Three patients with Chediak-Higashi syndrome underwent allogeneic bone marrow transplantation between the ages of 2 years 9 months and 7 years. The outcome was uneventful, with sustained mixed chimerism. No subsequent recurrent infections or hemophagocytic syndrome were observed. At the age of 22 to 24 years, these 3 patients developed a neurologic deficit combining difficulty walking, loss of balance, and tremor. Neurologic evaluation demonstrated cerebellar ataxia and signs of peripheral neuropathy. Moderate axon loss and rarefaction of large myelinated fibers were observed on semithin sections of peripheral nerve. Cerebellar atrophy was detected by cerebral magnetic resonance imaging in 2 patients. We also reviewed the very long-term outcome of the other 11 patients with Chediak-Higashi syndrome who had received bone marrow transplants at our center since 1981. All displayed neurologic deficits or low cognitive abilities.
Introduction
Chediak-Higashi syndrome (CHS) is a rare autosomal recessive disease characterized by partial oculocutaneous albinism and the occurrence of several dysfunctions of bone marrow-derived cells. This includes a fatal complication, known as accelerated phase or hemophagocytic syndrome, involving multivisceral infiltration by polyclonal activated CD8 T lymphocytes and macrophages.1-6 CHS1/LYST gene is ubiquitously expressed and known to be involved in controlling the exocytosis of secretory lysosomes.2,7,8 Ten years ago, we reported that HLA-identical bone marrow transplantation (BMT) was an acceptable curative treatment for CHS.9 Several other publications have reported similar favorable effects of BMT in patients with CHS.10,11
Various neurologic symptoms have been described in CHS, mostly in young adults.8,12-16 However, no large series of patients with CHS with neurologic expression has been reported because only 10% of patients have a milder form of the disease and survive childhood without BMT. It is unclear whether neurologic manifestations result directly from defective function of lysosome trafficking regulator protein (LYST) in neurons and glial cells, from lymphocyte infiltration into the central nervous system (CNS) in the accelerated phase of the disease, or both.
Study design
We reviewed the neurologic status of the 11 surviving patients with CHS who had undergone BMT at the Necker (10 patients) and Nancy (1 patient) hospitals since 1979. Seven of these patients were included in our initial report.9 Each patient underwent neurologic evaluation associated with a brain magnetic resonance imaging (MRI) study together with electrophysiologic evaluation and muscle and peripheral nerve biopsy when indicated. Chimerism was analyzed using DNA microsatellite markers9 or cytogenetic analysis. Informed consent was obtained from the patients or their families.
Case reports
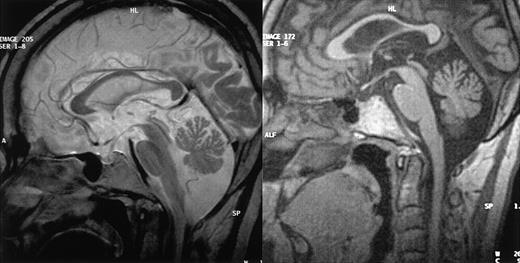
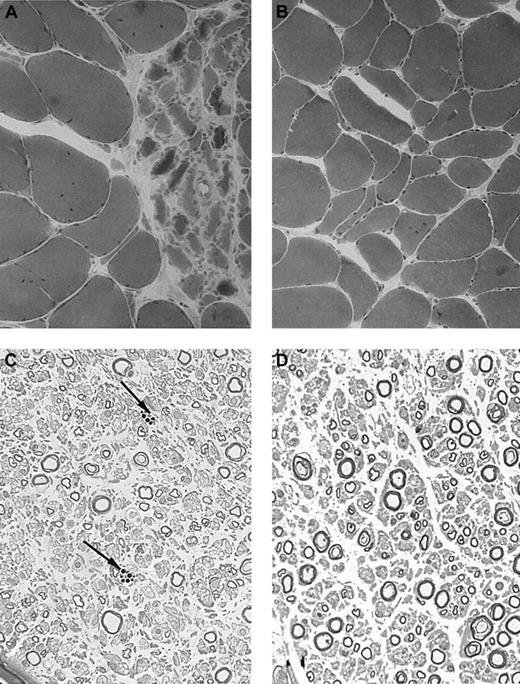
Patient 1. This patient was designated patient 1 in our initial report.9 The diagnosis of CHS was made when he was 2.5 years old and confirmed by the detection of a CHS1/LYST mutation (C3310T leading to R1103X, described in Certain et al5 as patient 2). The patient underwent allogeneic BMT at the age of 5 years. The initial outcome was largely uneventful with mixed chimerism (> 90% of donor-derived cells) that was stable over time.9 The patient is now 28 years old and has had no recurrent infections or hemophagocytic syndrome episodes in the last 23 years. He was unable to follow normal schooling and now works in a protected environment. At the age of 20 years, he developed type 2 diabetes. After the age of 22 years, he complained of increasing difficulty walking and climbing stairs and a loss of balance. Neurologic evaluation when he was 27 years old demonstrated moderate cerebellar ataxia, proximal weakness in all 4 limbs, and absent deep tendon reflexes, but no motor or sensory deficit. Electrophysiologic studies showed moderate distal motor-sensory axonal neuropathy. Cerebral MRI findings consisted of supratentorial and cerebellar volume loss (Figure 1A). A peripheral nerve biopsy specimen is shown in Figure 2B,D.
Patient 2. The patient was patient 2 in our initial report.9 The diagnosis of CHS was made when he was 15 months old; he underwent an allogeneic BMT at the age of 2 years 9 months. The outcome was uneventful, with sustained mixed chimerism (50% of donor-derived cells).9 The patient is now 23 years old and has had no recurrent infections or hemophagocytic syndrome in the last 20 years. His cognitive abilities proved sufficient for the completion of elementary school and to allow him to carry out unspecialized work. At the age of 20 years, he began to complain of difficulties walking, a loss of balance, and tremor of the hands. Neurologic evaluation demonstrated ataxia, nystagmus, and muscle weakness affecting the distal muscles of the arms and legs. Deep tendon reflexes were absent and the patient displayed pes cavus foot deformities and a marked distal sensory and motor deficit. Electrophysiologic evaluation confirmed that the patient had a motor-sensory axonal neuropathy. MRI results were normal. Muscle biopsy showed neurogenic atrophy, whereas peripheral nerve biopsy showed moderate axon loss, the absence of remyelinated fibers and very few endoneural macrophages with dense inclusions.
MRI results. MRI of CNS from patients 1 (left) and 3 (right), in sagittal view, showing cerebellar volume loss in both cases but normal brainstem size.
MRI results. MRI of CNS from patients 1 (left) and 3 (right), in sagittal view, showing cerebellar volume loss in both cases but normal brainstem size.
Patient 3. This patient was not included in our initial report. The diagnosis of CHS was made when she was 5 years old. She had incomplete bilateral blindness due to oculocutaneous albinism. One phase of hemophagocytic syndrome was treated with VP-16 and cyclophosphamide and the patient underwent HLA-identical BMT, with her brother as the donor, at the age of 7 years (conditioning regimen: 16 mg/kg busulfan, 200 mg/kg cyclophosphamide, and antithymocyte globulins). Her subsequent clinical condition was satisfactory. No manifestation of hemophagocytic syndrome occurred after transplantation and sustained mixed chimerism (95% of donor-derived cells) was demonstrated. The patient displayed moderate mental retardation and was able to work in a protected environment. At the age of 24 years, she began to suffer from gait abnormality and falls when walking, myoclonus, and tremor. Symptoms progressed over the next 2.5 years, and the patient is now unable to walk or to deal with daily life without constant help. Neurologic evaluation, when she was 28 years old, demonstrated nystagmus, massive cerebellar ataxia, and absent deep tendon reflexes but no motor or superficial sensory deficit. Electrophysiologic studies showed axon loss in the sural nerve. MRI and neuromuscular biopsy results are shown in Figures 1B and 2A,C.
Histologic results. Muscle and nerve histology in CHS patients 26 (patient 1) and 21 years (patient 3) after transplantation. (A-B) Muscle samples of patient 3 (A) and 1 (B) demonstrate areas of neurogenic atrophy of muscular fibers, which were more prominent in patient 3 (hematoxylin-eosin stain). (C-D) Semithin sections of samples from patient 3 demonstrate moderate rarefaction of large myelinated fibers, few remyelinated fibers, and endoneural macrophages containing dense osmiophilic inclusions (arrows in panel C; Toluidine blue stain). Images were visualized under a Laborlux 5 type 020505030 inverted microscope equipped with a dry periplan 10×/0.25 objective lens (Leitz, Portugal) and an Olympus OM-4T camera (Olympus, Melville, NY). Adobe Photoshop CS.8 software (Adobe, San Jose, CA) was used for image processing.
Histologic results. Muscle and nerve histology in CHS patients 26 (patient 1) and 21 years (patient 3) after transplantation. (A-B) Muscle samples of patient 3 (A) and 1 (B) demonstrate areas of neurogenic atrophy of muscular fibers, which were more prominent in patient 3 (hematoxylin-eosin stain). (C-D) Semithin sections of samples from patient 3 demonstrate moderate rarefaction of large myelinated fibers, few remyelinated fibers, and endoneural macrophages containing dense osmiophilic inclusions (arrows in panel C; Toluidine blue stain). Images were visualized under a Laborlux 5 type 020505030 inverted microscope equipped with a dry periplan 10×/0.25 objective lens (Leitz, Portugal) and an Olympus OM-4T camera (Olympus, Melville, NY). Adobe Photoshop CS.8 software (Adobe, San Jose, CA) was used for image processing.
Other patients
From 1981 to 2003, we carried out BMT in 14 patients with CHS; 11 survived the initial posttransplantation period9 but 2 displayed neurologic lesions immediately after transplantation. One patient died 2 years after transplantation during a relapse of hemophagocytic syndrome (patient 7 in our initial report9 ) and 1 patient was lost to follow-up. The 3 patients described herein were the oldest of the 7 remaining patients. Another patient, now 24 years old, had low academic achievement and started to suffer from gait abnormality, falls when walking, and decreased cognitive abilities at the age of 21. She had received HLA-nonidentical bone marrow from a single antigen-mismatched sibling, with low chimerism (4% of donor-derived cells, patient 8 in the initial report9 ). Three other patients, aged 17, 14, and 2 years, have borderline low IQ score but still have normal neurologic examination results.
Results and discussion
Three patients suffering from CHS developed cerebellar ataxia and peripheral axonal neuropathy more than 20 years after having undergone allogeneic BMT. Transplantation cured the CHS-associated immunologic disease because patients had neither recurrent infections nor manifestations of hemophagocytic syndrome after BMT.
The neurologic symptoms observed were identical to those previously described in adults with CHS with a mild clinical course of the disease and who did not undergo BMT. The very long delay between transplantation and neurologic symptoms and the absence of observed neurologic complications before or at the time of transplantation are not consistent with the possibility that neurologic symptoms resulted from neurologic toxicity of the transplantation procedure at the time of transplantation or from a previous bout of hemophagocytic syndrome in the CNS because hemophagocytic syndrome occurred in only 2 patients with only minor CNS involvement. A persistent intra-CNS hemophagocytic syndrome is also unlikely because no systemic manifestations or associated cerebrospinal fluid abnormalities were observed. Neurologic symptoms therefore most likely resulted from steady long-term progression, despite BMT, of the lysosomal defect in neurons and glial cells. Finally, evaluation of long-term outcome of the other CHS patients who had undergone BMT revealed neurologic deficits or low cognitive abilities in all. The immunologic benefits of BMT for CHS must therefore be weighed against the limitation of cognitive deficit and neurologic deficits occurring later in life despite transplantation.
Prepublished online as Blood First Edition Paper, March 24, 2005; DOI 10.1182/blood-2005-01-0319.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.