Abstract
RNA transfection of dendritic cells (DCs) was shown to be highly efficient in eliciting CD8+ and CD4+ T-cell responses. However, antigen presentation pathways involved in generation of human leukocyte antigen (HLA) class I and class II peptides have remained elusive. To analyze this we incubated mucin 1 (MUC1) RNA-transfected DCs with compounds known to inhibit HLA class I presentation and used these cells in chromium 51 (51Cr)–release assays. As effectors, we used cytotoxic T lymphocyte (CTL) lines specific for the MUC1 peptides M1.1 and M1.2. We observed that the presentation of HLA-A*02 epitopes is inhibited by brefeldin A and lactacystin. To determine the requirement of a functional transporter associated with antigen processing (TAP), we cotransfected DCs with MUC1 and infected cell peptide 47 (ICP47) RNA. ICP47 could only inhibit the presentation of the M1.1 but not the M1.2 peptide, indicating that this epitope derived from the signal sequence is presented independently of TAP. Cocultivation of MUC1 RNA-transfected DCs with MUC1-specific CD4+ T lymphocytes revealed that the presentation of HLA class II peptides is sensitive to proteasomal inhibitors and brefeldin A. Furthermore, the presentation pathway requires lysosomal and endosomal processing and is mediated by autophagy. Our results demonstrate that the efficient presentation of cytosolic proteins on major histocompatibility complex (MHC) class II combines the proteolytic and lysosomal pathways.
Introduction
Dendritic cells (DCs) are recognized as the most powerful antigen-presenting cells that are able to induce and maintain primary immune responses in vitro and in vivo.1-3
RNA transfection of DCs was demonstrated to be a highly efficient tool to elicit antigen-specific cytotoxic T lymphocytes (CTLs) capable of mediating tumor cell recognition and establishment of protective antitumor immunity.4-8 Furthermore, several recent in vitro and in vivo studies indicate that RNA-transfected DCs can generate both CD8+- and CD4+-mediated immune responses, thus suggesting that DCs can process epitopes for human leukocyte antigen (HLA) class II–restricted presentation derived from newly synthesized cytosolic proteins.9-13
Antigen processing for presentation on major histocompatibility complex (MHC) class II molecules involves a multitude of different compartments and proteases within the cell.14 MHC class II molecules predominantly present peptides derived from exogenous proteins as well as epitopes from plasma membranes or endosomes.15-17 However, biochemical and functional studies have shown that antigens localized within the cytoplasm can efficiently be processed for MHC class II–restricted presentation by human and murine antigen-presenting cells.18-21 Several pathways involved in this process have been discussed and analyzed. Degradation of the proteins by the proteasome, translocation of epitopes from cytoplasm into membrane organelles, and autophagy were implicated in several experimental settings using defined antigens and delivery systems.22,23
In our study, we addressed the pathways and requirements for processing and presentation of antigenic peptides for HLA class I and II presentation upon transfection of human monocyte-derived DCs with in vitro–transcribed RNA coding for a tumor-associated antigen. Our data demonstrate that the presentation of cytoplasmic proteins on HLA class II molecules requires the function of the proteasome, the involvement of lysosomal antigen degradation, processing in the lysosomal/endosomal vesicles, and autophagy.
Materials and methods
Reagents
Brefeldin A(5 μg/mL), N-acetyl-l-leucyl-l-leucyl-l-norleucinal (LLnL; 25 μM), N-acetyl-l-leucyl-l-leucyl-l-methioninal (LLM; 10 μM), chloroquine (50 μM), lactacystin (50 μM), leupeptin (200 μg/mL), 3-methyladenine (3-MA; 10 μM), and wortmannin (10 to 30 nM) were all purchased from Sigma-Aldrich (Taufkirchen, Germany). Cathepsin B inhibitor II (10 μM) was purchased from Calbiochem (Schwalbach, Germany).
Cell isolation and generation of dendritic cells from adherent peripheral blood mononuclear cells
In our study, we used buffy coats from healthy donors from the blood bank at the University of Tübingen, Germany. This was approved by our local ethics committee.
Generation of DCs from peripheral blood monocytes was performed as described previously.24 In brief, peripheral blood mononuclear cells (PBMNCs) were isolated by Ficoll/Paque (Biochrom, Berlin, Germany) density gradient centrifugation of HLA-A*02–positive buffy coat preparations of healthy volunteers from the blood bank of the University of Tübingen. Cells were resuspended in serum-free X-VIVO 20 medium (Cambrex, Verviers, Belgium) and allowed to adhere (1 × 107 cells per well) in 6-well plates (BD Falcon; Becton Dickinson, Heidelberg, Germany) in a final volume of 3 mL. After 2 hours of incubation at 37°C and 5% CO2, nonadherent cells were removed. Immature dendritic cells were generated by culturing the adherent blood monocytes in RP10 medium (RPMI 1640 with GlutaMAX-I supplemented with 10% heat-inactivated fetal calf serum and 100 IU/mL penicillin/streptomycin [Invitrogen, Karlsruhe, Germany]) supplemented with human recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF; 100 ng/mL; Leukine Liquid Sargramostim; Berlex Laboratories, Richmond, CA) and interleukin-4 (IL-4; 20 ng/mL; R&D Systems, Wiesbaden, Germany) for 6 days. For some experiments we used serum-free X-VIVO 20 medium (Cambrex), as indicated. The medium was replenished with cytokines every 2 to 3 days. For maturation, DCs were additionally cultured with tumor necrosis factor-alpha (TNF-α; 10 ng/mL; R&D Systems) for 24 hours. DCs were enumerated by flow cytometry as lineage (CD14, CD3, CD19) negative and human leukocyte antigen DR (HLA-DR) bright. Furthermore, analysis of the expression level of the DC markers CD1a and CD83 and costimulatory molecules CD80 and CD86 was performed.
Synthetic peptides
The mucin 1 (MUC1)–derived peptides M1.1 (amino acids 950 to 958: STAPPVHNV) and M1.2 (amino acids 12 to 20: LLLLTVLTV)25 and the irrelevant peptide control human immunodeficiency virus (HIV) (polymerase HIV-1 reverse transcriptase peptide; amino acids 476 to 484: ILKEPVHGV) were synthesized in an automated peptide synthesizer EPS221 (Abimed, Langenfeld, Germany) following the N-alpha-(g-fluorescylmethyl-oxycarbonyl)/tert-butyl (Fmoc/tBu) strategy and analyzed by high-performance liquid chromatography (HPLC) (Varian Star; Zinsser Analytic, München, Germany) and matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry (Future; GSG, Bruchsal, Germany). Peptides of less than 80% purity were purified by preparative HPLC.
Generation of mRNA by in vitro transcription
Enhanced green fluorescent protein (EGFP) in vitro transcript (IVT) was synthesized from the plasmid pSP64–Poly(A)–EGFP-2 (generously provided by V. F. I. Van Tendeloo, Antwerp, Belgium) as described previously.26,27 Full-length cDNA sequences of MUC1 and infected cell protein 47 (ICP47) were excised from pBS-PEM-tm (generously provided by S. Gendler, Imperial Cancer Research Fund, London, United Kingdom)28 and pBJ1neo-ICP47 (generously provided by H. Schild, Department of Immunology, Johannes Gutenberg University, Mainz, Germany), respectively, and subcloned into pSP64-Poly(A). For generation of MUC1 and ICP47 IVT, the plasmids pSP64-Poly(A)-MUC1 and pSP64-Poly(A)-ICP47, which allow in vitro transcription under the control of an SP6 promoter, were linearized using the restriction enzyme PvuII. The in vitro transcription was performed using the SP6 Cap Scribe kit (Roche, Mannheim, Germany) according to the protocol provided by the manufacturer. Purification of IVT was performed with RNeasy Mini anion-exchange spin columns (Qiagen, Hilden, Germany). Quantity and purity of generated IVT was determined by ultraviolet (UV) spectrophotometry and by formaldehyde/agarose gel electrophoresis. RNA was stored at –80°C in small aliquots.
Induction of antigen-specific CTL responses using HLA-A*02–restricted synthetic peptides
For CTL induction, 5 × 105 DCs were pulsed with 50 μg/mL synthetic peptide for 2 hours, washed, and incubated with 2.5 × 106 autologous PBMNCs in RP10 medium. After 7 days of culture, cells were restimulated with autologous peptide-pulsed PBMNCs. A total of 2 ng/mL human recombinant IL-2 (R&D Systems) was added on days 1, 3, and 5 after restimulation. The cytolytic activity of induced CTLs was analyzed on day 5 after the last restimulation in a standard chromium 51 (51Cr)–release assay.
CTL assay
Target cells were pulsed with 50 μg/mL synthetic peptide for 2 hours or electroporated as described below and labeled with [51Cr]sodium chromate in RP10 for 1 hour at 37°C and 5% CO2. A total of 1 × 104 cells were transferred to a well of a round-bottomed 96-well plate. Varying numbers of CTLs were added to a final volume of 200 μL and incubated for 4 hours at 37°C. At the end of the assay, supernatants (50 μL per well) were harvested and counted in a β-plate counter. The percentage of specific lysis was calculated as follows: 100 × (experimental release – spontaneous release/maximal release – spontaneous release). Spontaneous and maximal releases were determined in the presence of either RP10 medium or 2% Triton X-100, respectively.
Electroporation of immature DCs with in vitro transcripts
Electroporation of DCs with IVT was performed as described previously.27 In brief, prior to electroporation, immature DCs were washed twice with serum-free X-VIVO 20 medium (Cambrex) and resuspended to a final concentration of 2 × 107/mL. Subsequently, 200 μL cell suspension was mixed with 10 μg IVT and electroporated in a 4-mm cuvet using an Easyject Plus device (Peqlab, Erlangen, Germany). The physical parameters used were as follows: voltage of 300 V, capacitance of 150 μF, resistance of 1540 Ω, and pulse time of 231 milliseconds. After electroporation, cells were transferred immediately into RP10 medium supplemented with the cytokines GM-CSF (100 ng/mL) and IL-4 (20 ng/mL) and returned to the incubator.
Induction of MUC1-specific T-helper cells (CD4+) using RNA-transfected DCs
CD4+ T lymphocytes were isolated from PBMNCs from HLA-A*02–positive donors using the CD4+ T-cell isolation kit II (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the protocol provided by the manufacturer. Purity of CD4+ T cells was assessed after isolation by flow cytometry. Autologous immature DCs were transfected with MUC1 IVT by electroporation on day 6 as described. After transfection, DCs were incubated for 24 hours in RP10 medium supplemented with the cytokines GM-CSF (100 ng/mL), IL-4 (20 ng/mL), and 10 ng/mL TNF-α as maturation stimulus. For T-helper cell induction, 2.5 × 106 CD4+ T lymphocytes were coincubated with 5 × 105 RNA-transfected autologous DCs in X-VIVO 20 medium. On days 7 and 14 after T-cell induction, restimulations were performed by using 5 × 105 RNA-transfected autologous DCs. IL-2 (25 ng/mL) was added every other day following the first restimulation. The antigen specificity of the induced CD4+ T cell–mediated immune response was assessed on day 20 after T-cell induction in a [3H]thymidine proliferation assay.
Proliferation assay
A total of 2 × 105 responding cells (MUC1-specific CD4+ T lymphocytes) were cocultured in flat-bottomed 96-well microplates (Nunc, Wiesbaden, Germany) with 1 × 105 MUC1 RNA-transfected autologous DCs in X-VIVO 20 medium. Unstimulated CD4+ T lymphocytes and DCs electroporated with irrelevant Her2/neu IVT served as negative control. Inhibition of HLA class I or class II molecules was achieved by incubating DCs for 1 hour prior to the assay either with the monoclonal antibodies W6/32 (20 μg/mL) directed against HLA class I molecules or Tü39 (20 μg/mL) directed against HLA class II molecules (both antibodies were kindly provided by S. Stevanović, University of Tübingen). Thymidine incorporation was measured on day 5 by a 16-hour pulse with [3H]thymidine (1 μCi per well [0.037 MBq per well]; Amersham Life Sciences, Braunschweig, Germany).
Statistical analyses
All experiments were performed at least 3 times; representative experiments are presented. To analyze statistical significances, the Student t test was used.
Results
Generation of M1.1- and M1.2-specific cytotoxic T lymphocytes using peptide-pulsed DCs
We induced CTLs specific for the HLA-A*02–restricted epitopes M1.1 and M1.2 derived from MUC1 antigen in vitro using peptide-pulsed autologous DCs as antigen-presenting cells. The cytotoxic activity of the in vitro–induced CTLs was assessed in a standard 51Cr-release assay. The CTL lines obtained after 2 weekly restimulations demonstrated peptide-specific killing (Figure 1). T cells only recognized autologous DCs pulsed with the cognate MUC1 peptide, while they did not lyse target cells pulsed with an irrelevant peptide, confirming the specificity of the cytolytic activity.
Induction of MUC1-specific CTL responses in vitro. DCs generated from adherent PBMNCs of HLA-A*02–positive donors in the presence of GM-CSF, IL-4, and TNF-α were pulsed with synthetic peptides and used to generate a CTL response in vitro. Cytolytic activity of induced M1.1- (A) and M1.2- (B) specific CTLs was analyzed in a standard 51Cr-release assay using autologous DCs pulsed with the cognate peptide (M1.1 in A [♦]; M1.2 in B [▪]) or an irrelevant peptide (M1.2 in A [□]; M1.1 in B [⋄]; HIV peptide [▵] in both panels) as targets. E/T ratio, effector-target ratio.
Induction of MUC1-specific CTL responses in vitro. DCs generated from adherent PBMNCs of HLA-A*02–positive donors in the presence of GM-CSF, IL-4, and TNF-α were pulsed with synthetic peptides and used to generate a CTL response in vitro. Cytolytic activity of induced M1.1- (A) and M1.2- (B) specific CTLs was analyzed in a standard 51Cr-release assay using autologous DCs pulsed with the cognate peptide (M1.1 in A [♦]; M1.2 in B [▪]) or an irrelevant peptide (M1.2 in A [□]; M1.1 in B [⋄]; HIV peptide [▵] in both panels) as targets. E/T ratio, effector-target ratio.
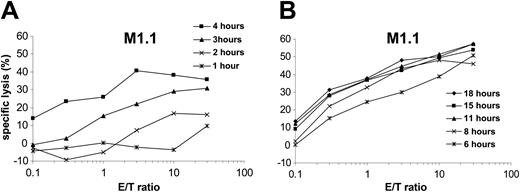
Presentation kinetics of MUC1-derived T-cell epitopes
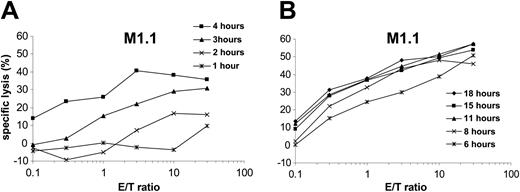
To analyze the time-dependent presentation of antigenic epitopes by human DCs after electroporation with in vitro–transcribed RNA, dendritic cells were transfected with MUC1 RNA and used as targets in a standard 51Cr-release assay after different time points. M1.1-specific CTLs were used as effectors. As shown in Figure 2, presentation of the processed M1.1 peptide was already detectable 2 to 3 hours after electroporation. The presentation maximum was reached after 6 hours, followed by a plateau (Figure 2B). Similar results were obtained for the M1.2 peptide (data not shown). For subsequent experiments we therefore chose to use RNA-transfected DCs 12 hours after transfection.
Kinetics of peptide presentation by RNA-transfected DCs. Monocyte-derived DCs generated from an HLA-A*02–positive donor were electroporated with in vitro–transcribed MUC1 RNA and used as targets in a standard 51Cr-release assay at different time points following transfection as indicated (A: *, 1 hour; ×, 2 hours; ▴,3 hours; ▪, 4 hours; B: *, 6 hours; ×, 8 hours; ▴, 11 hours; ▪, 15 hours; ♦, 18 hours). Autologous CTLs specific for the HLA-A*02–binding MUC1-derived peptide M1.1 were used as effector cells.
Kinetics of peptide presentation by RNA-transfected DCs. Monocyte-derived DCs generated from an HLA-A*02–positive donor were electroporated with in vitro–transcribed MUC1 RNA and used as targets in a standard 51Cr-release assay at different time points following transfection as indicated (A: *, 1 hour; ×, 2 hours; ▴,3 hours; ▪, 4 hours; B: *, 6 hours; ×, 8 hours; ▴, 11 hours; ▪, 15 hours; ♦, 18 hours). Autologous CTLs specific for the HLA-A*02–binding MUC1-derived peptide M1.1 were used as effector cells.
DCs use the cytosolic pathway for presentation of HLA class I–restricted epitopes upon electroporation with RNA
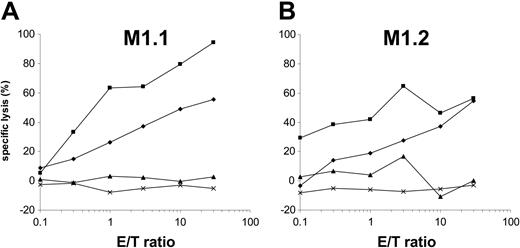
Cytosolic protein degradation is performed by the proteasome, a large multicatalytic protease complex. Lactacystin specifically inhibits the 20S and 26S proteasome activity by targeting the catalytic β subunit.29-32 To analyze if MUC1-derived HLA-A*02–binding epitopes require proteasome degradation, DCs were preincubated with lactacystin and used as targets in a standard 51Cr-release assay after MUC1 RNA transfection. Addition of lactacystin inhibited the presentation of MUC1-derived peptides (Figure 3) but had no effect on the presentation of synthetic peptides, thus excluding a toxic effect or unspecific alteration of DCs.
Presentation of MUC1-derived HLA-A*02–binding T-cell epitopes is sensitive to the proteasome inhibitor lactacystin. Autologous monocyte-derived DCs generated from an HLA-A*02–positive donor were electroporated with MUC1 RNA alone (♦) or additionally incubated with lactacystin (▴). These cells were used as target cells in a standard 51Cr-release assay. M1.1-specific CTLs (A) and M1.2-specific CTLs (B) do not lyse lactacystin-treated MUC1 RNA-transfected DCs. Lysis of the targets can be reestablished by additionally pulsing these DCs with the cognate synthetic peptide M1.1 (A, ▪) or M1.2 (B, ▪). DCs electroporated with irrelevant EGFP RNA (×) were used as control.
Presentation of MUC1-derived HLA-A*02–binding T-cell epitopes is sensitive to the proteasome inhibitor lactacystin. Autologous monocyte-derived DCs generated from an HLA-A*02–positive donor were electroporated with MUC1 RNA alone (♦) or additionally incubated with lactacystin (▴). These cells were used as target cells in a standard 51Cr-release assay. M1.1-specific CTLs (A) and M1.2-specific CTLs (B) do not lyse lactacystin-treated MUC1 RNA-transfected DCs. Lysis of the targets can be reestablished by additionally pulsing these DCs with the cognate synthetic peptide M1.1 (A, ▪) or M1.2 (B, ▪). DCs electroporated with irrelevant EGFP RNA (×) were used as control.
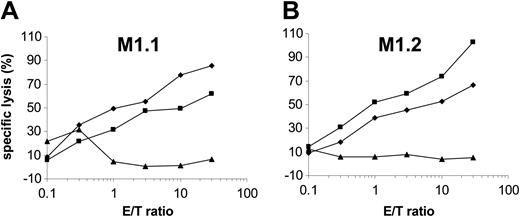
Newly synthesized HLA class I molecules–β2-microglobulin–peptide complexes are transported from the endoplasmic reticulum (ER) to the cell surface for presentation to and recognition by CD8+ CTLs.33,34 The fungal product brefeldin A blocks the MHC class I processing pathway by specifically inhibiting the vesicular egress from the ER and the Golgi complex.35,36 As demonstrated in Figure 4, incubation of DCs with brefeldin A reduced the lysis of MUC1 RNA-transfected DCs, while it had no effect on the recognition of brefeldin A–treated targets pulsed exogenously with the cognate antigenic peptide.
Brefeldin A blocks the presentation of HLA-A*02–binding peptides by RNA-transfected DCs. Autologous monocyte-derived DCs were electroporated with MUC1 RNA alone (♦) or additionally incubated with brefeldin A (▴). A standard 51Cr-release assay was performed using MUC1 IVT-transfected DCs as targets and MUC1-specific CTLs recognizing the M1.1 peptide (A) or the M1.2 peptide (B) as effector cells. To exclude an unspecific or toxic effect of the agent, brefeldin A–treated MUC1 RNA-transfected DCs were additionally pulsed with the synthetic cognate peptides (▪) and used as targets. DCs electroporated with irrelevant EGFP RNA (×) were used as control.
Brefeldin A blocks the presentation of HLA-A*02–binding peptides by RNA-transfected DCs. Autologous monocyte-derived DCs were electroporated with MUC1 RNA alone (♦) or additionally incubated with brefeldin A (▴). A standard 51Cr-release assay was performed using MUC1 IVT-transfected DCs as targets and MUC1-specific CTLs recognizing the M1.1 peptide (A) or the M1.2 peptide (B) as effector cells. To exclude an unspecific or toxic effect of the agent, brefeldin A–treated MUC1 RNA-transfected DCs were additionally pulsed with the synthetic cognate peptides (▪) and used as targets. DCs electroporated with irrelevant EGFP RNA (×) were used as control.
The addition of chloroquine, an agent that increases the pH of distal acidic vesicles and inhibits proteolysis in these compartments, had as expected no effect on the HLA class I presentation of MUC1 RNA-derived epitopes, indicating that these peptides were produced in the nonlysosomal, probably cytosolic, compartment (Figure 5).
Chloroquine has no effect on the presentation of HLA class I–restricted epitopes by RNA-transfected DCs. Autologous DCs were electroporated with MUC1 RNA alone (▪ in A, ♦ in B) and, after incubation with chloroquine, used as targets in a standard 51Cr-release assay (♦ in A, ▪ in B). MUC1-specific CTLs recognizing the M1.1 (A) or M1.2 (B) peptides were used as effector cells. DCs transfected with irrelevant EGFP RNA (▴) were included as controls.
Chloroquine has no effect on the presentation of HLA class I–restricted epitopes by RNA-transfected DCs. Autologous DCs were electroporated with MUC1 RNA alone (▪ in A, ♦ in B) and, after incubation with chloroquine, used as targets in a standard 51Cr-release assay (♦ in A, ▪ in B). MUC1-specific CTLs recognizing the M1.1 (A) or M1.2 (B) peptides were used as effector cells. DCs transfected with irrelevant EGFP RNA (▴) were included as controls.
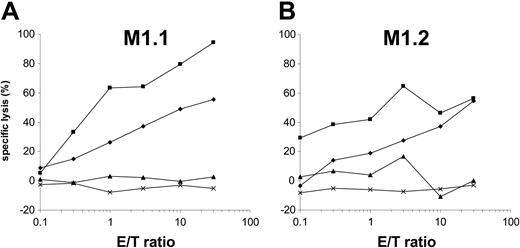
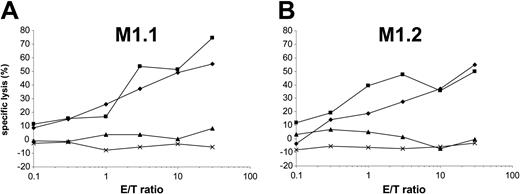
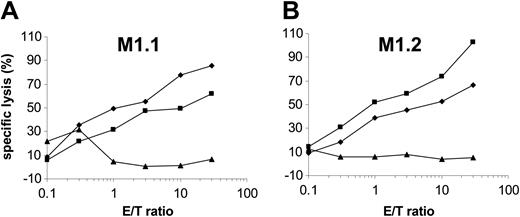
Presentation of the epitope M1.1 but not M1.2 is impaired by the viral TAP inhibitor ICP47
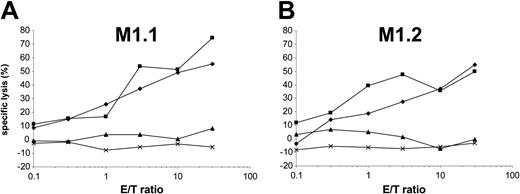
Peptides from 8 to 16 amino acid residues generated in the cytosol by proteasomal degradation are translocated into the lumen of the ER in an adenosine triphosphate (ATP)–dependent manner by transporter associated with antigen processing (TAP) molecules.37 To determine the requirement of a functional TAP molecule for peptide presentation by DCs, we cotransfected autologous DCs with in vitro–transcribed MUC1 RNA and ICP47 RNA. The ICP47 protein from herpes simplex virus binds to the cytosolic side of TAP and prevents the peptide transport from the cytosol into the ER.38-40 Interestingly, in our experiments ICP47 only inhibited the presentation of M1.1 (Figure 6A) but not of M1.2 (Figure 6B), thus indicating that M1.2 is presented TAP independently. This is most likely due to the deriving of the M1.2 peptide from the MUC1 signal sequence, because it was shown previously that peptides derived from the signal sequence can be presented independently of TAP.41,42 ICP47 had no effect on the recognition of targets exogenously pulsed with synthetic peptide. Cotransfection of DCs with MUC1 and ICP47 IVT did not result in complete inhibition of M1.1 presentation. This reflects the efficiency of RNA electroporation. In our previous experiments we found that only 30% to 40% of DCs are transfected with RNA by electroporation as analyzed by reporter gene expression.27
The M1.2 peptide deduced from the signal sequence of the MUC1 protein is presented TAP independently. To analyze if a functional TAP is required for the presentation of HLA class I epitopes, autologous DCs were cotransfected with MUC1 and ICP47 RNA (MUC1 alone, ×; ICP47 alone, ♦; both, ▴) and used as targets for the recognition by MUC1-specific CTLs (M1.1 [A] and M1.2 [B]). DCs pulsed with the synthetic cognate MUC1 peptide (▪) or electroporated with irrelevant EGFP RNA (×) were included as controls.
The M1.2 peptide deduced from the signal sequence of the MUC1 protein is presented TAP independently. To analyze if a functional TAP is required for the presentation of HLA class I epitopes, autologous DCs were cotransfected with MUC1 and ICP47 RNA (MUC1 alone, ×; ICP47 alone, ♦; both, ▴) and used as targets for the recognition by MUC1-specific CTLs (M1.1 [A] and M1.2 [B]). DCs pulsed with the synthetic cognate MUC1 peptide (▪) or electroporated with irrelevant EGFP RNA (×) were included as controls.
Presentation pathways involved in MHC class II presentation of cytosolic antigens by RNA-transfected DCs
We and others have recently shown that electroporation of DCs with RNA can induce CD4+ T-cell responses, suggesting that the introduction of RNA in the cytosolic compartment results in the synthesis of proteins that are processed for HLA class II presentation.9-12 We therefore explored the pathways involved in this phenomenon by using different inhibitors. As described (Figure 6), cytoplasmic proteases are required for the generation of peptide antigens for MHC class I presentation. However, the impact of these enzymes in supplying peptides for MHC class II presentation remains unclear.
To explore the role of the proteasome, a large multicatalytic complex found in the nucleus and cytoplasm well known to be of critical importance in MHC class I–restricted antigen presentation, we took advantage of various inhibitors of proteasomal degradation, including LLnL, LLM, and lactacystin.29-32,43 Of these compounds used in the assays, LLnL and LLM were reported to additionally block calpains and cathepsin B.44-46 However, they seem to have marked differences in their efficiency against the proteasome. The incubation of DCs electroporated with MUC1 RNA with lactacystin, LLnL, or LLM inhibited their presentation of MHC class II–restricted MUC1-derived epitopes significantly (Figure 7) as assessed by the stimulation of MUC1-specific CD4+ T cells.
HLA class II presentation pathway is in part mediated by autophagy and requires lysosomal processing. For the induction of antigen-specific CD4+ T-cell responses by autologous DCs transfected with MUC1 RNA, human CD4+ T lymphocytes were isolated from PBMNCs using magnetic bead technology and coincubated with MUC1 RNA-electroporated DCs. The antigen specificity of the elicited CD4+-mediated immune response was assessed after 2 restimulations on day 20 after T-cell induction in a [3H]thymidine proliferation assay. Blocking of HLA class I and II was performed by incubating DCs with specific monoclonal antibodies against MHC-I and -II, respectively, to confirm the HLA class II restriction. To analyze the pathways involved in the presentation of MUC1-derived HLA class II epitopes, DCs were incubated with agents known to inhibit the proteasome (LLM, LLnL, lactacystin), the egress from ER (brefeldin A), lysosomal acidification (chloroquine), autophagy (3-MA, wortmannin), and endosomal proteases (leupeptin, cathepsin B inhibitor II). In addition, DCs were electroporated with ICP47 IVT to determine the possible role of a functional TAP. DCs electroporated with irrelevant Her-2/neu RNA were included as control. The statistical significance of the proliferation reduction compared with DCs incubated with dimethyl sulfoxide (DMSO) plus anti-MHC class I antibody was analyzed in a Student t test. **P < .001, *P < .002. Error bars indicate standard deviation.
HLA class II presentation pathway is in part mediated by autophagy and requires lysosomal processing. For the induction of antigen-specific CD4+ T-cell responses by autologous DCs transfected with MUC1 RNA, human CD4+ T lymphocytes were isolated from PBMNCs using magnetic bead technology and coincubated with MUC1 RNA-electroporated DCs. The antigen specificity of the elicited CD4+-mediated immune response was assessed after 2 restimulations on day 20 after T-cell induction in a [3H]thymidine proliferation assay. Blocking of HLA class I and II was performed by incubating DCs with specific monoclonal antibodies against MHC-I and -II, respectively, to confirm the HLA class II restriction. To analyze the pathways involved in the presentation of MUC1-derived HLA class II epitopes, DCs were incubated with agents known to inhibit the proteasome (LLM, LLnL, lactacystin), the egress from ER (brefeldin A), lysosomal acidification (chloroquine), autophagy (3-MA, wortmannin), and endosomal proteases (leupeptin, cathepsin B inhibitor II). In addition, DCs were electroporated with ICP47 IVT to determine the possible role of a functional TAP. DCs electroporated with irrelevant Her-2/neu RNA were included as control. The statistical significance of the proliferation reduction compared with DCs incubated with dimethyl sulfoxide (DMSO) plus anti-MHC class I antibody was analyzed in a Student t test. **P < .001, *P < .002. Error bars indicate standard deviation.
In our previous experiments lactacystin also abolished MHC class I presentation of MUC1 peptides (Figure 3), indicating that proteasome-dependent degradation of the cytosolic MUC1 protein was affected. HLA class I presentation of exogenous synthetic peptides (Figure 3) was not inhibited by lactacystin, excluding a possible unspecific inhibition. Furthermore, these studies were performed using concentrations of lactacystin demonstrated not to affect cell viability or trigger apoptotic cell death22 (data not shown). Thus, HLA class II–restricted presentation of proteins endogenously synthesized in the cytosol upon transfection with RNA follows the MHC class I–restricted pathway and requires proteasomal activity for peptide generation.
However, although the differences between control DCs and cells treated with lactacystin are statistically significant, complete inhibition of endogenous presentation of HLA class II–restricted epitopes was not achieved in lactacystin-treated antigen-presenting cells (APCs), suggesting that other cytosolic enzymes may function in this processing pathway.
The critical role of TAP molecules in the presentation of HLA class I peptides from cytosolic antigens is well established.47 We examined if the presentation of HLA class II–restricted antigenic peptides generated in DCs upon transfection with MUC1 RNA requires a functional TAP. To address this we cotransfected DCs with MUC1 and ICP47 IVT as mentioned (Figure 6) and used these cells to stimulate MUC1-specific CD4+ T lymphocytes. As shown in Figure 7, ICP47 had no effect on the presentation of HLA class II epitopes. In contrast, the same experimental approach resulted in a significant reduction of the M1.1 HLA-A*02–binding peptide presentation (Figure 6A), confirming that cotransfection of ICP47 with MUC1 RNA inhibits the function of TAP molecules.
To further clarify the intracellular trafficking of HLA class II peptides derived from endogenously generated MUC1 protein, brefeldin A was used to block the transport of newly synthesized proteins from ER to the Golgi. Studies with exogenous antigens have demonstrated that 2 distinct processing pathways are involved in MHC class II–restricted presentation: the synthesis of new class II α/β heterodimers that get loaded with peptide ligands within the late endosomal- and lysosomal-like compartments and the transit of cell surface class II molecules through recycling early endosomal vesicles, where they acquire antigenic peptides by the process of peptide editing.48 Incubation of DCs with brefeldin A blocked the cytoplasmic MUC1 presentation to MUC1-specific CD4+ T cells and resulted in a significantly reduced proliferation of these cells (Figure 7), thus confirming that the HLA class II–restricted presentation of MUC1 epitopes requires newly synthesized HLA class II molecules and their transport to the cell surface.
To test whether the presentation of MUC1 peptides on HLA class II molecules was dependent on lysosomal proteases, DCs were treated with the lysosomotropic agent chloroquine that prevents acidification of the lysosomal compartment. Addition of chloroquine blocked the HLA class II–restricted presentation of MUC1-derived epitopes, resulting in a significantly reduced proliferation of MUC1-specific CD4+ cells (Figure 7), indicating that acidic lysosomal compartment is involved in the processing of endogenous MUC1 protein. This was not due to a toxic effect of chloroquine, because the same experimental approach did not affect the presentation of HLA class I–binding peptides (Figure 5).
Peptides derived from the MUC1 protein gain access to the endosomal compartment
To help localize the intracellular site of proteolysis for endogenously synthesized MUC1 antigen and determine if endosomal compartments are involved in this pathway, functional studies were performed using class-specific proteolytic inhibitors.
Leupeptin,29 which inactivates cysteine and serine proteases, including cathepsin S, L, and B, significantly reduced the presentation of MUC1 epitopes upon incubation with MUC1 RNA-transfected DCs, resulting in a significantly reduced proliferation of CD4+ T cells (Figure 7). To further determine the requirement of acidic cysteine proteases, presentation of MUC1-derived HLA class II–restricted epitopes was explored in DCs treated with cathepsin B inhibitor II, a leupeptin analog that preferentially inhibits cathepsin B.49 A reduction of MUC1 presentation was detected upon incubation of DCs with this compound as analyzed by antigen specific T-cell activation and proliferation, which was significantly reduced (Figure 7). These results demonstrate that the endogenously synthesized MUC1 protein or its fragments gain access to the endosomal compartment.
Autophagy is involved in the presentation of MUC1 peptides
It was previously demonstrated that degradation of cytosolic antigens is in part mediated by a process named autophagy that can be inhibited by a feedback mechanism of degradation products with 3-methyladenine (3-MA) or compounds blocking phosphatidylinositol-3 (PI-3) kinase, such as wortmannin.50-52 While higher wortmannin concentration affects several signal transduction pathways and the viability of the cells, lower wortmannin doses (less than 50 nM) selectively block autophagic sequestration.53 Incubation of DCs with 3-MA or low concentrations of wortmannin significantly reduced the stimulation and proliferation of MUC1-specific CD4+ T cells, suggesting that this mechanism is active during processing and presentation of antigenic peptides for HLA class II presentation by RNA-transfected DCs.
Discussion
In our study we analyzed the processing and presentation of antigenic peptides by DCs transfected with in vitro–transcribed MUC1 RNA. According to the cytosolic pathway of antigen presentation, electroporation of DCs with RNA should result in the synthesis of a cytosolic protein that is digested by a multicatalytic protease complex, the proteasome. The resultant peptides are transported by TAP, a heterodimeric complex composed of TAP1 and TAP2 subunits, from the cytosol into the ER. A small number of the generated peptides are selected by their ability to bind to the MHC class I molecules, thereby stabilizing the MHC class I–β2-microglobulin complex and allowing its transport to the cell surface, where the peptides are presented to and recognized by CD8+ CTLs.
In our study we found that the generation and presentation of HLA class I–restricted epitopes by DCs upon electroporation with in vitro–transcribed RNA coding for the tumor antigen MUC1 was sensitive to the inhibition of the proteasome and the transport from the ER to the Golgi, confirming that antigenic peptides use the classical cytosolic pathway of antigen presentation. This pathway requires functional TAP molecules, at least for most of the generated peptides. However, in cells lacking a functional TAP molecule (such as the human T2 cells), some of the MHC molecules contain antigenic peptides that are presented on the surface. In this context several mechanisms have been identified for the TAP-independent presentation of peptides derived from cytosolic antigens, including epitopes from signal sequences of membrane/secretory proteins.42,54,55 In line with these results we found that cotransfection of DCs with RNA coding for MUC1 and ICP47, a viral molecule that inhibits TAP, resulted in a reduced presentation of the HLA-A*02–binding MUC1-derived peptide M1.1, while it had no effect on the presentation of the M1.2 peptide derived from the signal sequence of the protein.
We and others have recently shown that DCs electroporated with RNA can elicit both CD8+- and CD4+-mediated T-cell responses.8-12 However, mechanisms underlying the presentation of HLA class II–restricted T-cell epitopes generated from cytosolic/endogenous antigens are not well understood and remain controversial. Several potential pathways have been implicated in the presentation of endogenous antigens by MHC class II molecules, including cytoplasmic processing of proteins and transport of the generated peptides into ER or, more recently, a process called autophagy.23,51 This process involves the sequestration of cytosolic proteins in the autophagolysosomes followed by a subsequent processing in the lysosomal compartment, where the degradation of the antigens and loading of MHC class II molecules takes place.
Here we show that proteins synthesized in DCs upon transfection with RNA are degraded in the cytosol by the proteasome for HLA class II presentation. To ascertain that proteasomal degradation is required for presentation of epitopes derived from endogenously synthesized MUC1 protein, proliferation assays using MUC1-specific CD4+ T cells were performed in the presence of LLnL, LLM, and lactacystin. In our experiments LLM and LLnL inhibited antigen presentation by RNA-transfected DCs to a greater extent than lactacystin. LLM and LLnL are potent inhibitors of the proteasome, endosomes, as well as other sites of cellular proteases,29,45,56 whereas lactacystin specifically blocks degradation of proteins by the proteasome without affecting lysosomal/endosomal proteolysis.30 Thus, these results indicate that proteasomal and endosomal compartments are involved in the presentation of HLA class II–restricted epitopes by RNA-transfected DCs.
In line with these observations, treatment of DCs with chloroquine, a compound that inhibits proteolysis by affecting the acidification in the lysosomal compartment, significantly altered HLA class II presentation, whereas it had no effect on the processing of class I–restricted peptides by MUC1 RNA-transfected DCs, thus excluding a nonspecific or toxic effect of this agent.
To further analyze if endosomal compartments are involved in the proteolysis of endogenously synthesized proteins, proliferation assays were performed using MUC1-specific CD4+ T cells and MUC1 IVT-transfected DCs treated with leupeptin, an inhibitor of serine and cysteine proteases such as trypsin, papain, and endosomal cathepsin B, L, and S.29 Moreover, a more specific inhibitor of cathepsin B, cathepsin B inhibitor II,49 was used. As expected, these agents reduced the presentation of endogenous MUC1 protein as analyzed by activation of MUC1-specific CD4+ T cells, thus confirming that the endosomal compartment is involved in the HLA class II–restricted processing of cytoplasmic antigens by RNA-transfected DCs. Next we determined if the Golgi apparatus is involved in the trafficking of HLA class II–peptide complexes. Antigen presentation of MUC1 RNA-electroporated DCs to MUC1-specific CD4+ T cells was analyzed in the presence of brefeldin A, which blocks the transit of newly synthesized proteins from the ER to the Golgi. Treatment of DCs with brefeldin A significantly inhibited stimulation of MUC1-specific CD4+ T cells, suggesting that egress of newly synthesized HLA class II molecules from the ER to the Golgi is required for the presentation of endogenous class II–restricted antigens by DCs upon electroporation with in vitro–transcribed RNA. However, a functional TAP molecule is not necessary for this presentation, according to our results from experiments with the herpes simplex virus–derived TAP inhibitor ICP47.
In the next set of experiments we wanted to analyze how the synthesized cytoplasmic MUC1 protein gained access into the vacuolar system. It was previously shown that autophagy mediates the nonspecific bulk degradation of cytoplasmic proteins and is involved in the HLA class II–restricted presentation of endogenously expressed neomycin phosphotransferase II (NeoR).23 Autophagy is recognized as a ubiquitous cellular activity of all eukaryotic cell types and is probably responsible for the turnover of most cytosolic antigens. This process is regulated by hormones, amino acids, growth factors, and by a feedback mechanism through degradation products.57,58
In our experiments, treatment of RNA-transfected DCs with compounds recognized to specifically block autophagy (like 3-MA or wortmannin)50,52,53,59 reduced the capacity of DCs to present HLA class II–restricted MUC1 peptides. These results imply an involvement of autophagy in the presentation of HLA class II epitopes by RNA-transfected DCs.
Collectively, the drug experiments performed in our study indicate that the HLA class II–restricted presentation of endogenous antigens by RNA-transfected DCs occurs via the combination of the cytosolic and lysosomal pathways and the endosomal impact of cytoplasmic antigens is mediated by autophagy.
Supported by grants from Deutsche Krebshilfe, Deutsche Forschungsgemeinschaft (SFB 510), and the Graduiertenkolleg grant entitled “Cellular mechanisms of immune-associated processes.”
D.D. and S.A. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Prepublished online as Blood First Edition Paper, December 23, 2004; DOI 10.1182/blood-2004-09-3556.
We thank Sylvia Stephan, Bruni Schuster, and Regina Heselmaier for excellent technical assistance as well as Christoph Driessen and Arthur Melms for critical reading of the manuscript.

![Figure 1. Induction of MUC1-specific CTL responses in vitro. DCs generated from adherent PBMNCs of HLA-A*02–positive donors in the presence of GM-CSF, IL-4, and TNF-α were pulsed with synthetic peptides and used to generate a CTL response in vitro. Cytolytic activity of induced M1.1- (A) and M1.2- (B) specific CTLs was analyzed in a standard 51Cr-release assay using autologous DCs pulsed with the cognate peptide (M1.1 in A [♦]; M1.2 in B [▪]) or an irrelevant peptide (M1.2 in A [□]; M1.1 in B [⋄]; HIV peptide [▵] in both panels) as targets. E/T ratio, effector-target ratio.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/8/10.1182_blood-2004-09-3556/6/m_zh80080576850001.jpeg?Expires=1768336299&Signature=NL7AYllKv5mgAzKh5p9f9FsDi~oh~UUdK7~xbzhvOQbi5586MOmXgOYHBrO7jWRYVspJJBlOW8VNz8Rr7Pm2EW4WjiGFZVd53hpbKHvfsqcKvGx3CyOVgsOrtx-64gzSGfhmPmpv6EtQO41hTSQEQfP96sE51ynymd0ywtp~vYO~-lhqxrIGP97lje~35tNWsmHZtqn7zCm3NLBnUi65Kdb~r0IILtNkjndng~2g6od0nFDdCZe0hpVUsTNAnTsXajwpVIyEE2HbTroSTEGmUHqGpjpSqufkoduMYSyoaockrE9eMbJ14a08ub8w2ec87KYvKRsMk58T5k0Ku3ULdQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. The M1.2 peptide deduced from the signal sequence of the MUC1 protein is presented TAP independently. To analyze if a functional TAP is required for the presentation of HLA class I epitopes, autologous DCs were cotransfected with MUC1 and ICP47 RNA (MUC1 alone, ×; ICP47 alone, ♦; both, ▴) and used as targets for the recognition by MUC1-specific CTLs (M1.1 [A] and M1.2 [B]). DCs pulsed with the synthetic cognate MUC1 peptide (▪) or electroporated with irrelevant EGFP RNA (×) were included as controls.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/8/10.1182_blood-2004-09-3556/6/m_zh80080576850006.jpeg?Expires=1768336299&Signature=Sl-xGx5vPhcm2luSc1TK9sgbs3kAG6wCZNyjCblm1p8cggINaw9LEZkG-d80p71~il8nVhRwcNM3~t0FcH-kEUPsY3Xtp2HxrMOnntmpoW7eOXqxIrpoIuL8rYkQqGHPVAl-C5lrXWXQUMfxgJL6kM-l42vjRhyXNaiKvB~PgjBT1~-e7FhRy0SQw~VjZnSXggtap2AuIY1s7vSYNy-1yYW3XGZpvE4mIALKr~gRR5nud~HkuEeQByHU9vtS7dlX7fA20oOCoGVku0xcLpeOJwUuplB1UIMkpQGj0oqYbjgGYYg7bpibRXI499ZqX5SdtzovC~XP4BgYMqqZX9QhQw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. HLA class II presentation pathway is in part mediated by autophagy and requires lysosomal processing. For the induction of antigen-specific CD4+ T-cell responses by autologous DCs transfected with MUC1 RNA, human CD4+ T lymphocytes were isolated from PBMNCs using magnetic bead technology and coincubated with MUC1 RNA-electroporated DCs. The antigen specificity of the elicited CD4+-mediated immune response was assessed after 2 restimulations on day 20 after T-cell induction in a [3H]thymidine proliferation assay. Blocking of HLA class I and II was performed by incubating DCs with specific monoclonal antibodies against MHC-I and -II, respectively, to confirm the HLA class II restriction. To analyze the pathways involved in the presentation of MUC1-derived HLA class II epitopes, DCs were incubated with agents known to inhibit the proteasome (LLM, LLnL, lactacystin), the egress from ER (brefeldin A), lysosomal acidification (chloroquine), autophagy (3-MA, wortmannin), and endosomal proteases (leupeptin, cathepsin B inhibitor II). In addition, DCs were electroporated with ICP47 IVT to determine the possible role of a functional TAP. DCs electroporated with irrelevant Her-2/neu RNA were included as control. The statistical significance of the proliferation reduction compared with DCs incubated with dimethyl sulfoxide (DMSO) plus anti-MHC class I antibody was analyzed in a Student t test. **P < .001, *P < .002. Error bars indicate standard deviation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/8/10.1182_blood-2004-09-3556/6/m_zh80080576850007.jpeg?Expires=1768336299&Signature=dVO17mn1TECx8qGnWq03e1ejQxRsG0fO6OJ15PooiigsRPX3UMf4F58iw3MB38AzvveVHD2V8RcrCKzdbj7IphN~k6jGbZU~Y8Apj2PTFg6j5zZRFcdxx8e1LclHuIVrrFCaOlLkYH7UKlvGdXHvGGKQBW0Pui~sFd3uXTdzAGAAJcvBueryPQnUn54qn2FGdudR5g44yoqS7lVfk6PwaL5SZkYjyqBxi-KfqBw1RCE~pOPpk~4Ab08Im0dIF470~Oth-qSiORjhBxk3zDbnNoLdqJhtnF~OEcd6XMY23dEXxwQTRytNMCGQdlM8LUBkai5KKU0E9Zlqg9cBFygNiA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 1. Induction of MUC1-specific CTL responses in vitro. DCs generated from adherent PBMNCs of HLA-A*02–positive donors in the presence of GM-CSF, IL-4, and TNF-α were pulsed with synthetic peptides and used to generate a CTL response in vitro. Cytolytic activity of induced M1.1- (A) and M1.2- (B) specific CTLs was analyzed in a standard 51Cr-release assay using autologous DCs pulsed with the cognate peptide (M1.1 in A [♦]; M1.2 in B [▪]) or an irrelevant peptide (M1.2 in A [□]; M1.1 in B [⋄]; HIV peptide [▵] in both panels) as targets. E/T ratio, effector-target ratio.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/8/10.1182_blood-2004-09-3556/6/m_zh80080576850001.jpeg?Expires=1768336300&Signature=mUyhWSFSpif19egyKHea-icFGhlIXQr-mCtnCP~4S~neJFM25JRbHwG2IZoj1PFgiCMFwgU5nkOtNHlYO6UqnL~HhmbDq-VSG2QEMLMyxJ9UCAO1-D2G-IxdcD7MO5jAL7Z3tpuLjkYvHM5R9jFC8g1sQBhAPXw0AIWswN6COpxGfTQsa-s36bEd2-yMEkf9m-FaUC4Jq1IGJXjun5USNERTfVSz9jyYpv5vVz8KTO7-Hg2BD93tQjFrXEOp-YYvdZitVcjj7H2vVgsu1g6M586XiuNonRdRLqYp-iX-peIQ4G-qM0YPAUXiPotS4mQz9x-lFnU6HRphfW5ZKTNNAA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. The M1.2 peptide deduced from the signal sequence of the MUC1 protein is presented TAP independently. To analyze if a functional TAP is required for the presentation of HLA class I epitopes, autologous DCs were cotransfected with MUC1 and ICP47 RNA (MUC1 alone, ×; ICP47 alone, ♦; both, ▴) and used as targets for the recognition by MUC1-specific CTLs (M1.1 [A] and M1.2 [B]). DCs pulsed with the synthetic cognate MUC1 peptide (▪) or electroporated with irrelevant EGFP RNA (×) were included as controls.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/8/10.1182_blood-2004-09-3556/6/m_zh80080576850006.jpeg?Expires=1768336300&Signature=imU32L3hCHdJsWG46LudNdDzdPYC~KhLrNGVUEByz4D4u173uAj7X8g~uTVW6FO0o55xn3QK1l6FY802yX4EvtikQ6kZOXkHMFP8iGhNW2yLLdwzmkYE9k69bm7Jx9xRfgduBUhAGwIIwSjTEBBKtLSpk2xc8Ex-r-w2gyIR2zmwQeNA5iskhIafjGJThmM06Aq1Mm79lj-qKaSiGYdef8rbWDY9AyQsR683OJZ6g0LPfAOwnIZlYpFELR0txLPOyS7FAMVWkahFjwlSjFcCcroWscc6ZvNL-bQzLCemOl8LrjGXCub6QBgYvP6wTsnY6mTOQP94-f5bERkY4Sdplg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. HLA class II presentation pathway is in part mediated by autophagy and requires lysosomal processing. For the induction of antigen-specific CD4+ T-cell responses by autologous DCs transfected with MUC1 RNA, human CD4+ T lymphocytes were isolated from PBMNCs using magnetic bead technology and coincubated with MUC1 RNA-electroporated DCs. The antigen specificity of the elicited CD4+-mediated immune response was assessed after 2 restimulations on day 20 after T-cell induction in a [3H]thymidine proliferation assay. Blocking of HLA class I and II was performed by incubating DCs with specific monoclonal antibodies against MHC-I and -II, respectively, to confirm the HLA class II restriction. To analyze the pathways involved in the presentation of MUC1-derived HLA class II epitopes, DCs were incubated with agents known to inhibit the proteasome (LLM, LLnL, lactacystin), the egress from ER (brefeldin A), lysosomal acidification (chloroquine), autophagy (3-MA, wortmannin), and endosomal proteases (leupeptin, cathepsin B inhibitor II). In addition, DCs were electroporated with ICP47 IVT to determine the possible role of a functional TAP. DCs electroporated with irrelevant Her-2/neu RNA were included as control. The statistical significance of the proliferation reduction compared with DCs incubated with dimethyl sulfoxide (DMSO) plus anti-MHC class I antibody was analyzed in a Student t test. **P < .001, *P < .002. Error bars indicate standard deviation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/8/10.1182_blood-2004-09-3556/6/m_zh80080576850007.jpeg?Expires=1768336300&Signature=xHqQgQzSj8UnWZHDkBAnpDhU0H33ACUHU0vsd~a2WsY1ACQW188cMOAFeCS6mo5Pbe1NZXiZnXV5j9h0jzo3tmEAC0kXCSBI-uSfyq9dmqDZeF219MT24zjOYn6kfgn7QvgEG6RgBq4SHpHtSor4ZAaBtCFL6dUPcyiTFKDereqYrBGWPY3RTBFpNzT4FfEmic0ucu1OvhJbjLOhZvW0Kjs6k18rLoWPHTh92PXwIuk8542h9cU38x1qHIPzaHBKecvqXsvrQlq5UkNWxohQqeHZSDd2SSadO2Acx9W7PaD4kaqNpvFEjVdlEE8zlv7Lj7u8FmusfW-8pFFf8f20bA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)