Abstract
Hematopoietic stem cell transplantation (HSCT) is associated with significant posttransplantation gonadotoxicity. This deficit has been mainly attributed to pretransplantation conditioning, but lower sperm counts in humans also appear to be associated with graft-versus-host disease (GVHD) following allogeneic HSCT. However, the mechanisms leading to diminished spermatocyte levels during GVHD remain unknown. Here we demonstrate that injury to intratesticular cells occurs in unconditioned F1 mice following the infiltration of donor alloreactive T cells during an acute graft-versus-host reaction (GVHR). Using computer-aided quantitative microscopic morphometry we demonstrate that the nadir of Leydig cell volume density coincides with the peak of intratesticular infiltration by donor T cells. Injury to Leydig cells correlates with an intratesticular inflammatory response characterized by interferon-γ and tumor necrosis factor-α production. These results demonstrate impairment of testosterone-producing Leydig cells during a local alloresponse, thus representing a mechanism that contributes to gonadal insufficiency following allogeneic HSCT.
Introduction
Hematopoietic stem cell transplantation (HSCT) is the preferred therapy for malignant and nonmalignant disorders.1,2 To maintain quality of life following HSCT, an appropriate reproductive status is required. A retrospective study conducted by the European Group for Blood and Marrow Transplantation (EBMT) determined, however, that the overall posttransplantation pregnancy rate is currently as low as 0.6%.3 These results suggested that female HSC transplant recipients become transiently or permanently infertile. Likewise, most male HSC transplant recipients are reversibly or irreversibly infertile due to germ cell injury and/or Leydig cell (LC) insufficiency.4-6
Posttransplantation gonadotoxicity has been attributed to the type and intensity of the pre-HSCT conditioning used. Here, myeloablative and nonmyeloablative reduced-intensity conditioning regimens can induce both spermatocyte injury and impaired LC function.5,7,8 Gonadal dysfunction has, however, also been associated with the development of graft-versus-host disease (GVHD) because sperm counts in long-term survivors of allogeneic HSCT are much lower in GVHD patients.9 GVHD remains a major transplantation-related toxicity that is initiated by the recognition of host alloantigens by donor-derived mature T cells, leading to injury in a restricted set of target tissues (ie, skin, liver, gastrointestinal tract).10,11 The mechanisms leading to diminished spermatocyte levels secondary to GVHD remain unknown. Here we tested the hypothesis that injury to intratesticular cells occurs following the infiltration of alloreactive donor T cells during an acute graft-versus-host reaction (GVHR).
Study design
Male B6.SJL-PtprcaPep3b/BoyJ mice (B6.CD45.1; H-2b, CD45.1+) were purchased from the Jackson Laboratories (Bar Harbor, ME) and male [C57BL/6 × DBA/2]F1 (B6D2F1, H-2bd) mice were obtained from Charles River (Les Oncins, France). Animals between 6 and 10 weeks of age were kept according to federal regulations. GVHD was induced in nonirradiated B6D2F1 mice by infusion of 60 × 106 allogeneic splenocytes from B6.CD45.1 mice containing approximately 15 × 106 T cells.12,13 This transplantation model allowed us to examine in a longitudinal study the acute GVHR-mediated testicular pathology independent of tissue injury effected by chemotherapy/radiotherapy. Control mice received the same number of cells from syngeneic B6D2F1 donors.
Testosterone in blood serum was measured by radioimmunoassay validated for murine samples.14 For analysis of the LC compartment, we used computer-aided quantitative microscopic morphometry. Transversal paraffin sections of paraformaldehyde-fixed testicular tissue (6 μm) were treated with 0.1% protease/0.5 M Tris (tris(hydroxymethyl)aminomethane) and labeled with an antibody directed against Cytochrome p450scc (Cy p450scc; Ab1244; VWR International Life Science, Lucerne, Switzerland). Cy p450scc is a key enzyme for steroid biosynthesis and, in the testis, it is exclusively localized to mitochondria in LCs.15 Bound antibody was revealed with biotin-conjugated goat antirabbit polyclonal antibody (Ab), ExtrAvidin (Sigma, Buchs, Switzerland), and aminoethylcarbazole. The aggregate area of the specific immunostaining within a random high-powered microscope field (758.6 × 508.4 μm2) was determined by detection of gray scale in digitized microscopic 2-dimensional (2-D) images (Leica DMRE microscope, Nidau, Switzerland; and Soft Imaging System, Lakewood, CO). The LC volume density (%) was then calculated by standard methods as described.16,17
For analysis of cytokine mRNA expression, quantitative polymerase chain reaction (PCR) was performed using SYBR Green (PE Biosystems, Rotkreuz, Switzerland), with glyceraldehyde phosphate dehydrogenase (GAPDH) as a reference.13 The following primers were used: interferon-γ (IFN-γ) sense: GCTGATGGGAGGAGATGTCTACAC, antisense: GACACATTCGAGTGCTGTCTGG; tumor necrosis factor-α (TNF-α) sense: AGGTCTACTTTGGAGTCATTGC, antisense: ACATTCGAGGCTCCAGTGAATTCGG; GAPDH sense: ACCATGTAGTTGAGGTCAATGAAGG, antisense: GGTGAAGGTCGGTGTGAACG. PCR data were analyzed using the comparative Ct method (ΔΔCt method) for relative quantitation of gene expression.
For immunohistochemical analyses of CD3ϵ and CD11b, frozen testicular sections (6 μm) were incubated with specific biotin-conjugated monoclonal antibodies (moAbs; clones 145-2C11 and M1/70, respectively; Pharmingen, San Diego, CA), ExtrAvidin, and aminoethylcarbazole. For confocal microscopy, frozen sections were simultaneously stained with biotin and fluorescein-conjugated moAbs to CD3ϵ and CD45.1 (clone A20), respectively. Strepatavidin-cyanin 3 (Cy3) was used as secondary reagent (Zymed Laboratories, Basel, Switzerland). Two-color immunofluorescent sections were analyzed using a Zeiss LSM510 confocal laser scanning microscope Axiovert 100 (Carl-Zeiss AG, Feldbach, Switzerland). A20 × objective lens with NA 0.5 was used. Data were analyzed with the LSM software version 3.2.
Results and discussion
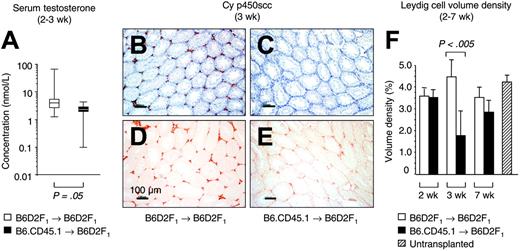
To evaluate gonadal insufficiency during acute GVHR, we assessed serum testosterone. Two to 3 weeks following the infusion of alloreactive T cells into unconditioned, haploidentical F1 mice, median serum testosterone levels were diminished relative to mice that received syngeneic transplants (1.7; range, 0.1-4.3 vs 3.4; range, 1.2-63.5 nM; Figure 1A). As lower systemic testosterone did not per se prove injury to intratesticular cells, however, we then analyzed testosterone-producing cells using expression of Cy p450scc as marker for LC.15 In recipients of alloreactive T cells, expression levels of Cy p450scc were lower than in mice that received syngeneic transplants (Figure 1B-E). To quantify this reduction, computer-aided microscopic morphometry was employed to determine the LC volume density (Figure 1F). We found that 3 weeks after transplantation, the LC volume density significantly decreased from a normal level of 4.70% ± 1.06% to a value of 1.78% ± 1.28% in mice with acute GVHD. Since transplant recipients had not received pretransplantation conditioning, this result indicated injury to LCs (decreased LC number or, alternatively, LC dysfunction as a result of mitochondrial damage) due to an acute GVHR. This testicular injury was temporally related to GVHD-associated impairment of other organs (ie, gastrointestinal tract and liver) as evidenced by histopathology at 3 weeks after T-cell transfer (data not shown). Similar to the other target organs in mice surviving GVHD (data not shown), the LC compartment had recovered from the insult at 7 weeks after transplantation (Figure 1F).
Injury to LC during an acute GVHR. Experimental acute GVHD was induced by transfer of 60 × 106 parental B6.CD45.1 splenocytes to unirradiated B6D2F1 mice (▪), whereas B6D2F1 mice that received syngeneic transplants (□) served as controls. (A) Determinations of serum testosterone by radioimmunoassay were done at the Institute for Reproductive Medicine (University of Muenster, Muenster, Germany). The graph represents pooled data from 8 (non-GVHD) and 7 (GVHD) mice, respectively, that were analyzed between 2 and 3 weeks after transplantation in 1 independent experiment. Three experiments were performed, with similar results. Medians are represented by horizontal bars within the quartile boxes, the ranges are given by vertical bars. Mann-Whitney U test, P = .05 syngeneic versus allogeneic transplantation. (B-E) Paraffin sections (6 μm) of testicular tissue were analyzed for LC-specific Cy p450scc expression (in red color) at 3 weeks after transplantation in mice without (B,D) and with GVHD (C,E). The nuclear counterstain (hematoxylin) is displayed in panels B and C for both groups in blue color. The horizontal bars represent 100 μm.A20×/0.5 HC-PL Fluotar objective lens was used (Leica). Photographs were taken with a KY-F55B-type JVC camera. (F) Sections from mice without and with GVHD were stained with antibody to Cy p450scc and were subjected to computer-aided quantitative morphometric analysis of the LC compartment. The LC volume density (%) was calculated from 5 random high-powered microscope fields per tissue section, with 5 sections per testis (serial sections separated by 30 μm; total of 25 fields per mouse). The figure depicts representative data (mean ± SD) from 1 (of 3) independent experiments, with 5 mice analyzed for each group. B6D2F1 mice infused with syngeneic (□) or allogeneic (▪) donor T cells. Unpaired t test, P < .005 syngeneic versus allogeneic transplantation. Untransplanted, naive B6D2F1 mice served as additional controls (▨).
Injury to LC during an acute GVHR. Experimental acute GVHD was induced by transfer of 60 × 106 parental B6.CD45.1 splenocytes to unirradiated B6D2F1 mice (▪), whereas B6D2F1 mice that received syngeneic transplants (□) served as controls. (A) Determinations of serum testosterone by radioimmunoassay were done at the Institute for Reproductive Medicine (University of Muenster, Muenster, Germany). The graph represents pooled data from 8 (non-GVHD) and 7 (GVHD) mice, respectively, that were analyzed between 2 and 3 weeks after transplantation in 1 independent experiment. Three experiments were performed, with similar results. Medians are represented by horizontal bars within the quartile boxes, the ranges are given by vertical bars. Mann-Whitney U test, P = .05 syngeneic versus allogeneic transplantation. (B-E) Paraffin sections (6 μm) of testicular tissue were analyzed for LC-specific Cy p450scc expression (in red color) at 3 weeks after transplantation in mice without (B,D) and with GVHD (C,E). The nuclear counterstain (hematoxylin) is displayed in panels B and C for both groups in blue color. The horizontal bars represent 100 μm.A20×/0.5 HC-PL Fluotar objective lens was used (Leica). Photographs were taken with a KY-F55B-type JVC camera. (F) Sections from mice without and with GVHD were stained with antibody to Cy p450scc and were subjected to computer-aided quantitative morphometric analysis of the LC compartment. The LC volume density (%) was calculated from 5 random high-powered microscope fields per tissue section, with 5 sections per testis (serial sections separated by 30 μm; total of 25 fields per mouse). The figure depicts representative data (mean ± SD) from 1 (of 3) independent experiments, with 5 mice analyzed for each group. B6D2F1 mice infused with syngeneic (□) or allogeneic (▪) donor T cells. Unpaired t test, P < .005 syngeneic versus allogeneic transplantation. Untransplanted, naive B6D2F1 mice served as additional controls (▨).
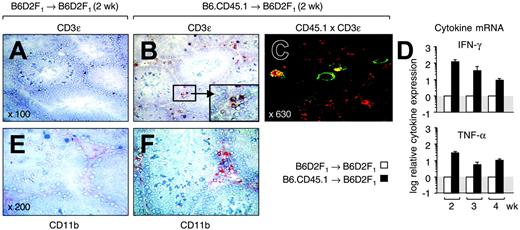
Following activation by host professional antigen-presenting cells (APCs) in secondary lymphoid organs, local APCs recruit primed alloreactive T cells, where they elicit cutaneous and hepatic GHVD during the disease effector phase.11,18-20 To establish whether donor T cells infiltrated the gonads, we examined testes from transplant recipients at 2 weeks after transplantation (Figure 2A-B). We found that CD3+ cells were very rare in normal testes but were abundant in the LC area of mice with GVHD (ie, the interstitial space separating the seminiferous tubules). Confocal microscopy confirmed that these T cells were CD45.1+ and were thus derived from the donor inoculum (Figure 2C). The infiltration of donor T cells peaked at 3 weeks after transplantation (data not shown), coinciding with the nadir in LC volume density. To further detail their function, testicular tissue was analyzed for cytokine expression because the activation of alloreactive T cells has been linked to secretion of the T-helper 1 (Th1) signature cytokine IFN-γ.21,22 Quantitative reverse transcriptase-PCR (RT-PCR) analysis revealed that intratesticular transcription of IFN-γ mRNA was enhanced by more than 100-fold as a result of the GVHR (Figure 2D top panel). Expression levels were up-regulated for the entire observation period although they decreased with time.
Alloreactive donor T cells infiltrate the testis during an acute GVHR. Acute GVHD was induced as in Figure 1. (A,B) Frozen sections from testicular tissues were analyzed at 2 weeks after transplantation for the presence of CD3ϵ+ cells in mice without (A) and with (B) GVHD. A cluster of CD3+ T cells present in the interstitial space between the seminiferous tubules is highlighted in panel B. Original magnification × 100 (magnification for inset × 300). (C) Donor T cells are present in testis. Colocalization of CD3ϵ and CD45.1 was detected by confocal microcopy in mice that received allogeneic transplants. The panel shows cells that stain simultaneously for red (CD3/Cy3) and green (CD45.1/FITC) color. Original magnification × 630. (D) Analysis of cytokine mRNA expression by quantitative PCR. Transcripts for IFN-γ and TNF-α were measured in frozen testicular tissue isolated from mice at 2 to 4 weeks after T-cell infusion. The y-axis represents the x-fold increase of cytokine mRNA in mice with acute GVHD when compared with mice without GVHD (normal transcriptional activity is set as 1). Mean ± SD. (E,F) Frozen sections from testicular tissues were analyzed at 2 weeks after transplantation for the presence of CD11b+ cells (shown in red) in mice without (E) and with (F) GVHD. Original magnification × 200. B6D2F1 mice infused with syngeneic (□) or allogeneic (▪) donor T cells.
Alloreactive donor T cells infiltrate the testis during an acute GVHR. Acute GVHD was induced as in Figure 1. (A,B) Frozen sections from testicular tissues were analyzed at 2 weeks after transplantation for the presence of CD3ϵ+ cells in mice without (A) and with (B) GVHD. A cluster of CD3+ T cells present in the interstitial space between the seminiferous tubules is highlighted in panel B. Original magnification × 100 (magnification for inset × 300). (C) Donor T cells are present in testis. Colocalization of CD3ϵ and CD45.1 was detected by confocal microcopy in mice that received allogeneic transplants. The panel shows cells that stain simultaneously for red (CD3/Cy3) and green (CD45.1/FITC) color. Original magnification × 630. (D) Analysis of cytokine mRNA expression by quantitative PCR. Transcripts for IFN-γ and TNF-α were measured in frozen testicular tissue isolated from mice at 2 to 4 weeks after T-cell infusion. The y-axis represents the x-fold increase of cytokine mRNA in mice with acute GVHD when compared with mice without GVHD (normal transcriptional activity is set as 1). Mean ± SD. (E,F) Frozen sections from testicular tissues were analyzed at 2 weeks after transplantation for the presence of CD11b+ cells (shown in red) in mice without (E) and with (F) GVHD. Original magnification × 200. B6D2F1 mice infused with syngeneic (□) or allogeneic (▪) donor T cells.
Our results support the premise that LCs are lost or dysfunctional as a consequence of a local alloresponse, representing a mechanism that contributes to gonadal insufficiency following HSCT. Similar to other target organs,20 tissue-resident professional or semiprofessional APCs23 may recruit activated T cells, which initiate a local inflammatory immune response, to the testis. Evidence for such intratesticular inflammation was provided by enhanced numbers of intratesticular macrophages (Figure 2E-F) and increased transcripts for TNF-α (Figure 2D bottom panel) in mice with GVHD. Due to the spatial proximity of donor T cells to major histocompatibility complex (MHC)-bearing host LCs, it is conceivable that direct T-cell-mediated cytotoxicity is responsible for target cell injury. Alternatively, inflammatory cytokines such as IFN-γ and TNF-α, which impair LC function,24 may affect LCs in an antigen-nonspecific mechanism similar to other target organs.25 In our experimental system, we did not find evidence for T-cell infiltration into the seminiferous tubules, a fact that correlated with the absence of overt abnormalities in this compartment (data not shown). Thus, it remains to be investigated whether spermatogenesis is indirectly altered during acute GVHD as a consequence of loss and/or dysfunction of Leydig cells.
Prepublished online as Blood First Edition Paper, December 2, 2004; DOI 10.1182/blood-2004-07-2646.
Supported by the Swiss National Science Foundation grants 31-068310.02 (G.A.H.) and 31-61782.00 (W.K.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank K. Hafen, H. Schaller, and B. Erne for their expert help with morphometric analyses, confocal microscopy, and animal work. Special thanks is also extended to Dr M. Simoni and R. Sandhowe (Institute for Reproductive Medicine, University of Muenster, Muenster, Germany) for the determination of testosterone.