Abstract
Dendritic cells (DCs), also referred to as the sentinels of the immune system, induce and coordinate important functions of immune surveillance. DCs acquire immunity-initiating capacity only after a process of maturation usually induced by ligands that bind to members of the tumor necrosis factor (TNF) or toll-like receptor families. Secretory phospholipase A2 (sPLA2), which hydrolyzes the sn-2 ester bond of glycerophospholipids, regulates a variety of cellular functions including migration of endothelial cells and neurite outgrowth. In the present study we investigated the role of sPLA2 in DC biology. We report that human monocyte-derived DC cultures lack sPLA2 activity but respond to exogenous sPLA2. sPLA2 alone and in cooperation with TNF-α and interleukin 1 β (IL-1β) induced fatty acid release from DC membranes, which was accompanied by upregulation of surface markers and by an increase in the migratory and immunostimulatory capacity of the DCs. Our findings indicate that secreted enzymes such as sPLA2 can contribute to DC maturation and emphasize the role of lipid mediators in the regulation of immune responses. This observation may also have implications for DC-based vaccine development.
Introduction
Phospholipases A2 (PLA2s; phosphatidylcholine-2-acylhydrolase, EC 3.1.1.4) represent a growing family of enzymes that catalyze the hydrolysis of the sn-2 fatty acyl ester bond of membrane glycero-3-phospholipids to release free fatty acids and lysophospholipids such as arachidonic acid (AA) and lysophosphatidylcholine (lyso-PC).1-3 PLA2s have been assigned to several groups and classified according to cellular localization, amino acid sequence, molecular mass, and calcium (Ca2+) requirement for enzymatic activity.4 The extracellular or secreted PLA2s (sPLA2s) are characterized by high disulfide bridge content, low molecular mass (∼14 kDa), the requirement of millimolar concentration of Ca2+ for catalysis, and wide fatty acid selectivity in vitro. sPLA2 products such as AA and lyso-PC are themselves potent mediators that have been implicated in the regulation of cellular functions.5 In addition, AA and lyso-PC are subject to further metabolism. While AA can be converted into eicosanoids including the prostaglandins, leukotrienes, thromboxanes, and lipoxins, lyso-PC can be metabolized to generate lysophosphatidic acid (LPA) or platelet-activating factor (PAF).5 Thus, sPLA2 activity eventually generates a wide array of bioactive lipid mediators.
Several mammalian and venom sPLA2s have been identified.6 The different venom sPLA2s have been classified into 4 groups based on their primary structures. The bee venom sPLA2 was the founding member of group III. The overall 3-dimensional structure of bee venom group III sPLA2 reveals the striking features of the PLA2 fold, including a well-defined Ca2+ loop, 3 large α helices, and a β wing–like structure. Group III sPLA2 from bee venom is made up of about 135 amino acids and has 5 disulfide bridges. Consistent with these catalytic and structural similarities between venom and mammalian sPLA2, evidence has been obtained that venom sPLA2 can indeed behave like mammalian sPLA2 and induce physiologic effects such as migration of endothelial cells and neurite outgrowth.7-9
Dendritic cells (DCs) affect both innate and adaptive immune responses.10 DCs elicit and tune antigen-specific T- and B-cell responses. According to a well-accepted concept that has emerged during recent years, the maturation state of DCs swings the decision between tolerance and immunity.11 While immature DCs maintain tolerance by silencing antigen-specific T lymphocytes or by actively converting them into regulatory T cells, mature DCs promote immunity. Maturation is induced by a multitude of signals frequently referred to as “danger” signals,12 and DCs have learned to respond to these signals using various receptors of the tumor necrosis factor (TNF) or toll-like receptor families.11 A hallmark of “danger” is inflammation. Infectious agents usually provoke inflammatory responses characterized by the production of many cytokines including TNF-α and interleukin 1 β (IL-1β), which are both known to trigger DC maturation.13 TNF-α and IL-1β are also among the most potent inducers of lipid mediator metabolism.5 TNF-α and IL-1β, for instance, augment the expression of PLA2 enzymes and cyclooxygenases (COXs), resulting in the production of prostaglandins (PGs) such as PGE2,2 which has also been described to contribute to DC maturation.14,15
In the present work we examined the role of sPLA2s in DC biology and found that sPLA2s can act on DC membranes to mobilize lipid mediators and induce DC maturation.
Materials and methods
Secretory phospholipase A2
sPLA2 from bee venom was obtained from Cayman Chemical Company (Ann Arbor, MI). To test for potential endotoxin (lipopolysaccharide [LPS]) contamination, sPLA2 was subjected to the Limulus amebocyte lysate (LAL) assay from Biowhittaker (Walkersville, MD). At the sPLA2 concentrations used in this study (1 μg/mL and 10 μg/mL) endotoxin concentrations were 1.1 pg/mL and 11 pg/mL, respectively. At these concentrations, LPS fails to induce DC activation (data not shown). sPLA2 activity was monitored using the colorimetric sPLA2 assay kit (Cayman Chemical Company), which is based on the synthetic substrate diheptanoyl thiophosphorylcholine. Results are expressed as time-dependent absorbance increase (mOD414nm/min) using 5 points in time with 1-minute increments. Mean values of triplicate measurements plus or minus standard deviation (SD) are shown.
DC culture
Blood monocytes were isolated to high purity using the magnetic-activated cell sorting (MACS) technology from Miltenyi Biotec (Bergisch Gladbach, Germany).16 Monocytes (3 × 106 cells/3 mL) were subsequently cultured for 5 days in 6-well plates in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS; vol/vol) in the presence of granulocyte macrophage–colony-stimulating factor (GM-CSF; 800 U/mL) and IL-4 (500 U/mL). In some experiments, lipoprotein-deficient (LPD) FCS was used (Sigma-Aldrich, Vienna, Austria). From day 5 to day 7, maturation was induced with TNF-α (100 U/mL) and IL-1β (1 ng/mL) in the presence or absence of sPLA2 (1 μg/mL or 10 μg/mL).
Fatty acid release
To assess fatty acid release, day-5 DCs were labeled overnight with [3H]-AA (1 μCi/mL) from Amersham Biosciences (Freiburg, Germany).16 The cells were washed to remove unincorporated radioactivity and reseeded in triplicate in RPMI 1640 medium supplemented with 1% FCS (vol/vol) and 1 mg/mL fatty acid–free bovine serum albumin (BSA; Sigma) for stimulation with TNF-α (100 U/mL) and IL-1β (1 ng/mL) in the presence or absence of sPLA2 (1 μg/mL or 10 μg/mL). After 90 minutes, supernatants and cells were harvested and radioactivity was determined by scintillation counting. The percentage release was calculated using the formula (S/[S+P]) × 100, where S and P represent the radioactivity measured in the supernatant and in the cell pellet, respectively. Values are mean values of quadruplicate measurements plus or minus SD.
Flow cytometry
To determine surface antigen expression, DCs were stained with anti-CD83–phycoerythrin (PE) or anti-CD86–fluorescein isothiocyanate (FITC) for 30 minutes on ice and then washed extensively. The endocytic activity of DCs was assessed using fluorescent tracer molecules. FITC-dextran was used to measure mannose receptor–mediated endocytosis and FITC-BSA to assess macropinocytosis. Cells (106) were incubated with FITC-dextran or FITC-BSA (0.5 mg/mL) for 30 minutes at 37°C (control at 0°C) and then washed extensively. Cells were analyzed using a FACSCalibur and Cell Quest Software from BD Biosciences (San Diego, CA).
Migration assay
The chemotactic capacity of the DCs was measured by assessing migration through a polycarbonate filter (6.5 mm in diameter and 8 μm pore size) in 24-well Transwell chambers. RPMI 1640 medium (600 μL) supplemented with 10% LPD-FCS with or without 300 ng/mL MIP-3β (= CCL19) were added to the lower chamber. DCs were added to the upper chamber (1 × 105 cells in a total volume of 100 μL) and incubated for 2 hours at 37°C. The number of migrated cells was determined by flow cytometry (FACSCalibur) as events acquired during 1 minute in an appropriate forward scatter/side scatter gate. Each experiment was performed in duplicate. The mean number of spontaneously migrated DCs (in the absence of macrophage inflammatory protein-3β [MIP-3β]) was subtracted from the total number of DCs that migrated in response to MIP-3β. Mean values of triplicate measurements plus or minus SD are shown.
Allogeneic mixed-leukocyte reaction (MLR)
CD14- peripheral blood mononuclear cells (PBMCs; 2 × 105/well) from 3 different donors were used as responder cells and stimulated with allogeneic, irradiated DCs at a ratio of 50:1. Cells were cultured in triplicate in 96-well, flat-bottomed, tissue-culture plates in a final volume of 200 μL/well in AIM-V medium. Proliferation was measured as [3H] thymidine incorporation (1 μCi/well [37 kBq/well]). Cells were pulsed during the last 16 hours of a 5-day culture period, and harvested onto nitrocellulose filters. Radioactivity was determined by scintillation counting. Results are expressed as the mean counts per minute (cpm) plus or minus SD.
Cytokine measurements
Interferon γ (IFN-γ), TNF-α, IL-2, IL-4, IL-5, and IL-10 were measured at 24 hours or 96 hours in MLR culture supernatants using the cytometric Cytokine Bead Array from BD Biosciences according the manufacturer's instructions. Samples were analyzed using the FACSCalibur and the appropriate software from BD Biosciences.
Results
DC-culture supernatants lack sPLA2 activity
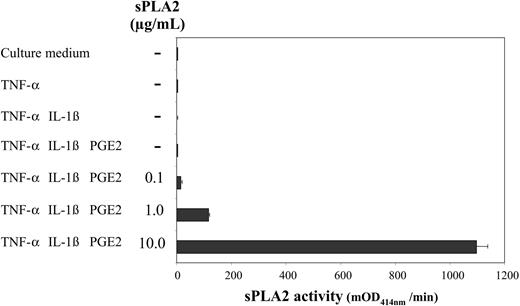
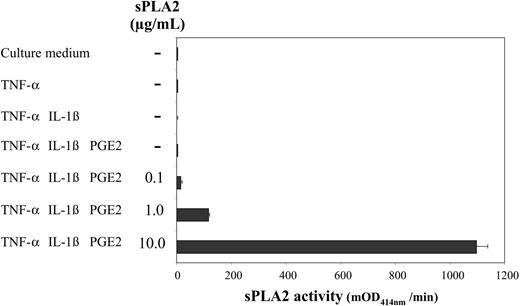
TNF-α, IL-1β, and PGE2, which cooperate to induce maturation of monocyte-derived DCs,14,15 have also been shown to efficiently promote AA mobilization in related cell types.17 To examine a potential role of endogenous sPLA2 in DC maturation we stimulated day-5 DCs with TNF-α alone (1000 U/mL) or in combination with IL-1β (5 ng/mL) with or without PGE2 (1 μM) for 48 hours and measured sPLA2 activity in culture supernatants (Figure 1). sPLA2 activity could not be detected under any condition. When increasing amounts of exogenous sPLA2 were added (0.1 μg/mL-10 μg/mL), sPLA2 activity could dose dependently be recovered after 48 hours (Figure 1) also indicating that the added sPLA2 was active throughout the 48-hour cell culture period.
DC-culture supernatants lack sPLA2 activity. Day-5 DCs were treated as indicated with TNF-α (1000 U/mL), IL-1β (5 ng/mL), and PGE2 (1 μM); sPLA2 was added at increasing concentrations (0.1 μg/mL-10.0 μg/mL). After a 48-hour culture supernatants were harvested and assessed for sPLA2 activity using a commercial sPLA2 assay kit. Results from 1 of 3 similar experiments are shown. mOD414 = (optical density at 414 nm per minute) × 10-3. Mean values of triplicate measurements ± SD are shown.
DC-culture supernatants lack sPLA2 activity. Day-5 DCs were treated as indicated with TNF-α (1000 U/mL), IL-1β (5 ng/mL), and PGE2 (1 μM); sPLA2 was added at increasing concentrations (0.1 μg/mL-10.0 μg/mL). After a 48-hour culture supernatants were harvested and assessed for sPLA2 activity using a commercial sPLA2 assay kit. Results from 1 of 3 similar experiments are shown. mOD414 = (optical density at 414 nm per minute) × 10-3. Mean values of triplicate measurements ± SD are shown.
Exogenous sPLA2 induces AA release from DC membranes
To assess the fatty acid–releasing function of exogenous sPLA2, day-5 DCs were pulsed overnight with [3H]-AA, which is incorporated into cellular membranes. Figure 2 shows that sPLA2 alone was able to release substantial amounts of [3H]-AA from DC membranes in a dose-dependent manner. Low doses of TNF-α (100 U) plus IL-1β (1 ng/mL) also stimulated [3H]-AA release, which was further enhanced dose dependently in the presence of sPLA2. TNF-α plus IL-1β and sPLA2 at 10 μg/mL mobilized more than 15% of AA within 90 minutes. Also of note was the observation that even unstimulated, immature DCs released several percent of AA within this short time (Figure 2).
sPLA2 induces AA release from DC membranes. Day-5 DCs were labeled overnight with [3H]-AA, washed, reseeded in triplicate, and stimulated as indicated for 90 minutes. Supernatants and cell pellets were harvested and radioactivity was determined by scintillation counting. Based on the distribution of radioactivity the percentage of total radioactivity released from cellular membranes into the supernatant was calculated. Results from 1 of 2 almost identical experiments are shown. Values are mean values of quadruplicate measurements ± SD.
sPLA2 induces AA release from DC membranes. Day-5 DCs were labeled overnight with [3H]-AA, washed, reseeded in triplicate, and stimulated as indicated for 90 minutes. Supernatants and cell pellets were harvested and radioactivity was determined by scintillation counting. Based on the distribution of radioactivity the percentage of total radioactivity released from cellular membranes into the supernatant was calculated. Results from 1 of 2 almost identical experiments are shown. Values are mean values of quadruplicate measurements ± SD.
Exogenous sPLA2 has no immediate effects on the endocytic acitvity of DCs
To study the influence of sPLA2 on the endocytic activity of DCs, endocytosis assays were performed in the presence or absence of sPLA2. Mannose receptor–mediated endocytosis assessed as uptake of FITC-dextran was dose-dependently inhibited in the presence of sPLA2 (data not shown), an effect attributable to binding of sPLA2 to the mannose receptor.18 The inhibition must therefore be considered a competitive inhibition and not a consequence of sPLA2 enzymatic activity. sPLA2 had little influence on macropinocytosis measured as uptake of FITC-BSA (data not shown). Taken together, no convincing evidence could be obtained that sPLA2 has short-term effects on the endocytosis of DCs.
DCs respond to exogenous sPLA2 and undergo maturation
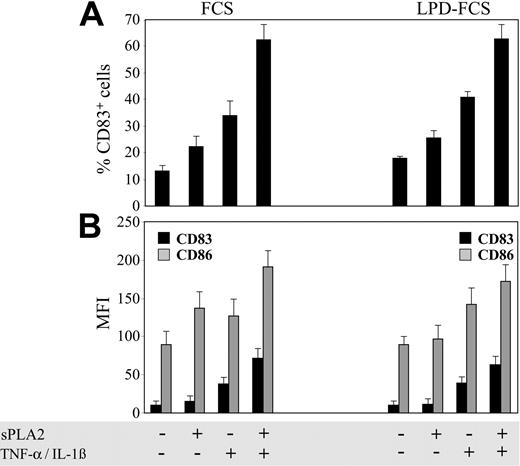
We of course wondered whether sPLA2 action on DC membranes is associated with the surface remodeling characteristic of DC maturation.10,11 We assessed expression of CD83, a very reliable marker of DC maturation, and expression of CD86, a well characterized costimulator of T-cell activation. Figure 3 demonstrates that sPLA2 alone (10 μg/mL) could increase the percentage of CD83+ cells (Figure 3A) and the mean fluorescence intensities (MFIs) of both CD83 and CD86 (Figure 3B). Since sPLA2 may also act on serum lipoproteins to release lipid mediators,19 identical experiments were performed in parallel using LPD-FCS. The percentages of CD83+ cells were similar or even modestly increased in LPD-FCS. In contrast, increases in CD83 and CD86 MFIs induced in FCS by sPLA2 alone were hardly detectable in LPD-FCS. However, increases in CD83 and CD86 MFIs induced by sPLA2 in combination with TNF-α plus IL-1β were still measurable in LPD-FCS, suggesting that the effects of sPLA2 alone on CD83 and CD86 MFIs occur mainly via sPLA2 digestion of serum lipoproteins, while sPLA2 effects in the presence of TNF-α plus IL-1β are a consequence of direct action of sPLA2 on DC membranes.
sPLA2 induces DC maturation. Day-5 DCs were treated for 48 hours in the presence of FCS or LPD-FCS with sPLA2 alone (10 μg/mL) or in combination with TNF-α (100 U/mL) plus IL-1β (1 ng/mL). Cells were stained with monoclonal antibodies specific for CD83 or CD86 and analyzed with a flow cytometer. The percentage of CD83+ cells (A) and the MFIs of CD83 (▪) and CD86 (▦) (B) are shown. Results from one experiment representative of 6 independent experiments are shown. LPD indicates lipoprotein-deficient; data are mean values of triplicate measurements ± SD.
sPLA2 induces DC maturation. Day-5 DCs were treated for 48 hours in the presence of FCS or LPD-FCS with sPLA2 alone (10 μg/mL) or in combination with TNF-α (100 U/mL) plus IL-1β (1 ng/mL). Cells were stained with monoclonal antibodies specific for CD83 or CD86 and analyzed with a flow cytometer. The percentage of CD83+ cells (A) and the MFIs of CD83 (▪) and CD86 (▦) (B) are shown. Results from one experiment representative of 6 independent experiments are shown. LPD indicates lipoprotein-deficient; data are mean values of triplicate measurements ± SD.
sPLA2 treatment enhances the migratory capacity of the DCs
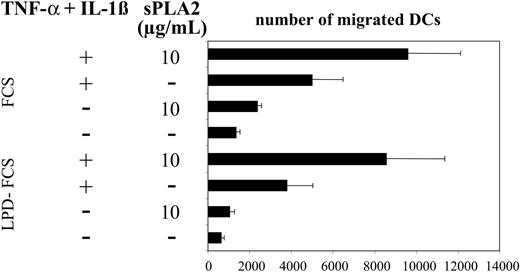
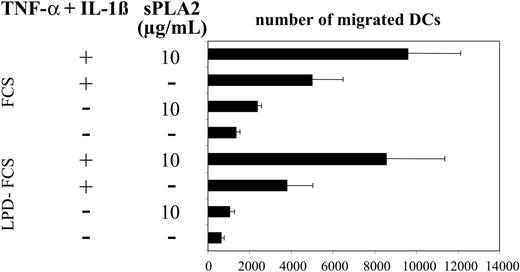
Previous work has shown that sPLA2 can affect the migratory activity of endothelial cells.7 To test the effects of sPLA2 on DC migration we used a well-established assay of MIP-3β–directed migration of DCs.20 Figure 4 shows enhancement of DC migration after treatment with sPLA2 alone or in combination with TNF-α plus IL-1β. Migratory responses were modestly reduced when treatments were performed in LPD-FCS but migratory patterns induced by the various stimuli were almost identical (Figure 4).
sPLA2 treatment increases DC migration in response to MIP-3β. Day-5 DCs were treated for 48 hours in the presence of FCS or LPD-FCS with sPLA2 alone (10 μg/mL) or in combination with TNF-α (100 U/mL) plus IL-1β (1 ng/mL). DCs (105 cells) were then added to wells of Transwell chambers containing MIP-3β in the lower chamber. The number of migrated cells present in the lower chamber was counted by flow cytometry as the number of events acquired during 1 minute. Mean values of duplicate measurements are shown. One of 3 experiments with consistent results is shown. LPD indicates lipoprotein-deficient. Mean values of triplicate measurements ± SD are shown.
sPLA2 treatment increases DC migration in response to MIP-3β. Day-5 DCs were treated for 48 hours in the presence of FCS or LPD-FCS with sPLA2 alone (10 μg/mL) or in combination with TNF-α (100 U/mL) plus IL-1β (1 ng/mL). DCs (105 cells) were then added to wells of Transwell chambers containing MIP-3β in the lower chamber. The number of migrated cells present in the lower chamber was counted by flow cytometry as the number of events acquired during 1 minute. Mean values of duplicate measurements are shown. One of 3 experiments with consistent results is shown. LPD indicates lipoprotein-deficient. Mean values of triplicate measurements ± SD are shown.
DCs treated with sPLA2 have enhanced immunostimulatory capacity
The primary purpose of DC maturation is enhancement of the immunostimulatory capacity. As a next step we examined whether the phenotypic changes induced by sPLA2 were accompanied by increased stimulatory capacity of the DCs in the allogeneic MLR. Figure 5 depicts [3H] thymidine incorporation data demonstrating that sPLA2 treatment of DCs enhances proliferative alloresponses. Proliferation data shown in Figure 5 indeed reflect the phenotypic changes shown in Figure 3.
sPLA2 treatment of DCs enhances their stimulatory capacity in the allogeneic MLR: proliferative responses. Day-5 DCs were treated in the presence of FCS or LPD-FCS for 48 hours with sPLA2 alone (10 μg/mL) or in combination with TNF-α (100 U/mL) plus IL-1β (1 ng/mL). Cells were washed and used as stimulators of 3 different preparations of allogeneic PBMCs depleted of CD14+ cells. Proliferation was determined as [3H] thymidine incorporation. Results from 1 of 2 almost identical experiments are shown. Mean values of triplicate measurements ± SD are shown. LPD indicates lipoprotein-deficient.
sPLA2 treatment of DCs enhances their stimulatory capacity in the allogeneic MLR: proliferative responses. Day-5 DCs were treated in the presence of FCS or LPD-FCS for 48 hours with sPLA2 alone (10 μg/mL) or in combination with TNF-α (100 U/mL) plus IL-1β (1 ng/mL). Cells were washed and used as stimulators of 3 different preparations of allogeneic PBMCs depleted of CD14+ cells. Proliferation was determined as [3H] thymidine incorporation. Results from 1 of 2 almost identical experiments are shown. Mean values of triplicate measurements ± SD are shown. LPD indicates lipoprotein-deficient.
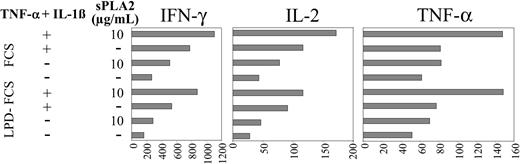
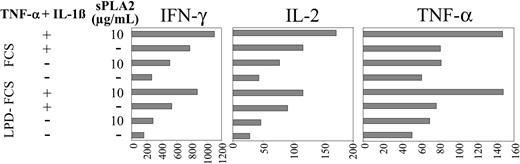
In addition, cytokines accumulating in the allogeneic MLR supernatants were measured after 24 hours. Figure 6 confirms that the phenotypic changes of DCs induced by sPLA2 (Figure 3), which result in enhanced proliferative responses (Figure 5), are also reflected in the cytokine profiles. Production of IFN-γ, IL-2, and TNF-α in the MLR are all up-regulated when DCs were pretreated with sPLA2 (Figure 6). The stimulatory effect of sPLA2 treatment was apparent in FCS and LPD-FCS; however, IFN-γ and IL-2 production in the MLR were reduced when sPLA2 treatment of DCs was performed in LPD-FCS (Figure 6).
sPLA2 treatment of DCs enhances their stimulatory capacity in the allogeneic MLR: cytokine responses. Day-5 DCs were treated in the presence of FCS or LPD-FCS for 48 hours with sPLA2 alone (10 μg/mL) or in combination with TNF-α (100 U/mL) plus IL-1β (1 ng/mL). Cells were washed and used as stimulators of 3 different preparations of allogeneic PBMCs depleted of CD14+ cells. Triplicate supernatants were harvested and pooled at 24 and 96 hours. Cytokine profiles were determined using a commercial cytokine bead array and a flow cytometer (both from BD Biosciences). The 24-hour values are shown. LPD indicates lipoprotein-deficient.
sPLA2 treatment of DCs enhances their stimulatory capacity in the allogeneic MLR: cytokine responses. Day-5 DCs were treated in the presence of FCS or LPD-FCS for 48 hours with sPLA2 alone (10 μg/mL) or in combination with TNF-α (100 U/mL) plus IL-1β (1 ng/mL). Cells were washed and used as stimulators of 3 different preparations of allogeneic PBMCs depleted of CD14+ cells. Triplicate supernatants were harvested and pooled at 24 and 96 hours. Cytokine profiles were determined using a commercial cytokine bead array and a flow cytometer (both from BD Biosciences). The 24-hour values are shown. LPD indicates lipoprotein-deficient.
Although low at 24 hours (= 16 pg/mL), IL-5 was regulated like IFN-γ, IL-2, and TNF-α (data not shown). After 96 hours, IFN-γ and IL-5 levels were increased manifold (17- to 50-fold; not shown), however, still presenting with the regulatory patterns apparent after 24 hours (Figure 6). In contrast, IL-2 and TNF-α levels were reduced at 96 hours and showed no pattern of regulation anymore. IL-10 and IL-4 remained low (= 20 pg/mL) and showed no pattern of regulation.
Taken together, sPLA2 treatment of DCs enhances production of IFN-γ, IL-2, TNF-α, and IL-5 in the MLR. The stimulatory effect of DC pretreatment with sPLA2 on the production of IFN-γ, IL-2, TNF-α, and IL-5 in the MLR was rapidly apparent (24 hours) but was transient for IL-2 and TNF-α and, at 96 hours, could only be detected for IFN-γ and IL-5. IL-4 and IL-10 production in the MLR were not regulated at all by sPLA2 pretreatment of DCs.
Discussion
This is one of the first studies that investigates the effects of a secreted enzyme on DCs. DCs generated from monocytes in the presence of IL-4 lack endogenous sPLA2 activity (Figure 1) but respond to exogenous sPLA2 (Figures 2, 3, 4, 5, 6). Exogenous sPLA2 induced AA release from DC membranes (Figure 2), up-regulation of surface markers (Figure 3), and an increase in migratory (Figure 4) and immunostimulatory capacities (Figures 5 and 6). Confirming evidence for stimulatory effects of sPLA2 on DCs has recently also been obtained by Perrin-Cocon and colleagues.21
A principle of DC maturation that emerges is the cooperation of 2 groups of mediators. Ligands of TNF or toll-like receptors initiate the process of DC maturation.11 The concurrent activation of PLA2 enzymes initiates phospholipid metabolism and generates lipid mediators5 that cooperate with the ligands of TNF or toll-like receptors to enhance DC maturation.
The major phospholipid of the outer leaflet of the plasma membrane is phosphatidylcholine (PC). Lipolysis of PC by sPLA2 thus results in the release of a free fatty acid such as AA and lyso-PC (Figure 7). Lyso-PC has recently been shown to activate DCs.22 AA can be metabolized by COX and the respective synthase toward PGE2, which cooperates with TNF-α in DC maturation.14 Using a sensitive radioimmunoassay we were unable to detect PGE2 in culture supernatants of DCs treated with sPLA2 with or without TNF-α plus IL-1β (data not shown). We therefore conclude that the maturation-enhancing effects of sPLA2 are mediated by lyso-PC.
Lipid mediator metabolism initiated by sPLA2. The major phospholipid of the outer leaflet of the plasma membrane is PC. Cleavage of PC by sPLA2 thus results in the release of a free fatty acid, such as AA and lyso-PC, which has recently been shown to activate DCs. AA can be metabolized by COX and the respective synthase toward PGE2, which cooperates with TNF-α in DC maturation.
Lipid mediator metabolism initiated by sPLA2. The major phospholipid of the outer leaflet of the plasma membrane is PC. Cleavage of PC by sPLA2 thus results in the release of a free fatty acid, such as AA and lyso-PC, which has recently been shown to activate DCs. AA can be metabolized by COX and the respective synthase toward PGE2, which cooperates with TNF-α in DC maturation.
In addition to the direct action on cellular membranes, sPLA2 may act on serum lipoproteins to release lipid mediators, including lyso-PC.19 Side-by-side analysis in complete FCS or LPD-FCS revealed that part of the observed sPLA2 effects are indeed mediated by lipoprotein-derived mediators (Figures 3, 4, 5, 6) indicating that sPLA2 may use either membrane phospholipids or serum lipoproteins as substrate. During inflammatory processes vasculary leakage results in the influx of serum lipoproteins which increase substrate availability for sPLA2.
Taken together, sPLA2 activity may lead to the concomitant generation of 2 different lipid mediators that both enhance DC maturation, lyso-PC and PGE2. However, monocyte-derived DCs generated in the presence of IL-4 lack endogenous sPLA2 activity (Figure 1) and indeed fail to produce PGE2.23 DCs obviously depend on exogenous sPLA2. In fact, one major function of mammalian secreted PLA2 may be to act on cells such as DCs which themselves lack endogenous enzyme activity. Such a mechanism of transcellular DC activation in which one cell, for instance a macrophage or stromal cell, secretes PLA2 which then induces transactivation of adjacent DCs has so far not been described. sPLA2 is considered an amplifier of inflammatory responses, which expands inflammation by activating neighboring cells. Our finding that sPLA2 activates DCs points to an important role of sPLA2 in the induction and amplification of immune responses. Transcellular DC activation may lead to more systemic DC maturation, which appears to be required for the induction of robust immunity. In this context the expression patterns of sPLA2 classes may also be of interest. While sPLA2 IIA is found at very high levels in inflammatory lesions, the group V enzyme is abundant in macrophages and group X sPLA2 is selectively expressed in spleen, thymus, and peripheral blood leukocytes.3 Future studies have to address the role of the various sPLA2 enzymes in immunity and tolerance.
sPLA2 activity depends on the physical state of the membrane.24 Agonist-stimulated alteration of membrane dynamics is believed to facilitate sPLA2 action on membrane phospholipids. In the present work we observed that sPLA2 alone could mediate [3H]-AA release from labeled DC membranes (Figure 2) and could increase the percentage of CD83+ cells even in LPD-FCS (Figure 3), suggesting that membrane dynamics of immature DCs enable sPLA2 action without further stimulation. Another endogenous activator of sPLA2-mediated phospholipolysis is 15-hydroperoxyeicosatetraenoic acid (15-HPETE).24 15-HPETE is the immediate product of 15-lipoxygenase action on free AA and can readily be taken up by monocyte-derived cells and incorporated into their membranes as shown 2 decades ago by Pawlowski et al.25 The esterified 15-HPETE appears to enhance sPLA2 action, membrane phospholipolysis, and eventually modulation of the inflammatory response. The link between these biochemical processes and DC biology relates to the fact that 15-lipoxygenase is strongly induced by IL-4.26 DCs generated from monocytes in the presence of IL-4 have abundant 15-lipoxygenase and indeed generate 15-HPETE.27,28 IL-4–induced and 15-lipoxygenase–mediated production of 15-HPETE may lead to the membrane dynamics that allow immediate sPLA2 action. Thus, immature DCs are primed to rapidly respond to sPLA2, which is abundant in inflammatory lesions and in lymphoid organs.
The immunity-initiating potential of DCs is currently exploited in clinical settings.29,30 In such studies DCs are generated from patient peripheral blood precursor cells in vitro, loaded with antigens, matured, and injected back into the patient to treat diseases like cancer. Bee venom sPLA2 may be attractive for DC-based vaccine development. Bee venom sPLA2 would serve 2 purposes at once. As an enzyme, sPLA2 would contribute to DC maturation leading to potent migratory and immunostimulatory cells (Figures 3, 4, 5, 6). As an antigen, bee venom sPLA2 would function as a helper and control antigen that supports antitumor immune responses and allows the monitoring of vaccine efficacy.
Prepublished online as Blood First Edition Paper, October 19, 2004; DOI 10.1182/blood-2004-08-3001.
Supported by grant 03a of the Kompetenzzentrum Medizin Tirol (KMT) (M.T.).
R.R. and T.P. contributed equally to this report.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Erika Artner-Dworzak and Andrea Casari for PGE2 measurements, Nikolaus Romani for helpful advice, and the Tilak GesmbH for continuous support.

![Figure 2. sPLA2 induces AA release from DC membranes. Day-5 DCs were labeled overnight with [3H]-AA, washed, reseeded in triplicate, and stimulated as indicated for 90 minutes. Supernatants and cell pellets were harvested and radioactivity was determined by scintillation counting. Based on the distribution of radioactivity the percentage of total radioactivity released from cellular membranes into the supernatant was calculated. Results from 1 of 2 almost identical experiments are shown. Values are mean values of quadruplicate measurements ± SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/9/10.1182_blood-2004-08-3001/6/m_zh80090577940002.jpeg?Expires=1766060637&Signature=Jh5V-Qqq1uZOVLIsctAjkw1nCoFf8STAtkVoXetqbzWlfAkY1LOAR1VEcBUAf8VHRrwHKSZVTfReDuQb6BxgesffoHlDiPWwmk2h6X4n0cNekZu41jkcEzXUGovWsD8P4GujgFb0kYqK4Td~TJol1POMGfBmmbFTW9PfN0NSLBpoW~7saJBucrUNSyk9hQ~70nGu~TiUZ43QQUf6ZHrvWRz1qFjQ1Cg6ww1RmWWvsqf34bIwxn7GiB2rIUB5h2PJHxIX-rGKHydFrpG0HC1~341QMbMhPzLn3uiXbw1PWo-8KiZ84aTOy7UNdmaFMTCP-HfETtcCiKvo4DtGsCfiPw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 5. sPLA2 treatment of DCs enhances their stimulatory capacity in the allogeneic MLR: proliferative responses. Day-5 DCs were treated in the presence of FCS or LPD-FCS for 48 hours with sPLA2 alone (10 μg/mL) or in combination with TNF-α (100 U/mL) plus IL-1β (1 ng/mL). Cells were washed and used as stimulators of 3 different preparations of allogeneic PBMCs depleted of CD14+ cells. Proliferation was determined as [3H] thymidine incorporation. Results from 1 of 2 almost identical experiments are shown. Mean values of triplicate measurements ± SD are shown. LPD indicates lipoprotein-deficient.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/9/10.1182_blood-2004-08-3001/6/m_zh80090577940005.jpeg?Expires=1766060637&Signature=PwYKe0VaWw2geujZREc5XqzLM4NE~4s3i3twUsZgN4JHC2XcqJ3I1fH5EgC16VQORprwKXOIX3m88yZc29GMy0cuvoURz8dzpNcgnCKxO7sJei6a16wNJZ5bzZL7eBuRV4UgyDWT4gdF3fDsp2KSHITw-qLE1ECkxPobLu2YbbJ3cEnIrwurWVoOqo6cNgmCdPr1vLEXwg6wZzhzltNLF7Rb9UA~dSdCmQQffhpRbBeDyKyoA2itQ1QGufO5srMkIp4xskKVte8M1~kSh1MiW4eLCRgi6R7xbMvIpBlAFEwUuSq05ZUMnYEumwqELfxHLgdNQhKwaL9Iggk2kDyxuQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Figure 2. sPLA2 induces AA release from DC membranes. Day-5 DCs were labeled overnight with [3H]-AA, washed, reseeded in triplicate, and stimulated as indicated for 90 minutes. Supernatants and cell pellets were harvested and radioactivity was determined by scintillation counting. Based on the distribution of radioactivity the percentage of total radioactivity released from cellular membranes into the supernatant was calculated. Results from 1 of 2 almost identical experiments are shown. Values are mean values of quadruplicate measurements ± SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/9/10.1182_blood-2004-08-3001/6/m_zh80090577940002.jpeg?Expires=1766060638&Signature=slG8PAFgot3TBUvIor0S0qhMaHNXXMSu8GYuYYyExoFftIpzVQY03zxjP-8NfMIIc-hY2EiHF2x6s2bEgaBEmAB1UrlI5OWY7LLb5gELckeNpX~AmRFxu2dqGHw6mtnbs0blW5Z6SIbaRVmfMTnV~efeJH8s4CcBQ-OWUmYOvATxAez2kl8gyzmtJgVXo-PZ~7vQFkUx1VmXYUYShjYJyz~ANjzqsHKAXZL9suscfDyebV1GSF9BPPjwahfwCggzxBrF9oXlXvDxvXAfXatIEPXc8dLVJ1uOVTT1fLuWu7AacjkCKFqBkzNS3pgJNllekii1Ta4~lFjxXZz8jpJTtA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. sPLA2 treatment of DCs enhances their stimulatory capacity in the allogeneic MLR: proliferative responses. Day-5 DCs were treated in the presence of FCS or LPD-FCS for 48 hours with sPLA2 alone (10 μg/mL) or in combination with TNF-α (100 U/mL) plus IL-1β (1 ng/mL). Cells were washed and used as stimulators of 3 different preparations of allogeneic PBMCs depleted of CD14+ cells. Proliferation was determined as [3H] thymidine incorporation. Results from 1 of 2 almost identical experiments are shown. Mean values of triplicate measurements ± SD are shown. LPD indicates lipoprotein-deficient.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/9/10.1182_blood-2004-08-3001/6/m_zh80090577940005.jpeg?Expires=1766060638&Signature=NrVc6BQJJJKwonIBdJHjXahEYstGFvyvzK~aoSUAUkj~ncKmokIHj3FZTKD1ZO609gSVoV75Vvg-jBuL0C36f06XJW~Ispi2GGDVrVQ7EifrAUlJuyqOrax6DPfxJt62-NksDwmNNGIR-1dAsnjCAWJec-~8AIOIUdaoSGPlfEst-8-SiqDZyLSmUQR4GKqsWQELe2QB5vzj~3wTSC9iaLYAJGrXdGd-XTjr02BHemgu3Bx3HOjkEWpyS2RcrDUhPVoIs7Kl0YL6DgqladQTSRSHRRqnpQ-aYf4xpiz-O1vGCDku3MWPp3QCZpU3OxFde-2ukRtxxz0lTjEZTnsTOw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)