Signal transducers and activators of transcription 1 (STAT1) is a critical mediator of interferon (IFN)–induced gene responses. Recently, STAT1 was found to become modified by small ubiquitin-like modifier 1 (SUMO-1) conjugation at Lys703 through the SUMO E3 ligase function of protein inhibitors of activated STAT (PIAS) proteins. However, the physiologic function of sumoylation in STAT1 is still unclear. Here, we show that mutations in the SUMO attachment site in STAT1 result in increased transcriptional activity in a fashion that is selective among IFN-γ target genes. The sumoylation-defective STAT1 mutant displayed increased induction of guanylate-binding protein 1 (GBP1) and transporters associated with antigen presentation 1 (TAP1) transcription but not interferon regulatory factor 1 (IRF1) transcription. Moreover, the sumoylation-defective mutant STAT1-KR showed a prolonged DNA-binding activity and nuclear localization in response to IFN-γ stimulation. These results suggest that sumoylation has a defined negative regulatory effect on selective STAT1-mediated transcription responses.
Introduction
Signal transducers and activators of transcription 1 (STAT1) is critical for interferon-gamma (IFN-γ)–mediated immune responses, and its activation requires phosphorylation on tyrosine 701 by the receptor-associated Janus kinases (JAKs).1 The function of STAT1 is also modulated by other posttranslational modifications, such as phosphorylation of Ser727 and, more recently, sumoylation at Lys703.2-4
Small ubiquitin-like modifier (SUMO)–1, –2, –3, and –4 are protein moieties covalently conjugated to specific lysine residues on substrate proteins through an enzymatic pathway.5 Recently, the regulatory enzymes in these reactions have been characterized, and protein inhibitors of activated STAT (PIAS) proteins, initially identified as regulators of STAT and androgen receptor activation, were shown to function as E3-type SUMO ligases.6,7 Sumoylation mediates divergent effects on transcription factors and can cause transcriptional repression or enhanced activation.8 Previously, we and others showed that PIAS proteins promote the sumoylation of STAT1 at Lys703, but the effect of the Lys703 mutation on reporter gene responses varied, leaving the functional role of this modification elusive.3,4 This study aimed to analyze the functional role of sumoylation in STAT1, and our results indicate that sumoylation mediates a negative, promoter-dependent regulatory function in STAT1-mediated transcription.
Study design
Reagents
Antibodies used were anti–SUMO-1 (mouse anti–GAP-modifying protein 1 [anti–GMP-1]; Zymed, San Francisco, CA); anti-HA (clone 16B12; Berkeley-Antibody, Richmond, CA); anti-Flag (anti-Flag M2; Sigma-Aldrich, St Louis, MO); anti-STAT1 (N-terminus; Transduction Lab, Becton Dickinson, Palo Alto, CA); anti–phospho-STAT1 (New England Biolabs, Beverly, MA); biotinylated antimouse (Dako A/S, Glostrup, Denmark); streptavidin-biotin horseradish peroxidase conjugate (Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom). Human IFN-γ (huIFN-γ) was purchased from Immugenex (Los Angeles, CA).
Plasmids
The SUMO-1, STAT1–wild-type–HA (STAT1-WT-HA), and STAT1-KR–HA plasmids have been previously described.3
STAT1-I702R-HA (Ile→Arg) and STAT1-E705A-HA (Glu→Ala) were constructed from STAT1-WT-HA using polymerase chain reaction (PCR) mutagenesis with the following primers: 5′-GGAACTGGATATAGGAAGACTGAGTTGATTTCTGTGTCTGAA-3′ and 5′-TTCAGACACAGAAATCAACTCAGTCTTCCTATATCCAGTTCC-3′; 5′-GGAACTGGATATATCAAGACTGCGTTGATTTCTGTGTCTGAA-3′ and 5′-TTCAGACACAGAAATCAACGCAGTCTTGATATATCCAGTTCC-3′.
Transfections and immunodetection
COS-7 cells were transfected using Fugene6 reagent and lysed in Triton-X lysis buffer supplemented with 5 mM NEM (N-ethylmaleimide; Sigma-Aldrich). HeLa cells were transfected using the calcium phosphate method. Immunodetection was performed as described.3
Quantitative RT-PCR and EMSA
Total RNA was extracted using TRIZOL (Gibco-BRL, Carlsbad, CA). Reverse transcription (RT) was performed using a First strands cDNA synthesis kit (MBI Fermentas, Burlington, ON, Canada). The primers for glyceraldehyde phosphate dehydrogenase (GAPDH), interferon regulatory factor 1 (IRF1), guanylate-binding protein 1 (GBP1), and transporters associated with antigen presentation 1 (TAP1) real-time PCR were previously described.2 Detection of IFN-γ–activated sequence (GAS)–binding proteins by electrophoretic mobility shift assay (EMSA) has been previously described.9
Immunofluorescence detection
Stably transfected U3A clones were serum starved and stimulated with 100 ng/mL huIFN-γ. Cells were fixed in p-formaldehyde (4%) and methanol (100%) and stained with anti–phospho-STAT1 antibody (1:300 dilution) and anti-STAT1 (N-term; 1:1000 dilution) overnight followed by Texas Red and Alexa 488 staining and microscopy fluorescence detection.
Results and discussion
STAT1 is modified by SUMO-1 conjugation at Lys703 in vitro and in vivo in fibroblasts3,4 and in hematopoietic myeloid cells (data not shown). Mutation of Lys703 to arginine (K→R) was found to enhance at variable degree the STAT1-dependent reporter gene activity in STAT1-deficient U3A cells, suggesting a negative regulatory function for sumoylation. To verify that the observed effect on STAT1 activity was due to and specific for SUMO modification, we mutated 2 amino acid residues within the SUMO-1 conjugation consensus IKTE (residues 702-705) motif of STAT1.5 The in vivo SUMO-1 conjugation to the mutants was analyzed by transfecting HA-tagged STAT1-E705A and STAT1-I702R together with SUMO-1 into COS-7 cells followed by immunoblotting with anti–SUMO-1 and anti-HA antibodies (Figure 1A). As expected, STAT1-E705A and STAT1-I702R both failed to conjugate SUMO-1, indicating that the Ile702 and the Glu705 are essential to create the sumoylation motif in STAT1. The transcriptional activities of STAT1-WT, STAT1-K703R, and STAT1-E705A were analyzed using STAT1-dependent GAS-reporter gene assays in U3A cells. Both the K703R and the E705A mutants showed a similar increased transcriptional activity when compared with STAT1-WT (Figure 1B), implying that sumoylation has a repressive role on STAT1 transcriptional activity.
We wished to analyze the underlying mechanism for the effect of sumoylation on transcription. The close vicinity of Lys703 to the phosphorylation site Tyr701 raised the possibility that the bulky SUMO-1 moiety could interfere with the phosphorylation/dephosphorylation events on Tyr701. The K703 is not required for Y701 phosphorylation, since the K703R mutation did not significantly affect the magnitude and kinetics of STAT1 tyrosine phosphorylation in U3A clones (Figure 1C top panel). However, the sensitivity of Western blotting may not be sufficient to detect subtle changes. In stable U3A clones, the STAT1-KR mutation showed an enhanced and prolonged DNA-binding activity after IFN-γ stimulation when compared with STAT1-WT (Figure 1C middle panel).
Next, we wanted to analyze the physiologic effect of sumoylation on some well-characterized IFN-γ–induced genes, such as IRF1, GBP1, TAP1, and TAP2.10-12 Quantitative RT-PCR using RNA from IFN-γ–treated U3A clones demonstrated an enhanced transcription of GBP1 and TAP1 in the STAT1-KR clones when compared with the wild-type clones (Figure 1D). Of interest, no significant differences between STAT1-WT and STAT1-KR were observed in the transcription of IRF1 gene. These results suggest that sumoylation of STAT1 has a selective effect on IFN-γ–meditated gene responses.
Mutation of the SUMO attachment site modulates STAT1-mediated transcription. (A) Point mutations in the SUMO consensus site affect SUMO-1 conjugation to STAT1. COS-7 cells were transfected with 0.7 μg of STAT1-E705A-HA, STAT1-I702R-HA, and STAT1-WT-HA together with SUMO-1 plasmid (0.2 μg) as indicated. After 36 hours the cells were lysed in Triton-X lysis buffer (50 mM tris(hydroxymethyl)aminomethane–HCl [Tris-HCl], pH 7.4; 150 mM NaCl; 1 mM EDTA [ethylenediaminetetraacetic acid]; 50 mM NaF; 5 mM NEM; 1% Triton-X 100; and 10% glycerol and protease inhibitors) and equal amounts of lysates were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted with anti–SUMO-1 and anti-HA antibodies. (B) K703R and E705A mutations increase the transcription activity of STAT1. STAT1-WT-HA, STAT1-K703R-HA, and STAT1-E705A-HA were transfected in U3A cells together with GAS–luciferase (luc) reporter plasmid (0.5 μg) and plasmid cytomegalovirus–βgalactosidase (pCMV-βGAL; 0.25 μg). After 24 hours the cells were starved and stimulated with 10 ng/mL huIFN-γ for 6 hours. The ± SD were calculated as a mean of 2 independent experiments. The bottom panel shows the STAT1 protein levels analyzed by Western blotting using anti-HA antibodies. RLU indicates relative luciferase units. (C) K703R mutation enhances DNA binding of STAT1. U3A STAT1-WT and STAT1-KR stable clones were treated with IFN-γ (100 ng/mL) as indicated and STAT1 DNA binding was analyzed by EMSA with 32P-labeled GAS-IRF1 probe. The bottom panel indicates the STAT1 protein levels by anti-HA immunoblotting. (D) Analysis of GBP1, TAP1, and IRF1 gene activation in U3A STAT1-WT and STAT1-KR stable clones. Total RNA isolated from U3A stable clones stimulated with IFN-γ (10 ng/mL) for different times as indicated was subjected to RT-PCR using specific primers for GBP1, TAP1, and IRF1. The target gene expression levels were normalized against the expression values of GAPDH3. The results were calculated as the mean ± SD of 2 independent experiments for each type of clone.
Mutation of the SUMO attachment site modulates STAT1-mediated transcription. (A) Point mutations in the SUMO consensus site affect SUMO-1 conjugation to STAT1. COS-7 cells were transfected with 0.7 μg of STAT1-E705A-HA, STAT1-I702R-HA, and STAT1-WT-HA together with SUMO-1 plasmid (0.2 μg) as indicated. After 36 hours the cells were lysed in Triton-X lysis buffer (50 mM tris(hydroxymethyl)aminomethane–HCl [Tris-HCl], pH 7.4; 150 mM NaCl; 1 mM EDTA [ethylenediaminetetraacetic acid]; 50 mM NaF; 5 mM NEM; 1% Triton-X 100; and 10% glycerol and protease inhibitors) and equal amounts of lysates were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted with anti–SUMO-1 and anti-HA antibodies. (B) K703R and E705A mutations increase the transcription activity of STAT1. STAT1-WT-HA, STAT1-K703R-HA, and STAT1-E705A-HA were transfected in U3A cells together with GAS–luciferase (luc) reporter plasmid (0.5 μg) and plasmid cytomegalovirus–βgalactosidase (pCMV-βGAL; 0.25 μg). After 24 hours the cells were starved and stimulated with 10 ng/mL huIFN-γ for 6 hours. The ± SD were calculated as a mean of 2 independent experiments. The bottom panel shows the STAT1 protein levels analyzed by Western blotting using anti-HA antibodies. RLU indicates relative luciferase units. (C) K703R mutation enhances DNA binding of STAT1. U3A STAT1-WT and STAT1-KR stable clones were treated with IFN-γ (100 ng/mL) as indicated and STAT1 DNA binding was analyzed by EMSA with 32P-labeled GAS-IRF1 probe. The bottom panel indicates the STAT1 protein levels by anti-HA immunoblotting. (D) Analysis of GBP1, TAP1, and IRF1 gene activation in U3A STAT1-WT and STAT1-KR stable clones. Total RNA isolated from U3A stable clones stimulated with IFN-γ (10 ng/mL) for different times as indicated was subjected to RT-PCR using specific primers for GBP1, TAP1, and IRF1. The target gene expression levels were normalized against the expression values of GAPDH3. The results were calculated as the mean ± SD of 2 independent experiments for each type of clone.
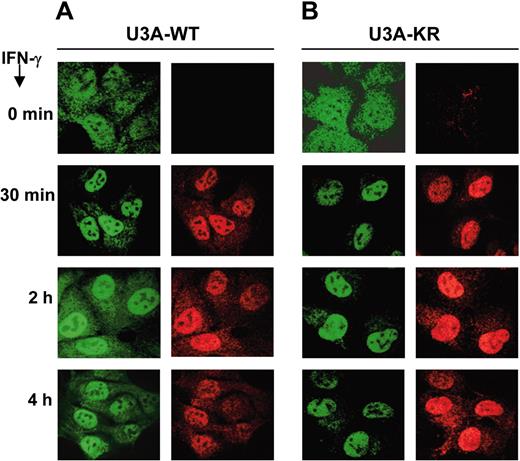
Nuclear translocation is a marker for STAT1 activation. Latent STAT1 resides mainly in the cytoplasm of unstimulated cells and undergoes a rapid and transient nuclear accumulation after IFN-γ stimulation.13 Nucleoplasmic STAT1 is actively exported to the cytoplasm in order to initiate another cycle of activation.14,15 We analyzed the subcellular localization of STAT1-WT and STAT1-KR during IFN-γ stimulation in U3A stable clones using anti-STAT1 and anti–phospho-STAT1 antibodies by immunofluorescence microscopy (Figure 2). After one hour of IFN-γ stimulation, STAT1 is mainly translocated to the nucleus in both types of clones. However, after 2 hours of stimulation, STAT1-WT begins to redistribute to the cytoplasm whereas the STAT1-KR remains predominantly in the nucleus up to 4 hours after the stimulation. These results suggest that SUMO-1 conjugation affects nuclear cytoplasmic redistribution of STAT1, which correlated with the observed differences in the DNA-binding activity. Nuclear export of STAT1 is dependent on functional nuclear export signal (NES) and requires dephosphorylation of STAT1 and dissociation from DNA.14 Even though we did not detect differences in dephosphorylation of STAT1-WT in comparison to STAT1-KR using an in vitro dephosphorylation assay (data not shown), it remains possible that sumoylation affects the dephosphorylation in vivo.
Differences in subcellular localization between STAT1-WT and STAT1-KR after IFN-γ stimulation. U3A STAT1-WT (A) and STAT1-KR (B) stable clones were serum starved overnight and stimulated with IFN-γ (100 ng/mL) for the indicated time points. STAT1 subcellular localization was visualized by immunofluorescence staining using specific anti-STAT1 antibody (left column of each panel) and anti–phospho-STAT1 (Y701) antibody (right column). Images were visualized under an Olympus IX70 confocal microscope equipped with a 100×/1.4 oil immersion objective lens (Olympus, Melville, NY) and an ULTRAPix ICX 085 camera (PerkinElmer, Wellesley, MA). Green emission was detected through 488 mm/10 mm and 525 mm/50 mm filters; red emission, through 568 mm/10 mm and 608 mm/45 mm filters (all filters from Perkin-Elmer Life Sciences, Cambridge, United Kingdom). UltraView 4.0 software (Perkin-Elmer) was used for image acquisition, and PhotoEditor software for Microsoft XP Office (Microsoft, Redmond, WA) was used for image analysis.
Differences in subcellular localization between STAT1-WT and STAT1-KR after IFN-γ stimulation. U3A STAT1-WT (A) and STAT1-KR (B) stable clones were serum starved overnight and stimulated with IFN-γ (100 ng/mL) for the indicated time points. STAT1 subcellular localization was visualized by immunofluorescence staining using specific anti-STAT1 antibody (left column of each panel) and anti–phospho-STAT1 (Y701) antibody (right column). Images were visualized under an Olympus IX70 confocal microscope equipped with a 100×/1.4 oil immersion objective lens (Olympus, Melville, NY) and an ULTRAPix ICX 085 camera (PerkinElmer, Wellesley, MA). Green emission was detected through 488 mm/10 mm and 525 mm/50 mm filters; red emission, through 568 mm/10 mm and 608 mm/45 mm filters (all filters from Perkin-Elmer Life Sciences, Cambridge, United Kingdom). UltraView 4.0 software (Perkin-Elmer) was used for image acquisition, and PhotoEditor software for Microsoft XP Office (Microsoft, Redmond, WA) was used for image analysis.
Posttranslational modifications of STAT1 are highly specific processes as exemplified by the selective effect of STAT1-Ser727 phosphorylation on the expression of IFN-γ–induced genes.2 The physiologic role of sumoylation of STAT1 is also interestingly related to the inhibitory function of PIAS1 on STAT1-mediated gene activation. Recent results from PIAS1–/– mice indicate that PIAS1 can function as a negative regulator of IFN-responsive genes and differentially affect the binding of STAT1 to various promoters of STAT1-dependent genes. PIAS1 had a profound impact on genes that contain weak STAT1 binding sites (GBP1) without affecting promoters with stronger affinity binding sites (IRF1).16 The precise molecular mechanism underlying the PIAS-mediated inhibition of DNA-binding activity is currently not known, but the inhibition is observed in EMSA where no complex formation between PIAS1 and STAT1 is detectable. This finding suggests that PIAS1 may induce modification of STAT1 that would diminish the DNA-binding activity. Our results with the sumoylation-defective STAT1 mutants are in line with the results from PIAS1–/– mice and support the concept that the SUMO E3-ligase function is a physiologic function of PIAS1, although they do not exclude the possibility that PIAS1 could also affect the STAT1 function through other mechanisms.
Prepublished online as Blood First Edition Paper, March 10, 2005; DOI 10.1182/blood-2004-11-4514.
Supported by grants from the Medical Research Council of Academy of Finland, Medical Research Foundation of Tampere University Hospital, the Finnish Foundation for Cancer Research, Tampere Tuberculosis Foundation, and the Sigrid Jusélius Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank P. Kosonen, M. Lehtinen, and M. Paakkunainen for excellent technical assistance.

![Figure 1. Mutation of the SUMO attachment site modulates STAT1-mediated transcription. (A) Point mutations in the SUMO consensus site affect SUMO-1 conjugation to STAT1. COS-7 cells were transfected with 0.7 μg of STAT1-E705A-HA, STAT1-I702R-HA, and STAT1-WT-HA together with SUMO-1 plasmid (0.2 μg) as indicated. After 36 hours the cells were lysed in Triton-X lysis buffer (50 mM tris(hydroxymethyl)aminomethane–HCl [Tris-HCl], pH 7.4; 150 mM NaCl; 1 mM EDTA [ethylenediaminetetraacetic acid]; 50 mM NaF; 5 mM NEM; 1% Triton-X 100; and 10% glycerol and protease inhibitors) and equal amounts of lysates were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted with anti–SUMO-1 and anti-HA antibodies. (B) K703R and E705A mutations increase the transcription activity of STAT1. STAT1-WT-HA, STAT1-K703R-HA, and STAT1-E705A-HA were transfected in U3A cells together with GAS–luciferase (luc) reporter plasmid (0.5 μg) and plasmid cytomegalovirus–βgalactosidase (pCMV-βGAL; 0.25 μg). After 24 hours the cells were starved and stimulated with 10 ng/mL huIFN-γ for 6 hours. The ± SD were calculated as a mean of 2 independent experiments. The bottom panel shows the STAT1 protein levels analyzed by Western blotting using anti-HA antibodies. RLU indicates relative luciferase units. (C) K703R mutation enhances DNA binding of STAT1. U3A STAT1-WT and STAT1-KR stable clones were treated with IFN-γ (100 ng/mL) as indicated and STAT1 DNA binding was analyzed by EMSA with 32P-labeled GAS-IRF1 probe. The bottom panel indicates the STAT1 protein levels by anti-HA immunoblotting. (D) Analysis of GBP1, TAP1, and IRF1 gene activation in U3A STAT1-WT and STAT1-KR stable clones. Total RNA isolated from U3A stable clones stimulated with IFN-γ (10 ng/mL) for different times as indicated was subjected to RT-PCR using specific primers for GBP1, TAP1, and IRF1. The target gene expression levels were normalized against the expression values of GAPDH3. The results were calculated as the mean ± SD of 2 independent experiments for each type of clone.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/1/10.1182_blood-2004-11-4514/4/m_zh80130580400001.jpeg?Expires=1767925111&Signature=ILkieRg954ePHT0J-4~Wohm6dSD6LAU7WW0K7Pj0GW8tBAYiZlHqqIXBuOgDngQgkKqjRk3CHQmfIl93z1YToMPQgL9wmIBcHLTm4Dg4lYtswPcondsfNrTSSRGZm-DY33YACJgxqTg2Y-vM7vJfp2Htgsm3YnJGT~PHlSI9Zxqswi58EPhTvT4e3sePAqnPjRgveRF9LRI8FDBg1oLj7OeLcwSV-J0o3v7q5i8Wv~F14GiYkbpc3zpwWspPLqBdsP26g7VK0~-fxe2dYN4Krfw2lYGrGmbppm5ImFP5kZRUbIdeggt-XJ6xIlY2vKMt-peEqd1G0tlKyiYeG~AqoQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)