Oncogenic mutations of the Kit receptor tyrosine kinase occur in several types of malignancy. Juxtamembrane domain mutations are common in gastrointestinal stromal tumors, whereas mutations in the kinase activation loop, most commonly D816V, are seen in systemic mastocytosis and acute myelogenous leukemia. Kit activation-loop mutants are insensitive to imatinib mesylate and have been largely resistant to targeted inhibition. We determined the sensitivities of both Kit mutant classes to the adenosine triphosphate (ATP)–based inhibitors AP23464 and AP23848. In cell lines expressing activation-loop mutants, low-nM concentrations of AP23464 inhibited phosphorylation of Kit and its downstream targets Akt and signal transducer and activator of transcription 3 (STAT3). This was associated with cell-cycle arrest and apoptosis. Wild-type Kit–and juxtamembrane-mutant–expressing cell lines required considerably higher concentrations for equivalent inhibition, suggesting a therapeutic window in which cells harboring D816V Kit could be eliminated without interfering with normal cellular function. Additionally, AP23464 did not disrupt normal hematopoietic progenitor-cell growth at concentrations that inhibited activation-loop mutants of Kit. In a murine model, AP23848 inhibited activation-loop mutant Kit phosphorylation and tumor growth. Thus, AP23464 and AP23848 potently and selectively target activation-loop mutants of Kit in vitro and in vivo and could have therapeutic potential against D816V-expressing malignancies.
Introduction
Kit is a type III receptor tyrosine kinase (RTK), a subgroup characterized by 5 extracellular immunoglobulin domains and a split kinase domain.1 Other type III RTKs include Fms-like tyrosine kinase 3 (Flt3), platelet-derived growth factor receptor (PDGFR), and colony-stimulating factor (CSF-1) receptor.1 Binding of stem-cell factor (SCF) to Kit induces dimerization and subsequent activation through autophosphorylation of the receptor.2 This event activates several signaling pathways critical to cell survival and proliferation including Src kinases, mitogen-activated protein (MAP) kinases, c-Jun N-terminal kinase (Jnk), signal transducers and activators of transcription (STATs), and phosphatidylinositol 3 (PI3)–kinase/Akt.2
Kit is expressed in hematopoietic progenitors, mature mast cells, germ cells, melanocytes, and interstitial cells of Cajal.1,3 Oncogenic Kit mutations have been reported in diverse malignancies of nearly all tissue types in which Kit is expressed, including gastrointestinal stromal tumors (GISTs), systemic mastocytosis (SM), acute myelogenous leukemia (AML), sinonasal natural killer/T-cell lymphoma, seminomas, and dysgerminomas.3,4 Mutations of Kit that constitutively activate the kinase have been observed in several distinct domains of the receptor including extracellular domains, intracellular regulatory domains, and within the kinase domain.3 The site of mutation generally correlates with the cell type in which it is observed. For example, 50% to 70% of GISTs contain a point mutation or small deletion in the intracellular juxtamembrane domain of Kit,3 a region critical for autoinhibition of the receptor.5 In contrast, SM is characterized by mutations in the activation loop of the Kit kinase domain that constitutively activate the kinase independently of ligand binding, alter substrate specificity, and aberrantly activate multiple signaling pathways.6-10 Nearly all cases of SM express an aspartate-to-valine substitution at codon 816 (D816V).11 Additional substitutions at codon 816 have been reported at lower frequency, including tyrosine (D816Y), phenylalanine (D816F), and histidine (D816H).11,12 Additionally, mutations of the activation loop or exon 8 (coding for part of the extracellular domain) have been observed in some patients with AML, typically concurrent with core binding factor (CBF) mutations.13,14
Current treatment of SM is primarily symptomatic; however, cladribine and interferon alpha have shown some therapeutic success.15,16 Additionally, while CBF-mutant AML generally carries a good prognosis, the presence of a D816V Kit mutation seems to increase the risk of relapse.13 Identification of a potent and selective inhibitor of Kit activation-loop mutants may, therefore, dramatically improve therapeutic options for these patients.
Many adenosine triphosphate (ATP)–competitive inhibitors of wild-type Kit have activity against a subset of Kit mutants and some have been used successfully for the treatment of malignances bearing the respective mutants.3,17 Sensitivity to small-molecule inhibitors, however, depends on the type of mutation. For example, wild-type Kit and juxtamembrane domain mutants of Kit are highly sensitive to imatinib mesylate (hereafter referred to as imatinib).18,19 Consistent with this, patients with GISTs harboring these mutations have shown a 30% to 70% response rate to imatinib.20 In contrast, codon 816 mutants of Kit are completely resistant to imatinib inhibition19,21,22 and patients with SM with D816V Kit mutations are unresponsive to imatinib therapy.23
Several Flt3 inhibitors are active against Kit, including MLN518,24,25 SU5416,26 and PKC412,17 all of which are currently in clinical trials for AML. While MLN518 and some indolinone compounds have activity against D816V,27,28 this mutant is several-fold less sensitive than wild-type Kit, juxtamembrane mutant Kit, and Flt3. Clinically effective doses may, therefore, be difficult to achieve without side effects. Thus far, no Kit inhibitors have been successful in the treatment of SM or D816V-expressing AML.
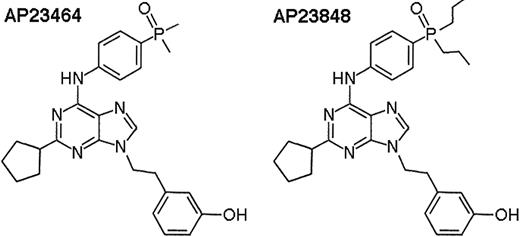
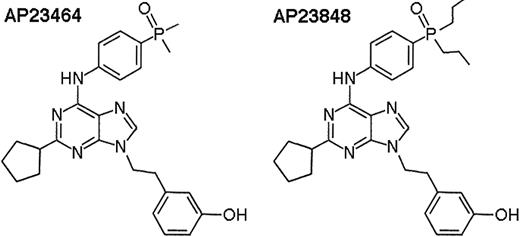
In the present study, we examine the sensitivities of wild-type Kit, juxtamembrane mutant Kit, and D816 mutant Kit to the ATP-based 2,6,9-trisubstituted purines AP2346429 and AP23848 (Figure 1). We demonstrate that these compounds are potent inhibitors of D816 mutant Kit in vitro and in vivo and exhibit selectivity for activation-loop mutant versus wild-type Kit. Our findings introduce this class of inhibitors as strong candidates for future clinical development to target D816V-harboring diseases such as SM and AML.
Materials and methods
Cell lines
Ba/F3 murine pro-B-cells, Mo7e human cells, and P815 murine mastocytoma cells expressing murine D814Y (m-D814Y) Kit were purchased from ATCC (Manassas, VA). The HMC-1 human mast cell line expressing a V560G Kit juxtamembrane mutation and D816V Kit Ba/F3 cells were described previously.28 D816Y- and D816F-expressing Ba/F3 cells were created as follows. D816Y Kit pcDNA3 was kindly provided by Dr Jack Longley (University of Wisconsin). D816F was introduced into full-length, wild-type Kit pcDNA3 (kindly provided by Michael Heinrich) by polymerase chain reaction amplification with primers 5′-CTAGCCAGATTCATCAAGAAT-3′ and 5′-ACCAAAATCACAAATCTTTG-3′ to generate the entire plasmid containing the mutation. Both mutants were cloned as BamHI cassettes into the pLXSN mammalian expression vector (Invitrogen, Carlsbad, CA). Bosc23 (ATCC) cells were transiently transfected with the pLXSN constructs to generate retrovirus. Ba/F3 cells were then transduced with retroviral supernatants and selected first for G418 resistance then for interleukin 3 (IL-3)–independent growth. All transduced Ba/F3 cell lines were maintained in RPMI1640 with 10% fetal bovine serum (FBS). Parental Ba/F3 cells were additionally supplemented with WEHI–conditioned media as a source of IL-3, unless otherwise specified. P815 and HMC-1 cells were maintained in Dulbecco modified Eagle medium (DMEM) with 10% FBS. Mo7e cells were maintained in RPMI1640 and 20% FBS and supplemented with granulocyte-macrophage colony-stimulating factor (GM-CSF) unless otherwise specified.
Chemical structures of the ATP-based trisubstituted purines AP2346429and AP23848.
Chemical structures of the ATP-based trisubstituted purines AP2346429and AP23848.
KIT phosphorylation assays
Cells (107 per inhibitor concentration) were serum starved for 3 hours then treated for 1 hour with AP23464 (0 to 100 nM: D816V Ba/F3, D816Y Ba/F3, D816F Ba/F3, and P815; 0 to 500 nM: HMC-1, Mo7e). Mo7e cells were additionally stimulated with 150 ng/mL human stem-cell factor (Stem Cell Technologies, Vancouver, BC, Canada) for the final 15 minutes of the incubation. Kit was immunoprecipitated from nonidet P–40 (NP40) cell lysates with 2 μg anti-Kit Ab-1 (Oncogene, Cambridge, MA), and the immunoprecipitate was split in half and run on simultaneous gels. Kit phosphorylation was determined by 4G10 (Upstate Biotechnology, Lake Placid, NY) immunoblots. Anti-Kit (Ab-1) was used for loading control. Bands were detected by enhanced chemiluminescence and quantitated on a Lumi-Imager (Roche, Indianapolis, IN). Phosphorylation levels were normalized for Kit loading and reported as the percent of the signal of untreated cells. Graphs and 50% inhibitory concentration (IC50) values are given as the average of 3 independent assays.
In vitro kinase assays
The purified intracellular domains (amino acids 544-end) of wild-type and D816V Kit, fused to glutathione-S–transferase (GST), were purchased from Upstate Biotechnology. Kinase assays were performed according to the SignaTECT Protein Tyrosine Kinase Assay System protocol (Promega, Madison, WI) using biotinylated protein tyrosine kinase peptide 2 from the assay kit as a substrate for both enzymes. Reactions were carried out in the following modified conditions: 8 mM MOPS (3-[N-Morpholino]propanesulphonic acid), pH 7.0; 10 mM Tris-Cl (tris(hydroxymethyl)aminomethane–Cl), pH 7.5; 0.05 mM EDTA (ethylenediaminetetraacetic acid); 0.005 mM EGTA (ethyleneglycotetraacetic acid); 10 mM MnCl2; 13.5 mM sucrose; 0.05 mM benzamidine; 0.01 mM PMSF (phenylmethylsulfonyl fluoride); 0.25% glycerol; 0.002% Brij-35; 0.01% β-mercaptoethanol; 1000 μM ATP; 0.2 μCi (0.0074 MBq) 32Pγ-ATP (Perkin Elmer, Shelton, CT; 3000 Ci/mmol [11.1 × 1013 Bq/mmol]). AP23464 was additionally added to the reaction (0 to 400 nM) and allowed to pre-equilibrate for 5 minutes prior to the addition of ATP. Specific activities for each enzyme were calculated as pmol phosphate transferred/min as analyzed by scintillation counting of the biotinylated peptide bound to streptavidin-coated filters. Counts were corrected for background binding to filters as determined by omitting peptide substrate. Reaction rates were converted to percent inhibition relative to the reaction without inhibitor. Assays were repeated 3 times and averaged.
Cell proliferation assays
Tetrazolium-based proliferation assays were performed as described.30 Briefly, cell viability following 48-hour treatment with AP23464 (0 to 50 nM: D816V Ba/F3, D816Y Ba/F3, D816F Ba/F3, and P815; 0 to 500 nM: Mo7e and HMC-1; 0 to 5 μM: Ba/F3) was determined by spectrophotometric measure of MTT (3-(4,5-cimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) reactivity. Assays were repeated 3 times, each plated in triplicate and averaged.
Cell-cycle analysis
The percentage of cells at each stage of the cell cycle was determined following 24-hour AP23464 treatment (0 to 50 nM: D816V Ba/F3 and P815; 0 to 1000 nM: HMC-1; 0 to 5 μM: Ba/F3). Cells were washed with phosphate-buffered saline (PBS) and fixed in 0.45% NaCl and 45% ethanol and stained with propidium iodide using the Cellular DNA Flow Cytometric Analysis Reagent Set (Roche), and DNA content was analyzed on a Guava PCA (Guava Technologies, Hayward, CA). Histograms were fit for cell-cycle ratios using ModFit software (Verity Software, Turramurra, NSW, Australia). Three independent assays were averaged to determine the percent G0/G1, S, and G2/M at each dose. Cells in the sub-G1 compartment, representing dead cells, were excluded.
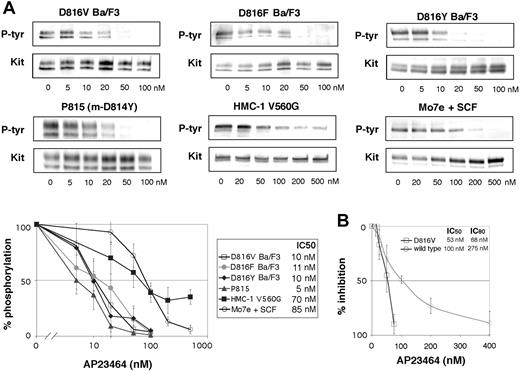
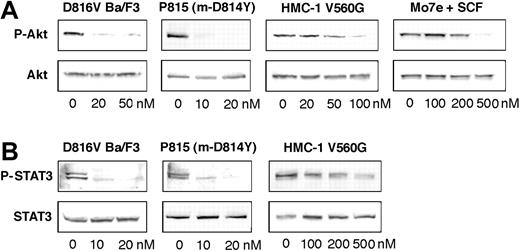
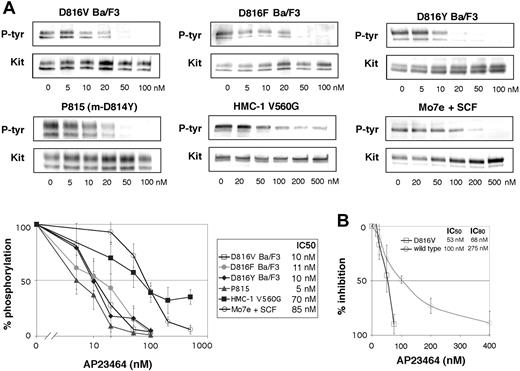
Inhibition of Kit phosphorylation and Kit kinase activity by AP23464. (A) Inhibition of Kit phosphorylation by AP23464. Kit immunoprecipitates from cell lines expressing various Kit mutants were immunoblotted for phosphotyrosine (P-tyr) content in escalating doses of AP23464. Mo7e cells stimulated with SCF were used as a model of activated wild-type Kit. Immunoprecipitates were divided to simultaneously produce total Kit immunoblots as a loading control. Phospho-Kit levels were normalized for total Kit and 3 independent experiments were averaged to calculate IC50 values and to generate the graph shown at bottom. Representative gels are shown for each cell line. (B) Inhibition of Kit kinase activity by AP23464. In vitro kinase assays of peptide substrate phosphorylation were performed with purified intracellular Kit or D816V Kit in escalating concentrations of AP23464. Graphs represent the mean reaction rates shown as percentages of the uninhibited reaction rate. Error bars depict standard deviation of the mean.
Inhibition of Kit phosphorylation and Kit kinase activity by AP23464. (A) Inhibition of Kit phosphorylation by AP23464. Kit immunoprecipitates from cell lines expressing various Kit mutants were immunoblotted for phosphotyrosine (P-tyr) content in escalating doses of AP23464. Mo7e cells stimulated with SCF were used as a model of activated wild-type Kit. Immunoprecipitates were divided to simultaneously produce total Kit immunoblots as a loading control. Phospho-Kit levels were normalized for total Kit and 3 independent experiments were averaged to calculate IC50 values and to generate the graph shown at bottom. Representative gels are shown for each cell line. (B) Inhibition of Kit kinase activity by AP23464. In vitro kinase assays of peptide substrate phosphorylation were performed with purified intracellular Kit or D816V Kit in escalating concentrations of AP23464. Graphs represent the mean reaction rates shown as percentages of the uninhibited reaction rate. Error bars depict standard deviation of the mean.
Apoptosis assays
Induction of apoptosis was determined by analyzing the binding of annexin-V and the incorporation of propidium iodide using the annexin-V–Fluos Staining Kit (Roche) and a FACSAria (BD Biosciences, San Jose, CA) flow cytometer following 48-hour AP23464 treatment (0 to 50 nM: D816V Ba/F3 and P815; 0 to 1000 nM: HMC-1; 0 to 5 μM: Ba/F3). Three independent assays were averaged to determine the percentage of annexin-V–positive cells at each dose.
STAT3 and Akt phosphorylation
STAT3 phosphorylation levels were determined by phospho-tyrosine-705 STAT3 (Santa Cruz Biotechnology, Santa Cruz, CA) and total STAT3 (Santa Cruz Biotechnology) immunoblots of cell lysates treated with AP23464 (0 to 20 nM: D816V Ba/F3 and P815; 0 to 500 nM: HMC-1), as described for Kit phosphorylation assays. Akt phosphorylation levels were determined by phospho-serine-473 Akt (Cell Signaling Technology, Beverly, MA) and total Akt (Cell Signaling Technology) immunoblots. Two or 3 independent assays were performed.
Hematopoietic colony-forming assays
Mononuclear cells from 4 independent commercially available normal bone marrow samples (AllCells, Berkeley, CA) were cultured in duplicate plates with doses of AP23464 ranging from 0 to 1 μM. Cells were cultured in methylcellulose media containing 50 ng/mL SCF, 10 ng/mL GM-CSF, 10 ng/mL IL-3 (Stem Cell Technologies), as previously described,31 to assess granulocyte/macrophage colony formation (CFU-GM). Erythropoietin (3 U/mL; Stem Cell Technologies) was additionally included to assess formation of erythrocyte colonies (BFU-Es). Colonies were counted following 14 days of culture, with more than 50 cells/colony as the criteria for positive colony scoring.
Tumor growth and Kit phosphorylation in a murine tumor model
The following formulation was used for in vivo AP23848 dosing: 15% dimethylacetamide, 14% vitamin E, 5% Tween-80, 26% PEG-400, 40% water, 20 mg/mL AP23848.
Eight- to 10-week-old female DBA/2 mice (Charles River Laboratories, Wilmington, MA) were subcutaneously injected in the hind flank with 1 × 106 syngeneic P815 cells in 100 μL PBS. Mice were monitored daily for tumor formation. On average, tumors were first visible on day 5 and reached 13 mm × 11 mm on day 14. Once tumors were established (day 14), 8 mice were treated 3 times daily by oral gavage with 100 mg/kg AP23848 (100 μL dosing solution) and 3 with carrier for 3 days. Following the final dosing, tumor weight was measured based on the tumor dimensions: weight (mg) = [length (mm) × width2 (mm2)]/2. Four mice from the treatment cohort were killed at 1 hour and 8 hours after dose. Peripheral blood from the treatment and control mice was harvested through heart puncture and plasma was isolated to determine the plasma drug levels. Additionally, tumors were excised and cells were resuspended and lysed in NP40 lysis buffer. Kit phosphorylation status was determined by simultaneous immunoblots with a phospho-Kit–specific antibody, Y568/Y570, and Kit antibody, Ab-1. Phosphorylation levels were normalized for total Kit loading.
Results
AP23464 inhibits cellular phosphorylation and kinase activity of Kit activation-loop mutants
Activation of the Kit kinase through oncogenic mutation results in constitutive receptor autophosphorylation and provides docking sites for the binding and activation of many signaling molecules.2 We examined the effect of AP23464 treatment on the intracellular Kit phosphorylation levels in several cell lines expressing mutant Kit, including Ba/F3 cells engineered to express the activation-loop mutants D816V, D816F, and D816Y, the murine mastocytoma cell line P815 that endogenously expresses m-D814Y (the murine homolog of D816Y), and the human mast-cell line HMC-1 that endogenously expresses a juxtamembrane Kit mutant (V560G). As a model of activated wild-type Kit, we used Mo7e cells stimulated with SCF.32 We observed striking differences in the response to AP23464 between activation-loop mutants and Kit with a wild-type catalytic domain. In Ba/F3 cells expressing D816V, D816F, and D816Y and in P815 cells, AP23464 inhibited Kit phosphorylation at low concentrations, with IC50 values ranging from 5 to 11 nM (Figure 2A), whereas the IC50 for wild-type Kit was 85 nM (Figure 2A). Addition of SCF to D816V Ba/F3 cells did not change Kit phosphotyrosine levels or the IC50 value (data not shown). Juxtamembrane mutant Kit from the human mast-cell line HMC-1 showed a 50% reduction of phosphotyrosine levels at 70 nM. Unexpectedly, however, Kit phosphotyrosine levels did not decrease below 30% of untreated when the concentration was increased (Figure 2A), suggesting that another kinase that is not a target of AP23464 may partially phosphorylate Kit in the HMC-1 cell line. Nonetheless, intracellular Kit phosphorylation in the activation-loop mutants was, on average, 9-fold more sensitive to inhibition by AP23464 than wild-type Kit or juxtamembrane mutant Kit. As autophosphorylation is the primary mechanism of cellular Kit phosphorylation,2 these data demonstrate not only potent inhibition of activation loop Kit mutants by AP23464 but also significant differential sensitivity between wild-type Kit and the Kit mutants.
To verify that the inhibition of Kit phosphorylation was due to direct inhibition of Kit, in vitro kinase assays were performed using purified wild-type and D816V Kit intracellular domains. We observed a dose-dependent inhibition of kinase activity in low-nM concentrations of AP23464, demonstrating that Kit is a direct target of AP23464. Consistent with cellular phosphorylation assays, D816V was more sensitive than wild type to inhibition by this compound. IC50 values for inhibition of peptide substrate phosphorylation were 100 nM and 53 nM for wild-type and D816V Kit, respectively, in the presence of 1 mM ATP (Figure 2B). More pronounced sensitivity differences were apparent at higher inhibitor concentrations, with wild-type and D816V Kit IC80 values at 275 nM and 68 nM, respectively (Figure 2B). While the differential sensitivity between wild type and mutant was not as pronounced as the cellular phosphorylation assays would predict, our in vitro kinase assay does not account for differences in the kinetic parameters of the enzymes. Thus, expanded kinetics studies would be required to precisely define the differences in inhibitor affinities between wild-type and D816V Kit. Nonetheless, we demonstrate that both wild-type and D816V Kit are targets of AP23464.
AP23464 inhibits proliferation of Kit activation-loop mutant–expressing cells
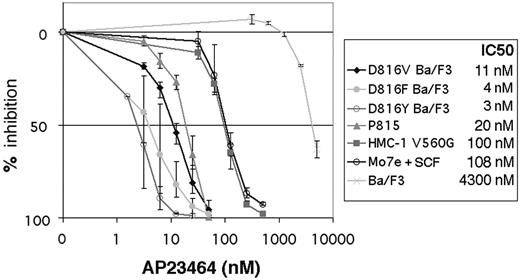
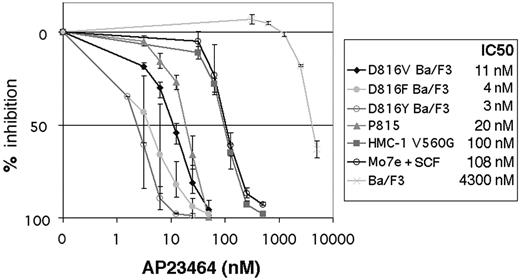
We next determined the effect of AP23464 on the proliferation of cell lines expressing activation-loop mutant and juxtamembrane domain mutant Kit. Mo7e cells were assayed in the presence of SCF as the sole cytokine to determine wild-type Kit–dependent effects. Following 48-hour treatment with AP23464, Ba/F3 cells expressing D816V, D816F, and D816Y and P815 cells (expressing D814Y) demonstrated IC50 values for cell-growth inhibition ranging from 3 nM for D816Y to 20 nM for P815 (Figure 3). The relatively higher IC50 of P815 cells may be the result of higher levels of Kit protein compared with the transduced Ba/F3 cells (data not shown). The human mast-cell line HMC-1, containing a juxtamembrane mutation, and Mo7e cells had IC50 values of 100 nM and 108 nM, respectively (Figure 3), consistent with the higher concentrations of AP23464 required to inhibit Kit activity in kinase assays. In parental Ba/F3 cells, nonspecific toxicity occurred only at several-fold higher concentrations, with an IC50 value for this cell line of 4.3 μM (Figure 3).
AP23464 induces cell-cycle arrest and apoptosis in cells expressing activation-loop mutant Kit
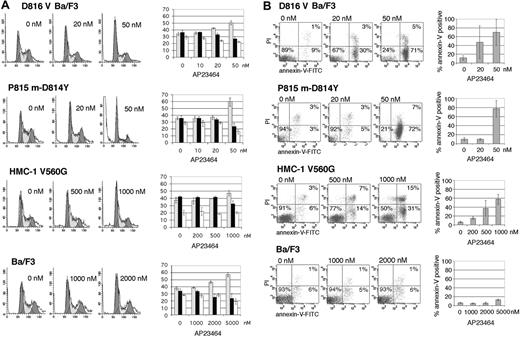
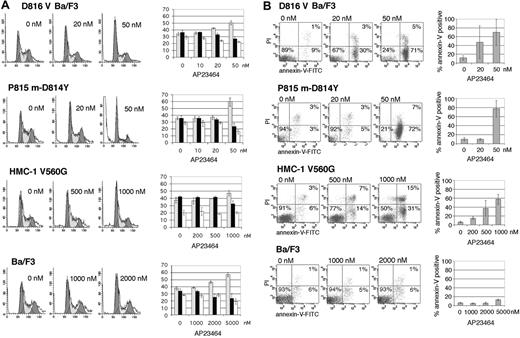
We wished to determine whether loss of mutant Kit activity would induce cell-cycle arrest and apoptosis. Propidium iodide staining following 24-hour treatment with AP23464 showed a dose-dependent increase in the proportion of cells in G0/G1. At 50 nM AP23464, the average number of cells in G0/G1 increased from 35% to 51% in D816V Ba/F3 cells and from 35% to 60% in P815 cells (Figure 4A). Consistent with the proliferation assays, cell-cycle effects in juxtamembrane mutant HMC-1 cells were demonstrable only at higher doses, with a modest increase of the G0/G1 fraction from 37% to 48% following treatment with 1 μM AP23464 (Figure 4A). Parental Ba/F3 cells demonstrated changes in cell-cycle ratios only at AP23464 concentrations of at least 2 μM (Figure 4A). Treatment of D816V Ba/F3 and P815 cells with 50 nM AP23464 for 24 hours resulted in 45% annexin-V–positive cells, whereas 48-hour treatment resulted in an average of 70% and 78% annexin-V–positive cells, respectively (Figure 4B), indicating rapid and pronounced induction of apoptosis. In the juxtamembrane mutant cell line, 48-hour treatment with 1 μM AP23464 resulted in an average of 62% annexin-V–positive cells (Figure 4B). Nonspecific apoptotic effects were not seen in the Ba/F3 parental cell line up to 5 μM AP23464 (Figure 4B). These results further highlight the difference in sensitivity to AP23464 between the activation-loop mutants and the juxtamembrane Kit mutant and indicate that concentrations that have little impact on wild-type Kit–dependent proliferation and SCF-induced phosphorylation can effectively induce cell-cycle arrest and apoptosis in D816V Kit–expressing cells.
Inhibition of Kit-dependent cell proliferation by AP23464. SCF-stimulated Mo7e, juxtamembrane, and activation-loop mutant cell lines as well as Ba/F3 cells (as a control) were grown in graded concentrations of AP23464 for 48 hours, and viable cells were measured by MTT assay. Results are expressed as % of control. Graphs represent the average of 3 independent experiments. Error bars depict standard deviation of the mean.
Inhibition of Kit-dependent cell proliferation by AP23464. SCF-stimulated Mo7e, juxtamembrane, and activation-loop mutant cell lines as well as Ba/F3 cells (as a control) were grown in graded concentrations of AP23464 for 48 hours, and viable cells were measured by MTT assay. Results are expressed as % of control. Graphs represent the average of 3 independent experiments. Error bars depict standard deviation of the mean.
AP23464 abrogates Kit-dependent signaling in cells expressing activation-loop mutants
PI3 kinase has been shown to associate via the p85 subunit with phosphorylated tyrosine 719 in the kinase insert region of Kit.2 Abrogating this interaction or inhibiting PI3 kinase activity can have multiple cell-type–specific effects that include partial impairment of cell proliferation, adhesion, membrane ruffling, actin assembly, and Kit trafficking.2 In D816V mutant–expressing cells, loss of PI3 kinase activation dramatically impairs cell proliferation and induces murine D814Y–driven tumor regression,33 demonstrating that the Kit/PI3 kinase interaction is critical to D816V-mediated transformation. We used Akt phosphorylation as a surrogate marker to assess PI3 kinase activity in response to AP23464 treatment. Akt phosphorylation was substantially reduced at 20 nM AP23464 in D816V Ba/F3 cells and completely eliminated in P815 cells (Figure 5A). Approximately 500 nM AP23464 was required to completely abrogate Akt phosphorylation in Mo7e cells stimulated with SCF (Figure 5A), consistent with higher concentrations required to affect proliferation and Kit phosphorylation. In HMC-1 cells, Akt phosphorylation was down-regulated at somewhat lower concentrations of AP23464 than wild type (Figure 5A), however, no effect was apparent at 20 nM, thus demonstrating a consistent pattern of differential sensitivity between the different mutant classes.
Cell-cycle arrest and induction of apoptosis in Kit-mutant cell lines following AP23464 treatment. (A, left) Juxtamembrane mutant HMC-1 and activation-loop mutant D816V Ba/F3 and P815 cell lines were analyzed for cell-cycle distribution by propidium iodide (PI) staining following 24-hour AP23464 treatment. Ba/F3 cells were used as a control. (Right) The percentages of cells in the G0/G1 (▦), S(▪), and G2/M (□) phases were calculated by fitting flow cytometry data with ModFit software. Cells in sub-G1 were not included in the calculation. Three independent assays were averaged to generate the graphs. Representative histograms are shown. For histograms, x axis indicates DNA content and y axis indicates cell count; for bar graphs, y axis indicates the percentage of cells. (B) Juxtamembrane mutant HMC-1 and activation-loop mutant D816V Ba/F3 and P815 m-D814Y cell lines were assessed for annexin-V binding and PI incorporation as a measure of apoptosis following 48-hour AP23464 treatment. Ba/F3 cells are shown as a control. Three independent assays were averaged to generate graphs. Representative dot plots are shown. Error bars depict standard deviation of the mean. FITC indicates fluorescein isothiocyanate.
Cell-cycle arrest and induction of apoptosis in Kit-mutant cell lines following AP23464 treatment. (A, left) Juxtamembrane mutant HMC-1 and activation-loop mutant D816V Ba/F3 and P815 cell lines were analyzed for cell-cycle distribution by propidium iodide (PI) staining following 24-hour AP23464 treatment. Ba/F3 cells were used as a control. (Right) The percentages of cells in the G0/G1 (▦), S(▪), and G2/M (□) phases were calculated by fitting flow cytometry data with ModFit software. Cells in sub-G1 were not included in the calculation. Three independent assays were averaged to generate the graphs. Representative histograms are shown. For histograms, x axis indicates DNA content and y axis indicates cell count; for bar graphs, y axis indicates the percentage of cells. (B) Juxtamembrane mutant HMC-1 and activation-loop mutant D816V Ba/F3 and P815 m-D814Y cell lines were assessed for annexin-V binding and PI incorporation as a measure of apoptosis following 48-hour AP23464 treatment. Ba/F3 cells are shown as a control. Three independent assays were averaged to generate graphs. Representative dot plots are shown. Error bars depict standard deviation of the mean. FITC indicates fluorescein isothiocyanate.
STAT3 is constitutively tyrosine phosphorylated in cell lines expressing juxtamembrane or activation loop Kit mutants10,34 and most primary GIST samples bearing Kit mutations.35 Additionally, expression of a dominant-negative STAT3 severely impairs D816H-mediated cell proliferation and tumor formation, implying that STAT3 is critical for the tumorigenicity of D816 mutants.9,10 In contrast, SCF-induced STAT3 tyrosine phosphorylation is weak or absent in wild-type Kit–expressing cells and variable between cell types.10,34,36 Consistent with a previous report, we did not observe tyrosine phosphorylation of STAT3 in Mo7e cells following SCF stimulation.36 Constitutive STAT3 phosphorylation in both D816V Ba/F3 and P815 cells was eliminated by 20 nM AP23464 (Figure 5B). Although STAT3 phosphorylation in the juxtamembrane mutant was reduced, it was still apparent at 500 nM, mirroring the incomplete inhibition of Kit autophosphorylation observed at this concentration (Figure 5B). Taken together, these data demonstrate that low concentrations of AP23464 abrogate aberrantly activated signaling pathways critical to D816V-mediated cell survival. Additionally, considerably higher concentrations of AP23464 are required to achieve comparable inhibition of juxtamembrane mutant–and wild-type Kit–induced signal transduction, suggesting that therapeutically active concentrations would not disrupt normal Kit signaling.
Down-regulation of mutant Kit–dependent signaling by AP23464. Cell-lysate immunoblots for (A) phospho-Akt-serine 473 and (B) phospho-STAT3-tyrosine 703 were performed for D816V Ba/F3, P815 m-D814Y, the juxtamembrane mutant HMC-1, and SCF-stimulated Mo7e cell lines following treatment with escalating AP23464 concentrations. SCF stimulation of Mo7e cells did not induce STAT3 tyrosine phosphorylation and thus is not shown. Simultaneous total Akt and total STAT3 blots were done as loading controls. Representative gels are shown.
Down-regulation of mutant Kit–dependent signaling by AP23464. Cell-lysate immunoblots for (A) phospho-Akt-serine 473 and (B) phospho-STAT3-tyrosine 703 were performed for D816V Ba/F3, P815 m-D814Y, the juxtamembrane mutant HMC-1, and SCF-stimulated Mo7e cell lines following treatment with escalating AP23464 concentrations. SCF stimulation of Mo7e cells did not induce STAT3 tyrosine phosphorylation and thus is not shown. Simultaneous total Akt and total STAT3 blots were done as loading controls. Representative gels are shown.
AP23464 does not inhibit normal hematopoietic cell growth at concentrations that target Kit activation-loop mutants
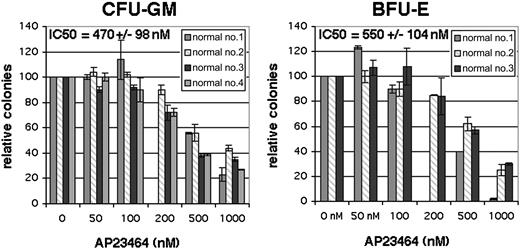
A complete profile of targets of AP23464 and their relative sensitivities to the compound has not been determined. We therefore examined the impact of therapeutically relevant concentrations of AP23464 on hematopoietic colony formation by normal progenitor cells. Bone marrow mononuclear cells from healthy individuals were assessed for colony-forming units–granulocyte/macrophage (CFU-GMs) and burst-forming units–erythroid (BFU-Es) in the presence of escalating doses of AP23464. The IC50 values for inhibition of CFU-GM and BFU-E colony formation were 470 ± 98 nM and 550 ± 104 nM, respectively (Figure 6). At concentrations of 500 nM or greater, a decrease in colony size was apparent in addition to the decrease in colony numbers. No inhibition of colony formation or colony size was observed at concentrations of 100 nM or less AP23464, indicating that doses capable of eliminating cells expressing D816V mutant Kit are unlikely to impact normal hematopoiesis.
Effect of AP23464 on hematopoietic colony formation. Normal bone marrow mononuclear cells were incubated in semisolid media in the presence of SCF, GM-CSF, and IL-3, with or without erythropoietin, with escalating AP23464 concentrations. Granulocyte/macrophage (CFU-GM) and erythrocyte (BFU-E) colony numbers are reported for 4 and 3 bone marrow samples, respectively, as a percentage of the untreated culture. Each data point represents the average of duplicate cultures. IC50 values and standard deviation were calculated from the average of all samples. Error bars depict standard deviation of the mean.
Effect of AP23464 on hematopoietic colony formation. Normal bone marrow mononuclear cells were incubated in semisolid media in the presence of SCF, GM-CSF, and IL-3, with or without erythropoietin, with escalating AP23464 concentrations. Granulocyte/macrophage (CFU-GM) and erythrocyte (BFU-E) colony numbers are reported for 4 and 3 bone marrow samples, respectively, as a percentage of the untreated culture. Each data point represents the average of duplicate cultures. IC50 values and standard deviation were calculated from the average of all samples. Error bars depict standard deviation of the mean.
AP23848 inhibits activation-loop mutant Kit phosphorylation and tumor growth in vivo
Lastly, we determined the efficacy of an AP23464 analog against tumors expressing activation-loop mutant Kit in a murine model. For these studies AP23848 was used based on preliminary pharmacokinetics and metabolism studies (T.K.S. and W.C.S., unpublished data, March 1, 2004) to identify candidates for in vivo testing. Cell proliferation assays demonstrated a comparable inhibitory profile for AP23464 and AP23848 against the relevant Kit mutants (Figure S1A; see the Supplemental Figure link at the top of the online article, at the Blood website). Additionally, AP23848 showed a similar effect on hematopoietic colony formation as AP23464 (Figure S1B). P815 murine mastocytoma cells, syngeneic with DBA/2 mice, have been previously shown to induce tumors following subcutaneous injection.37 Injection of 1 × 106 P815 cells produced tumors in 100% of mice by day 5. Once tumors were established, AP23848 was administered by oral gavage at 100 mg/kg 3 times daily for 3 days. Preliminary pharmacokinetics and toxicity studies on this compound indicated that this dose was optimal to provide proof-of-concept testing in this model. Additionally, an extension of the treatment period was not feasible, since the aggressive DBA/2 mice tolerated the frequent oral gavage regimen poorly, with a 12% weight loss in both treatment and control groups after 3 days of treatment.
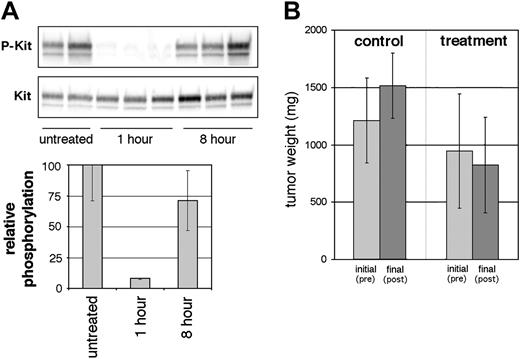
Following the 3-day treatment, m-D814Y Kit tyrosine phosphorylation levels in the tumor cells were assessed at 1 hour and 8 hours following the last dose. Phosphorylation levels at 1 hour were dramatically reduced to an average of 9% of untreated m-D814Y Kit (Figure 7A), indicating successful target inhibition in the tumor tissue. The corresponding average plasma concentration at this time point was 1117 nM. While this value is well above AP23848 concentrations necessary to effectively inhibit Kit phosphorylation in the isolated cell line, it is unknown what fraction of drug is protein bound and thus the actual free drug concentration may be much lower. At 8 hours, the plasma concentration dropped to an average of 70 nM, indicating a short plasma half-life of approximately 2 hours, as well as lack of accumulation over the course of the 3-day treatment regimen. Correspondingly, phosphotyrosine levels in the tumor tissue were restored to the level of untreated mice at the 8-hour time point (Figure 7A). While plasma concentrations at 8 hours after dose would be predicted from the in vitro studies to be high enough to effectively inhibit Kit phosphorylation, it is likely that drug delivery to the largely necrotic tumors was not efficient.
We concurrently monitored tumor size during AP23848 treatment. At the start of treatment there was no significant difference in tumor size between the treated and control groups (P = .27), although the average tumor size was slightly larger in the group that would not receive compound. There was, however, a statistically significant (P = .02) difference in tumor size following treatment in spite of a short half-life and the consequent lack of continuous target inhibition (Figure 7B). Average tumor size of the control group increased by 25% during the 3-day regimen. The average tumor size in the mice that received AP23848 decreased slightly, however, this change was not significant. The difference in tumor growth between the control mice and the mice that received compound was statistically significant (P = .008). This observation indicates that AP23848 inhibits D814Y-dependent tumor growth in vivo.
In vivo inhibition of Kit phosphorylation and tumor growth by AP23848. (A) Murine tumors were induced by subcutaneous injection of the activation-loop mutant cell line P815. Following tumor establishment, mice were treated 3 times daily with 100 mg/kg AP23848. Tumor tissue was obtained from treated mice, 3 at 1 hour and 3 at 8 hours after dose, and from 2 untreated mice, and (top) lysates were immunoblotted with a phospho-specific antibody for Kit Y568/Y570. (Bottom) Phosphotyrosine levels were averaged for each cohort to generate the graph shown. (B) Tumor weight was calculated from the measured dimensions of the tumors before treatment and after treatment at the time that the mice were killed. Average tumor weight for the treated and untreated groups was calculated to generate the graph shown. P values (Student t test) show a statistically significant difference in tumor size between the control and treatment groups following therapy. Error bars depict standard deviation of the mean.
In vivo inhibition of Kit phosphorylation and tumor growth by AP23848. (A) Murine tumors were induced by subcutaneous injection of the activation-loop mutant cell line P815. Following tumor establishment, mice were treated 3 times daily with 100 mg/kg AP23848. Tumor tissue was obtained from treated mice, 3 at 1 hour and 3 at 8 hours after dose, and from 2 untreated mice, and (top) lysates were immunoblotted with a phospho-specific antibody for Kit Y568/Y570. (Bottom) Phosphotyrosine levels were averaged for each cohort to generate the graph shown. (B) Tumor weight was calculated from the measured dimensions of the tumors before treatment and after treatment at the time that the mice were killed. Average tumor weight for the treated and untreated groups was calculated to generate the graph shown. P values (Student t test) show a statistically significant difference in tumor size between the control and treatment groups following therapy. Error bars depict standard deviation of the mean.
Discussion
Activation-loop mutants of the Kit kinase have thus far been largely resistant to inhibition by small-molecule ATP-competitive inhibitors. We demonstrate that the ATP-based trisubstituted purines AP23464 and AP23848 potently inhibit the kinase activity of Kit activation-loop mutants both in vitro and in vivo. Treatment of cell lines expressing activation-loop mutants with 50 nM AP23464 completely eliminated cellular Kit autophosphorylation, inhibited cell proliferation, arrested the cell cycle, and induced apoptosis. In vitro kinase assays revealed direct inhibition of the activation-loop mutant and wild-type Kit kinase, indicating that the biologic effects of AP23464 are the direct result of Kit inhibition.
In addition to constitutive activation of the Kit kinase, mutation of D816V has been shown to amplify the intrinsic catalytic activity38 and alter the substrate specificity of Kit.6 As a consequence, this mutant constitutively stimulates signaling via pathways such as STAT3 and PI3 kinase/Akt, whose activity under normal physiologic conditions is dependent on stimulation with SCF. Abrogation of either STAT3 or PI3 kinase activity downstream of D816V Kit has been shown to severely impair the transforming capacity of this mutant in vitro and in vivo.8-10,33 In activation-loop mutant–expressing cell lines, 50 nM AP23464 eliminated phosphorylation of Akt and STAT3, demonstrating effective suppression of downstream pathways that are crucial to cell proliferation and survival.
In vivo studies showed that AP23848, an analog of AP23464, inhibited activation loop Kit mutant–driven tumor growth and eliminated tumor Kit phosphorylation, further demonstrating the therapeutic potential of this class of compounds. While there was complete inhibition of tumor growth in mice receiving this compound compared with a 25% increase in tumor size in control mice, we did not observe any significant decrease in tumor size in response to treatment. This is likely due to the lack of sustained inhibition of Kit kinase resulting from a short plasma half-life of approximately 2 hours and is reminiscent of the effects of imatinib on tumors expressing breakpoint cluster region–Abelson murine leukemia fusion (Bcr-Abl), where tumor regression requires continuous inhibition of the kinase.39 Consistent with these results, we observed that P815 cells, in culture, require continuous exposure to 100 nM AP23848 for a minimum of 72 hours to prevent resumption of proliferation once the inhibitor is removed (data not shown). Unfortunately, a more intense regimen or more prolonged treatment was poorly tolerated by the DBA2 mice due to toxicity and gavage trauma. However, compounds related to AP23848 with superior pharmacokinetics are currently being developed to overcome this issue.
We additionally demonstrate that higher concentrations of AP23464 inhibit cell proliferation and Kit, Akt, and STAT3 phosphorylation and induce cell-cycle arrest and apoptosis in cell lines expressing wild-type and juxtamembrane mutant Kit. Approximately 10-fold higher concentrations were required to achieve comparable overall inhibition of wild-type Kit–dependent processes compared with cells expressing activation-loop mutants. The heightened sensitivity of D816V versus wild-type Kit suggests that ATP-based trisubstituted purines could not only potently target malignant cells at low doses but also likely do so without disrupting normal SCF/Kit signaling. This property of AP23464 is similar to gefitinib, an epidermal growth factor receptor inhibitor that exhibits far greater inhibitory potential against p-loop and activation-loop mutants of the kinase than the wild-type protein.40 Correspondingly, clinical results of gefitinib treatment of small-cell lung cancer demonstrate that the inhibitor is highly effective only in tumors bearing the sensitizing mutations.40,41 Thus, selectively targeting activating mutations of a Kit with analogs of AP23464 and AP23848 could be equally successful in the treatment of D816V Kit–bearing malignancies.
Crystallographic studies of the Kit kinase domain demonstrate that the fidelity of codon 816 is critical to maintaining the stability of the inactive conformation of the activation loop, thus tightly regulating the kinase activation status.5 In fact, mutation of this residue to nearly any other amino acid activates the kinase independent of ligand binding and increases the catalytic activity by shifting the equilibrium to an open, ATP-accessible conformation of the activation loop.5,7,38 It is likely this feature of the mutant that underlies its resistance to imatinib. In fact, cocrystallization of Kit with imatinib demonstrated that imatinib requires an inactive conformation of the activation loop to bind to the kinase,5 and disruption of this conformation by mutation at codon 816 would thus abrogate imatinib binding. Molecular modeling of the Kit structure based on previous cocrystallization of Kit and imatinib (inactive conformation) and cocrystallization of Src and AP23464 (active conformation) (personal communication with ARIAD Pharmaceuticals) suggests that the inhibitor preferentially binds to the active conformation of Kit. The cyclopentyl group of AP23464 may interfere with binding to the inactive conformation. While this accounts for the inhibitor's activity against D816V, it is unclear why the mutant is hypersensitive relative to wild-type Kit. Mutation of the juxtamembrane region of Kit deregulates autoinhibition of the kinase by that domain; however, the ATP-binding pocket, predicted to contact AP23464, is not altered and this may indicate why wild-type and juxtamembrane mutants of Kit have similar inhibitory profiles. Cocrystallization of wild-type, juxtamembrane mutant, and D816V Kit with AP23464 will be critical to understanding the differences in inhibitory effects against the different mutant classes.
The very similar topologies of the ATP-binding pockets of active tyrosine kinase conformations often result in a loss of selectivity for compounds that target the active conformation.42 It is conceivable that simultaneous inhibition of several cellular kinases increases nonspecific effects and thus toxicity. In separate in vitro kinase assays, IC50 values for AP23464 inhibition of type III receptor tyrosine kinases were 10 nM, 50 nM, and 272 nM for PDGFRa, PDGFRb, and Flt3, respectively (T.K.S., personal e-mail communication with ARIAD pharmaceuticals, February 7, 2005), indicating that the compound does in fact have multiple targets that are hematopoietic kinases. In the presence of multiple cytokines, however, we observed no inhibition of hematopoietic colony formation at concentrations sufficient to inhibit D816V Kit, suggesting that kinases critical to hematopoiesis are either not sufficiently inhibited by AP23464 at low-nM concentrations or that there is enough redundancy of function to overcome the inhibitory effects. This suggests a potential therapeutic window in which malignant cells could be eliminated without disrupting hematopoiesis.
Thus far, only 2 Kit kinase inhibitors, including the indolinone derivatives SU11652, 54, and 55, and the quinazoline-based MLN518, have been shown to have activity against activation-loop mutants of Kit.27,28 While MLN518 is currently in clinical trials for AML with Flt3 juxtamembrane mutations,25 in vivo activity against D816V had not yet been demonstrated, and no clinical studies with D816V-harboring malignancies have been done. Indolinone compounds are also in clinical trials for AML,26 however, SU11652, 54, and 55, specifically, have not been introduced in human studies and their potential for drug development is unknown. These compounds have several-fold lower activity against D816V than against wild-type or juxtamembrane mutant Kit, as well as Flt3 and PDGFR,24,27,28 suggesting that clinically relevant doses, which are higher than currently necessary for treatment of AML, could be difficult to achieve without side effects. Additionally, the IC50 values of both of these compounds against D816V Kit mutants in cellular phosphorylation assays were approximately 150 to 250 nM,27,28 20-fold higher than those of AP23464 and AP23848, suggesting that potent target inhibition by this series of ATP-based small molecules could be achieved at considerably lower doses compared with other potential therapies that are currently in development.
The ATP-based trisubstituted purines AP23464 and AP23848 are the first class of compounds shown to inhibit activation-loop mutants of Kit more potently than wild-type Kit and represent the first example of in vivo target inhibition of this mutant. The in vitro and in vivo efficacy against activation-loop mutants of Kit identifies this class of small-molecule kinase inhibitors as a promising candidate for future clinical development for the treatment of malignancies harboring D816-mutant Kit such as SM or AML.
Prepublished online as Blood First Edition Paper, March 3, 2005; DOI 10.1182/blood-2004-12-4771.
Supported by Howard Hughes Medical Institute, The Leukemia and Lymphoma Society, and The Doris Duke Charitable Foundation. M.W.N.D. is a recipient of a junior faculty award of the American Society of Hematology.
Several of the authors (Y.W., C.A.M., R.S., W.C.S., J.S., S.W., D.D., J.I., and T.S.) are employed by ARIAD Pharmaceuticals, whose product was studied in the present work.
A.S.C., B.J.D., and M.W.N.D. designed the research; A.S.C., S.D., and I.J.G. performed the research; Y.W., C.A.M., R.S., W.C.S., T.S., and M.C.H. contributed vital new reagents; A.S.C., S.D., I.J.G., J.S., S.W., D.D., and J.I. analyzed the data; and A.S.C. and M.W.N.D. wrote the paper.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Chris Koontz and Sarah Anderson for administrative assistance.