot exclude that ontogenic characteristics of neonatal hematopoiesis still play a role at the patients' young age (below 1 year of age). SCID-X1 pathogenesis itself, by causing an early block in T/NK lymphocyte differentiation, could be responsible for an increased number of actively cycling stem cells. Both phenomena can also account for the relatively high percentage of γc gene–corrected LTC-ICs detected in this trial. Transduction of a limited number of immature progenitor or stem cells, as achieved in this clinical trial, will probably not suffice for disease correction in settings where transgene expression is not providing any selective advantage and where corrected cells would be short-lived. Improvements in gene transfer technology based on the usage of retrovirus vectors enabling the transduction of noncycling cells,43,44 combined with myeloablation2 and potentially with the usage of factors capable of inducing stem cell amplification such as HoxB4 or Wnt,45-47 could eventually solve this problem.
Transduction of some self-renewing multipotent progenitor cells can be detected in the SCID-X1 gene therapy trial, thus demonstrating that such cells do exist in the human CD34+ compartment and play a role in long-term engraftment after transplantation.
Prepublished online as Blood First Edition Paper, December 7, 2004; DOI 10.1182/blood-2004-07-2648.
Supported by grants from INSERM, Bundesministerium für Bildung und Forschung (BMBF), Deutsche Forschungsgemeinschaft (DFG), Association Française contre les Myopathies, Programme Hospitalier de Recherche Clinique of the Health Ministry, Assistance Publique—Hôpitaux de Paris, EC contract no. QLK3-CT 2001 (coordinator, G. Wagemaker), the Jeffrey Modell Foundation, and AMGEN-France (Christian Cailliot).
M.S. and S.H.-B.-A. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are indebted to families of the patients for their continuous support; to the medical and nursing staff of the Unité d'Immunologie et d'Hématologie Pédiatriques, Hopital des Enfants Malades, for patient care; to L. Coulombel, W. Vainchenker, P. Kourilsky, J. P. de Villartay, and D. Favy for their helpful discussion; and to F. Gross and E. Morillon for technical help.

![Figure 2. Evaluation of recent thymic emigrants over time. (A) Longitudinal kinetics of TREC quantification in peripheral blood mononuclear cells of treated patients (P1, P2, P4 to P9). Kinetics of P4 and P5 are shown up to the time of the clonal lymphoproliferation.24 Day 0 corresponds to the date of the treatment. (B) Phenotypic quantification of naive CD45RA (▪) and memory CD45RO (▦) CD4+ T cells at 12 months (left panel) and at last follow-up for P1 (M [months] + 47), P2 (M + 53), P4 (M + 30), P5 (M + 30), P7 (M + 24), P8 (M + 22), P9 (M + 12) (right panel). In age-matched healthy children, the mean value of naive T cells is 66% ± 10% for children younger than 1 year of age and 60% ± 5% for children 1 year and older.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/7/10.1182_blood-2004-07-2648/6/m_zh80070576190002.jpeg?Expires=1766452426&Signature=LKQt60oMZinXZjiJuujCXfFqJLBjk48SrFY~Ej9x~lLe-ENfe5NAwGtW14HWjbgOKGJyWSfTLkAGJ8TzmSPoFhjDVvUqYJb8luls~-GinODJcECtwdUYR67UigdhPQCdxCV0AbeRkxR39Ev0Be40HOGMxyZodIMEHNl1D7kT5afdyBuqOf2wfuCnSxkcVVSOz-k3ii-lFpjTWTUhz-RakTcrEVxC~w0ZWtZ095D2dEqFI0Ye6bkO57DOKIwcpd7Ur-m34mFoM0pILggFdwTloVYSbe7S4nMB~wPscrYa18XOk1upHSl5TClmsvdlc3cZUJ2Tj2Gt-0m6rWOyQYNXVA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)