Little is known about the long-term consequences of overexpression of the human telomerase reverse transcriptase (hTERT) gene in T lymphocytes. To address this issue, we transduced polyclonal as well as clonally derived populations of naive and memory CD4+ T cells from 2 healthy donors (aged 24 and 34 years) with retroviral vectors encoding green fluorescence protein (GFP) and hTERT (GFP-hTERT) or GFP alone. After transduction, cells were sorted on the basis of GFP expression and cultured in vitro until senescence. T cells transduced with hTERT exhibited high stable telomerase activity throughout the culture period. Relative to GFP controls, minor changes in overall gene expression were observed yet the proliferative lifespan of the hTERT-transduced populations was significantly increased and the rate of telomere loss was lower. Nevertheless, hTERT-transduced cells showed progressive telomere loss and had shorter telomeres at senescence than controls (2.3 ± 0.3 kilobase [kb] versus 3.4 ± 0.1 kb). Furthermore, a population of cells with 4N DNA consisting of binucleated cells with connected nuclei emerged in the hTERT-transduced cells prior to senescence. We conclude that overexpression of hTERT in CD4+ T cells provides a proliferative advantage independent of the average telomere length but does not prevent eventual genetic instability and replicative senescence.
Introduction
Telomeres are specialized structures at the end of eukaryotic chromosomes, which consist of tandem repeats of the sequence T2AG31 and associated proteins2 folded into a telomere loop structure.3 Telomeres are required to maintain chromosomal integrity and prevent end-to-end fusions of chromosomes. When telomeric ends become too short, DNA damage signals from telomeres4 can induce apoptosis or a state of replicative senescence and telomere shortening has been proposed to limit the proliferation of most human cells.5 Telomeres shorten with each round of cell division as a result of failure to completely replicate the 3′ end of chromosomes6,7 as well as other causes.8 The average telomere length in cells from most human tissues decreases with age in vivo and with culture in vitro.9-11 The loss of telomeric DNA with age in T lymphocytes exceeds that in other cell types.12 Limitations in the proliferative potential of the cells of the immune system are consistent with the recent finding that the telomere length in human nucleated blood cells is significantly correlated with mortality caused by infectious diseases.13
The ribonucleoprotein enzyme telomerase is able to synthesize terminal T2AG3 telomeric repeats de novo and thus extend telomeres (for reviews, see Collins and Mitchell14 and Smogorzewska and de Lange2 ). In contrast to most somatic cells with little or no telomerase activity, the cells of the immune system show transient telomerase activity allowing mitogenic and antigen stimulation.15-17 Nevertheless, T lymphocytes maintained in culture have a finite lifespan and undergo replicative senescence typically after 30 to 50 population doublings (PDs) of in vitro culture (for a review, see Effros and Pawelec18 ). Difficulties in propagating T lymphocytes may limit their use for clinical applications that require large cell numbers such as adoptive transfer therapy of infections and malignancies.
Telomerase activity can be stably reconstituted in cells by ectopic overexpression of the hTERT gene, which encodes the catalytic reverse transcriptase subunit of the telomerase enzyme complex. The ectopic expression of hTERT is sufficient to permit some cell types, such as fibroblasts, retinal pigment epithelial cells, and endothelial cells19,20 to overcome the senescence checkpoint and to proliferate indefinitely without detectable changes characteristic of malignant transformation.21,22
We have recently shown that inhibition of endogenous telomerase activity (by expressing a dominant-negative hTERT gene) shortens the lifespan of both human CD8+ and CD4+ T cells.23 These findings support the idea that endogenous telomerase activity is essential for cytogenetic stability and telomere function in human T cells. However, expression of endogenous hTERT requires mitogenic stimulation and does not prevent telomere loss in vivo and in vitro.24,25 In contrast, ectopic expression of hTERT is able to reconstitute a constant high level of telomerase activity and extend the lifespan of T cells.23,26-28
In the present study, we show that ectopic hTERT expression extends the replicative lifespan of CD4+ T cell without preventing overall loss of telomeric DNA. Interestingly, the telomere length in late passage hTERT-transduced cells was shorter than was ever measured in controls. Furthermore, many binucleated cells with chromatin bridges between the nuclei were observed in such late-passage hTERT-transduced CD4+ T cells. We speculate that the observed failure to separate chromosomes and nuclei in such cells reflects loss of telomere function that occurs despite high levels of telomerase. Our results indicate that telomere length in CD4+ T cells is very tightly regulated and that overexpression of hTERT provides only a modest replicative advantage, does not prevent telomere erosion, and does not protect against eventual replicative senescence and chromosomal abnormalities.
Materials and methods
Isolation of naive and memory CD4+ T cells from peripheral blood
Density centrifugation with Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) was used to obtain peripheral blood mononuclear cells (PBMCs) from healthy male and female individuals who gave informed consent. The PBMCs (2-4 × 107 cells) were stained with CD4-phycoerythrin (PE; Becton Dickinson, San Jose, CA) and CD45RA-cyanine 5 (Cy5). Naive CD4+CD45RA+ and memory CD4+CD45RA– T lymphocytes were sorted by fluorescence-activated cell sorting (FACStar Plus, Vantage SE; Becton Dickinson). Cultures were initiated with large numbers (> 100) of cells as well as with single cells and expanded as described (see “T-cell cloning and culture”).
T-cell cloning and culture
Single T cells were deposited by single-cell sorting (FACStar Plus, Vantage SE; Becton Dickinson) into 96 U-bottom plates (Falcon, Becton Dickinson). Cells were cultured in RPMI 1640 medium (Gibco, Grand Island, NY) containing 10% human serum (HS) supplemented with phytohemagglutinin (PHA; Gibco), 100 U/mL recombinant interleukin 2 (rIL-2, Roche, Nutley, NJ), and 1 × 106/mL irradiated (3000 rad) allogeneic mononuclear feeder cells. After 12 to 14 days of culture, positive wells were transferred into 24-well plates in RPMI 1640 medium containing 10% HS and 100 U/mL rIL-2. After one additional round of stimulation with PHA, irradiated feeder cells (1 × 106/mL), and rIL-2 (100 U/mL), enough cells (1-2 × 107) were obtained for transduction and for telomere length analysis. Population doublings (PDs) were calculated from the average cell count, using the following equation: PDs = 10log (number of cells counted after expansion) – 10log (number of cells seeded)/10log2.
Retrovirus-mediated transduction of the hTERT gene into T cells
Gene transfer was achieved using retrovirus-mediated gene transduction. We constructed murine stem-cell virus (MSCV)–based retroviral vectors29 containing the gene for enhanced green fluorescent protein (GFP; Clontech, Palo Alto, CA) under the control of phosphoglycerate kinase (PGK) promoter with or without an internal ribosomal entry site (IRES) element and the full-length hTERT. Helper-free retrovirus pseudotyped with the Gibbon ape leukemia virus envelope for efficient infection of human cells was generated using PG13 packaging cells.30
T cells were stimulated with PHA (Gibco) and irradiated allogeneic mononuclear feeder cells prior to transduction. After 2 to 3 days, the cells were harvested and counted. A total of 0.5 to 1 × 106 T cells of either cloned or polyclonal cultures were then incubated on nontissue culture 24-well plates (Falcon) coated with 6 to 10 μg/cm2 RetroNectin (Takara Shuzo, Otsu, Japan) preloaded with 2 mL retrovirus-containing supernatant supplemented with 100 U/mL rIL-2 (Roche). Plates were coated either at 4°C overnight or at 37°C for 2 hours, subsequently blocked with 2% bovine serum albumin (BSA) for 30 minutes at 37°C, and then washed once with phosphate-buffered saline (PBS) prior to use. After 4 hours, cells were harvested from the wells by vigorous pipetting and washed once with PBS. Cells were resuspended in RPMI 1640 medium (Gibco) containing 10% HS supplemented with 100 U/mL rIL-2 (Roche) and kept in 24-well plates (Falcon) overnight. The transduction procedure was repeated on 2 consecutive days.31 After transduction, cells were expanded for 5 to 6 days and then sorted for GFP expression. The efficiency of transduction was estimated by the percentage of cells expressing GFP to be around 1% to 40%.
Telomeric repeat amplification protocol assay
Telomerase activity was measured using the telomeric repeat amplification protocol (TRAP) assay with an end-labeled telomerase substrate (TS) primer as described.32,33 Cell extracts were obtained from a positive control cell line (K562) and from 1 × 105 quiescent T cells present in cultures 10 days after initial stimulation with PHA (Gibco), 100 U/mL rIL-2 (Roche), and 1 × 106/mL irradiated feeder cells. Extension of the TS primer by telomerase was performed for 30 minutes at room temperature, and the products generated were amplified by 30 cycles of polymerase chain reaction (PCR) at 95°C for 60 seconds, 50°C for 45 seconds, and 72°C for 60 seconds using the ACX-anchored return primer. Half of the amplified products were resolved on a 12% polyacrylamide gel and visualized by a phosphoimaging system (Storm 820; Molecular Dynamics, Sunnyvale, CA).
Analysis of GFP expression
Cells (2-4 × 105) were washed once with PBS (StemCell Technologies, Vancouver, BC, Canada) and resuspended in Hanks balanced salt solution (StemCell Technologies) containing 2% fetal calf serum (FCS; StemCell Technologies) and propidium iodide at 1 μg/mL. Samples were analyzed on a FACSSort flow cytometer (Becton Dickinson).
Telomere fluorescence in situ hybridization and flow cytometry
The average length of telomere repeats at chromosome ends in individual cells from T lymphocyte clones was measured by automated fluorescence in situ hybridization (FISH) and flow cytometry (flow-FISH) as described previously.34
Cell-cycle analysis and cell sorting
Cells (1 × 106) were washed once with PBS (StemCell Technologies) and resuspended in permeabilizing buffer (100 mM Tris [tris(hydroxymethyl)-aminomethane], pH 7.4, 154 mM NaCl, 1 mM CaCl2, 0.5 mM MgCl2, 0.1% Nonidet P-40) containing 5 μM propidium iodide. Samples were analyzed and sorted on the basis of DNA content on a FACStar Plus (Becton Dickinson) cell sorter.
To sort viable lymphocytes on the basis of DNA content, cells were stained with Hoechst 33342. Cells (1 × 106) were washed once with PBS (StemCell Technologies) and resuspended in prewarmed medium. Hoechst 33342 was added to a final concentration of 5 μg/mL. Cells were mixed well and placed in a 37°C water bath. After 30 minutes, cells were spun down in the cold and resuspended in cold Hanks balanced salt solution (StemCell Technologies) containing 2% FCS (StemCell Technologies) and sorted on a FACStar Plus (Becton Dickinson).
Cytospin preparation
Sorted cells were resuspended in 100 μL RPMI 1640 medium (Gibco) containing 10% BSA (Calbiochem-Novabiochem, San Diego, CA) and spun at 200g for 1 minute with high acceleration in a cytocentrifuge (Cytospin-2, Thermo Shandon, Pittsburgh, PA). The slides were air-dried, stained with Wright-Giemsa stain (Hematek 2000; Bayer Diagnostics, Tarrytown, NY) or DAPI (4,6 diamidino-2-phenylindole; Vectashield, Vector Laboratories, Burlingame, CA), and analyzed with an Axioplan 2 light and fluorescence microscope (Zeiss Canada, Toronto, ON, Canada) equipped with an Axiocam MRM digital camera (both from Zeiss Canada, Toronto, ON, Canada). Digital images of cells observed with a 63×/1.4 oil objective lens were acquired using Isis software (Metasystems, Altlussheim, Germany).
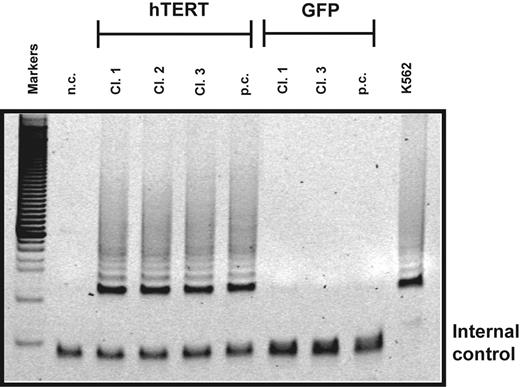
Telomerase activity in transduced T lymphocytes. Telomerase activity in cultured clones (cl.) and polyclonal (p.c.) populations of CD4+ T cells transduced with hTERT or a GFP control vector. After transduction, cells were sorted for GFP expression and further expanded. Two thousand transduced cells were analyzed for telomerase activity using the TRAP assay. T lymphocytes transduced with hTERT exhibited high telomerase activity 10 days after stimulation. At this time point no telomerase activity was detected in GFP control cells. Negative control (n.c.) indicates no cell extract; positive control, extracts obtained from K562 cell line. Internal control shows PCR products amplified from an internal control primer to ensure equal loading of the samples.33 Results shown are representative of 3 separate experiments.
Telomerase activity in transduced T lymphocytes. Telomerase activity in cultured clones (cl.) and polyclonal (p.c.) populations of CD4+ T cells transduced with hTERT or a GFP control vector. After transduction, cells were sorted for GFP expression and further expanded. Two thousand transduced cells were analyzed for telomerase activity using the TRAP assay. T lymphocytes transduced with hTERT exhibited high telomerase activity 10 days after stimulation. At this time point no telomerase activity was detected in GFP control cells. Negative control (n.c.) indicates no cell extract; positive control, extracts obtained from K562 cell line. Internal control shows PCR products amplified from an internal control primer to ensure equal loading of the samples.33 Results shown are representative of 3 separate experiments.
Affymetric gene-expression analysis
RNA from 1 to 2 × 108 expanded T cells was extracted 10 days after initial stimulation, purified, and used for cDNA synthesis using standard procedures. The cDNA was used to synthesize biotinylated cRNA using the BioArray High Yield RNA Transcript Labeling Kit (Enzo Diagnostics, Farmingdale, NY). Labeled cRNA was purified using the RNeasy mini kit (Qiagen, Hilden, Germany). Fragmentation of cRNA, hybridization to HuGeneFL microarrays (Affymetrix, Santa Clara, CA), and washing, staining, and scanning of the arrays in a GeneArray scanner (Agilent, Palo Alto, CA) were performed as recommended in the Affymetrix Gene Expression Analysis Technical Manual. Signal intensities (MAS5 signal) and detection calls for statistical analysis were determined using the Microarray Suite (MAS 5.0) software (Affymetrix). Scaling across all probe sets of a given array to an average intensity of 1000 U was included to compensate for variations in the amount and quality of the cRNA samples and other experimental variables.
Results
hTERT expression increases the lifespan of human CD4+ T cells
Progressive telomere loss limits the expansion of human T lymphocytes in vitro and probably in vivo. Overexpression of hTERT in CD8+ human T lymphocytes results in constitutive telomerase activity and extends their replicative lifespan.23,27,28 To test whether the replicative lifespan of human CD4+ T lymphocytes could also be extended, naive and memory CD4+ T-cell clones as well as naive and memory polyclonal populations of such cells from different donors were purified and transduced with MCSV-based retroviral vectors encoding wild-type hTERT (GFP-hTERT) or GFP as control. After transduction cells were sorted for GFP expression and further expanded by stimulation with PHA and irradiated feeders.
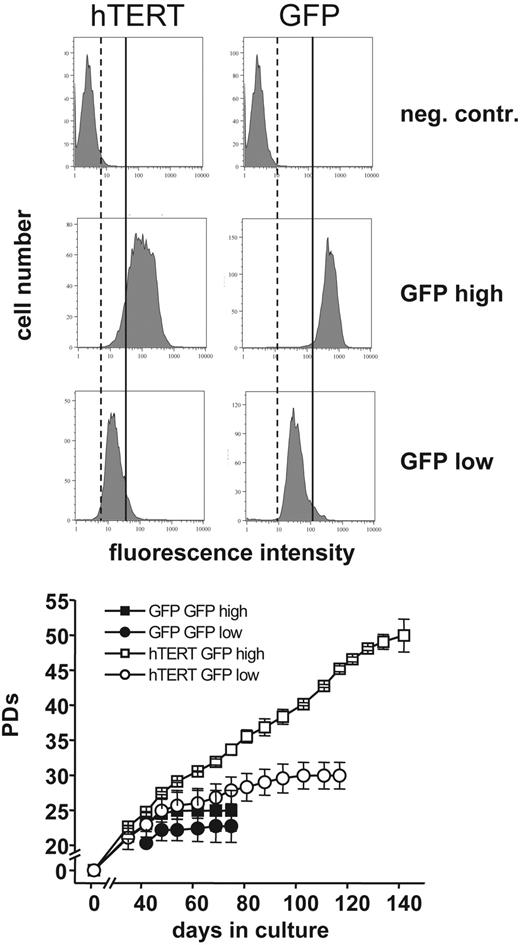
T cells transduced with hTERT showed high and stable telomerase activity 10 days after stimulation (Figure 1). At that time point, no telomerase activity was detectable in GFP control samples. In most of the transduced cells, GFP expression (results not shown) as well as telomerase activity were stably expressed throughout the entire culture period (Figure 2). Populations of CD4+ T cells transduced with the GFP control vector could not be expanded beyond 25 to 60 PDs. In contrast, the proliferative lifespan of hTERT-transduced populations was significantly increased in some cases to over 120 PDs (Figure 3A-B). However, the majority of hTERT-transduced cells eventually died. Similar results were found for naive as well as memory CD4+ T-cell populations (Figure 3C). We also transduced the progeny of a CD4+ T-cell clone with hTERT and GFP to test the significance of transgene expression levels on proliferation. Cells were sorted for high and low GFP expression and subclones were generated. Two subclones of each group were selected and expanded until senescence. No difference in PDs was found for the GFP control-transduced T lymphocytes with high or low GFP expression and the clones reached senescence after 24.9 ± 2.9 PDs and 22.7 ± 2.3 PDs, respectively. Subclones transduced with hTERT and high GFP expression exhibited a significantly higher proliferative potential compared to hTERT-transduced subclones with low GFP expression (49.9 ± 2.9 PDs versus 29.9 ± 1.9 PDs; Figure 4). Interestingly, no differences in telomerase activity between the hTERT-transduced clones were detectable using the TRAP assay. However, analysis of hTERT expression by reverse transcription–PCR (RT-PCR) revealed significant higher expression levels of the transgene in T lymphocytes sorted on the basis of high GFP expression (data not shown). These observations suggest that GFP expression levels correlate with expression levels of the transgene as expected and that high expression levels of exogenous hTERT are required for extending the proliferative capacity of hTERT-transduced CD4+ T lymphocytes.
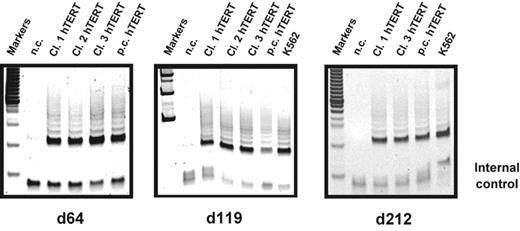
Telomerase activity in hTERT-transduced T lymphocytes during long-term culture. Telomerase activity in cultures of CD4+ naive T-cell clones as well as polyclonal populations transduced with hTERT was analyzed using the TRAP assay. After transduction cells were sorted for GFP expression and further expanded. Two thousand cells of each population were analyzed at different time points during long-term expansion. High stable telomerase activity was detected for all the analyzed samples 10 days after stimulation as indicated throughout the whole culture period (day 64, 119, and 212 after transduction). Negative control (n.c.) indicates no cell extract; positive control, extracts obtained from K562 cell line.
Telomerase activity in hTERT-transduced T lymphocytes during long-term culture. Telomerase activity in cultures of CD4+ naive T-cell clones as well as polyclonal populations transduced with hTERT was analyzed using the TRAP assay. After transduction cells were sorted for GFP expression and further expanded. Two thousand cells of each population were analyzed at different time points during long-term expansion. High stable telomerase activity was detected for all the analyzed samples 10 days after stimulation as indicated throughout the whole culture period (day 64, 119, and 212 after transduction). Negative control (n.c.) indicates no cell extract; positive control, extracts obtained from K562 cell line.
Overexpression of hTERT increases the lifespan of CD4+ T lymphocytes. Polyclonal populations as well as cells derived from a single sorted naive and memory CD4+ lymphocyte from 2 different donors (aged 34 years [A] and 24 years [B]) were transduced with hTERT (right panels) and a GFP control vector (left panels) on the indicated days and cultured until no further growth was obtained. Population doublings (PDs) were determined by counting viable cells once a week. The proliferative lifespan of the hTERT-transduced populations was increased to over 120 PDs in polyclonal as well as clonally derived naive CD4+ cells (A and B) as well as CD4+ memory T cells (C, results obtained with cells from donor B) transduced with hTERT compared with GFP controls that stopped proliferation after 25 to 60 PDs. Accumulated total PDs are shown in panel C, whereas the number of PDs relative to the days in culture are shown in panels A and B.
Overexpression of hTERT increases the lifespan of CD4+ T lymphocytes. Polyclonal populations as well as cells derived from a single sorted naive and memory CD4+ lymphocyte from 2 different donors (aged 34 years [A] and 24 years [B]) were transduced with hTERT (right panels) and a GFP control vector (left panels) on the indicated days and cultured until no further growth was obtained. Population doublings (PDs) were determined by counting viable cells once a week. The proliferative lifespan of the hTERT-transduced populations was increased to over 120 PDs in polyclonal as well as clonally derived naive CD4+ cells (A and B) as well as CD4+ memory T cells (C, results obtained with cells from donor B) transduced with hTERT compared with GFP controls that stopped proliferation after 25 to 60 PDs. Accumulated total PDs are shown in panel C, whereas the number of PDs relative to the days in culture are shown in panels A and B.
PDs and GFP expression of hTERT-transduced T lymphocytes. The progeny of one naive CD4+ T-cell clone was transduced with hTERT and a GFP control vector. After transduction cells were sorted for GFP expression and subclones with high and low GFP expression were obtained. Two subclones of each were selected and further expanded. GFP expression was analyzed and PDs were determined by counting viable cells once a week. The subclone transduced with hTERT showing high GFP expression exhibited a significantly higher proliferative potential compared to the subclone with low GFP expression (49.9 ± 2.3 PDs versus 29.9 ± 1.9 PDs). Dotted and solid vertical lines are shown for comparison; left of dotted line indicates no GFP expression, between dotted and solid lines indicates low levels of GFP expression, and right of solid line indicates high GFP expression. No difference in PDs was found for the GFP control-transduced T-lymphocyte clones with high or low GFP expression (24.9 ± 2.9 PDs versus 22.7 ± 2.3 PDs). Error bars indicate mean ± standard deviation of duplicate measurements.
PDs and GFP expression of hTERT-transduced T lymphocytes. The progeny of one naive CD4+ T-cell clone was transduced with hTERT and a GFP control vector. After transduction cells were sorted for GFP expression and subclones with high and low GFP expression were obtained. Two subclones of each were selected and further expanded. GFP expression was analyzed and PDs were determined by counting viable cells once a week. The subclone transduced with hTERT showing high GFP expression exhibited a significantly higher proliferative potential compared to the subclone with low GFP expression (49.9 ± 2.3 PDs versus 29.9 ± 1.9 PDs). Dotted and solid vertical lines are shown for comparison; left of dotted line indicates no GFP expression, between dotted and solid lines indicates low levels of GFP expression, and right of solid line indicates high GFP expression. No difference in PDs was found for the GFP control-transduced T-lymphocyte clones with high or low GFP expression (24.9 ± 2.9 PDs versus 22.7 ± 2.3 PDs). Error bars indicate mean ± standard deviation of duplicate measurements.
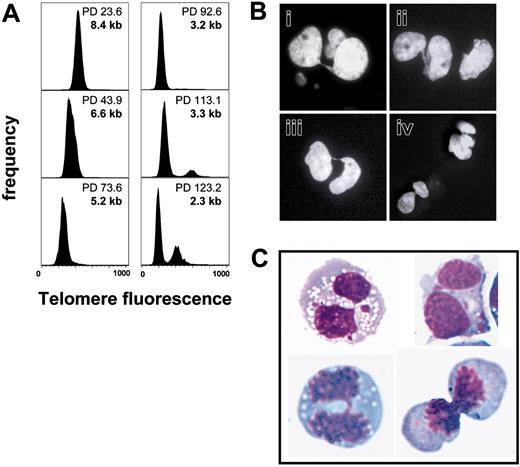
Telomere length analysis of transduced CD4+ T lymphocytes. Representative results of polyclonal CD4+ T cells transduced with GFP control (A) and hTERT (B) as well as a clone transduced with hTERT (C) measured by automated flow-FISH. The GFP control-transduced polyclonal population exhibited a decline of telomere length (111 bp/PD) and stopped at 54.0 PDs as indicated at a telomere length of approximately 3.4 ± 0 kb. There was also a decline of telomere length for the hTERT-transduced polyclonal population. The telomere loss was less over time compared to the GFP control (59 bp/PD [B], 58 bp/PD [C]). At later passages, hTERT-transduced polyclonal populations and clones exhibited shorter telomeres than were ever measured in the GFP controls (2.9 ± 0.25 kb [B], 2.3 ± 0.3 kb [C] versus 3.4 ± 0 kb [(A]). Error bars indicate mean ± standard deviation of duplicate measurements.
Telomere length analysis of transduced CD4+ T lymphocytes. Representative results of polyclonal CD4+ T cells transduced with GFP control (A) and hTERT (B) as well as a clone transduced with hTERT (C) measured by automated flow-FISH. The GFP control-transduced polyclonal population exhibited a decline of telomere length (111 bp/PD) and stopped at 54.0 PDs as indicated at a telomere length of approximately 3.4 ± 0 kb. There was also a decline of telomere length for the hTERT-transduced polyclonal population. The telomere loss was less over time compared to the GFP control (59 bp/PD [B], 58 bp/PD [C]). At later passages, hTERT-transduced polyclonal populations and clones exhibited shorter telomeres than were ever measured in the GFP controls (2.9 ± 0.25 kb [B], 2.3 ± 0.3 kb [C] versus 3.4 ± 0 kb [(A]). Error bars indicate mean ± standard deviation of duplicate measurements.
Lifespan extension without net telomere lengthening in hTERT-transduced T lymphocytes
The average telomere length was measured in serial passages of transduced T-cell clones by automated flow-FISH. As shown in Figure 5, a progressive decline in telomere length was observed in all populations that were analyzed indicative of telomere loss over time. The control population of GFP+ polyclonal CD4+ T cells reached senescence after 54.0 PDs at a telomere length of approximately 3.4 ± 0 kilobase (kb) (Figure 5A) with a calculated telomere attrition rate of 111 base pair (bp)/PD. Despite high and stable telomerase activity, loss of telomere repeats over time was also observed for all hTERT-transduced populations. However, the calculated telomere loss/PD was lower than in the GFP controls (59 bp/PD [Figure 5B] and 58 bp/PD [Figure 5C] versus 111 bp/PD [Figure 5A]) and the lifespan of most of the hTERT-transduced populations was increased. Interestingly, hTERT-transduced populations at later passages exhibited shorter telomeres than were ever measured in the GFP controls (2.9 ± 0.3 kb and 2.3 ± 0.3 kb versus 3.4 ± 0.1 kb in panels B, C, and A, respectively).
Binucleated cells in late passages of hTERT-transduced CD4+ T lymphocytes
Telomere length measurement of hTERT-transduced CD4+ T lymphocytes revealed a progressive decline of telomere length during expansion in long-term culture. Surprisingly, telomere fluorescence measurements of late-passage hTERT-transduced cells revealed a population of cells with twice average the telomere fluorescence once the average telomere length reached approximately 3.3 kb. Moreover, the size of this population increased with further population doublings and continued shortening of the average telomere length (Figure 6A). Cells with double the amount of telomere fluorescence were never observed in control cells transduced with GFP alone. DNA analysis of late-passage hTERT-transduced cells showed a high percentage of cells with 4N DNA, which were sorted and analyzed by microscopy. Many binucleated cells with chromatin bridges between nuclei were observed (Figure 6B). Similar results were obtained with live cells sorted on the basis of staining with Hoechst 33342 (Figure 6C).
Telomere length analysis by automated flow-FISH of CD4+ T-cell clones transduced with hTERT. Naive CD4+ T-cell clones were transduced with hTERT and a GFP control vector. After transduction cells were sorted for GFP expression and further expanded. Telomere length was measured by automated flow-FISH. (A) Telomere fluorescence histograms at different PDs. Once the average telomere length reached approximately 3.3 kilobase (kb), a population of cells with double the telomere signal was observed. Interestingly, this population also increased in size on further culture. (B) Cell-cycle analysis also revealed an increasing fraction of cells with 4N DNA with further increase of PDs. The cells were sorted and cytospins were prepared. DNA was stained with DAPI and analyzed with a Zeiss Axioplan II fluorescence microscope. Chromatin bridges between nuclei were observed, suggesting that cells failed to complete anaphase. (C) To analyze live cells, samples were also stained with Hoechst 33342. Cells were sorted for their DNA content, cytospins were prepared and stained with Wright-Giemsa stain. Again, binucleated cells with chromatin bridges between nuclei were observed.
Telomere length analysis by automated flow-FISH of CD4+ T-cell clones transduced with hTERT. Naive CD4+ T-cell clones were transduced with hTERT and a GFP control vector. After transduction cells were sorted for GFP expression and further expanded. Telomere length was measured by automated flow-FISH. (A) Telomere fluorescence histograms at different PDs. Once the average telomere length reached approximately 3.3 kilobase (kb), a population of cells with double the telomere signal was observed. Interestingly, this population also increased in size on further culture. (B) Cell-cycle analysis also revealed an increasing fraction of cells with 4N DNA with further increase of PDs. The cells were sorted and cytospins were prepared. DNA was stained with DAPI and analyzed with a Zeiss Axioplan II fluorescence microscope. Chromatin bridges between nuclei were observed, suggesting that cells failed to complete anaphase. (C) To analyze live cells, samples were also stained with Hoechst 33342. Cells were sorted for their DNA content, cytospins were prepared and stained with Wright-Giemsa stain. Again, binucleated cells with chromatin bridges between nuclei were observed.
Gene expression profiles in early- and late-passage transduced CD4+ T cells
Ideally, the consequences of hTERT overexpression should be studied using relevant functional T-cell assays. We did observe that both GFP- and hTERT-GFP–transduced CD4+ T cells maintained a strict requirement for IL-2 and irradiated allogeneic cells to stimulate their proliferation. To explore possible differences in gene expression resulting from the hTERT overexpression, we compared the gene expression in the clonal progeny of a CD4+ T cell before and after transduction with GFP and hTERT-GFP vectors. The overall gene expression pattern was strikingly similar between the 4 cell populations tested (National Center for Biotechnology Information GSE2230) and of 20 000 transcripts tested fewer than 200 were significantly increased or decreased between the different cell types. The most striking differences in gene expression between cells that overexpress hTERT and untransduced or GFP-transduced cells are shown in Tables 1 and 2. However, no major differences between hTERT or control cells or between early or late-passage hTERT-transduced cells were observed in the expression of some of the genes known to be involved in DNA repair or cell-cycle control (Table 3). This observation does not exclude differences in the activity of the proteins encoded by these genes because in many cases this activity is known to be controlled at the protein level.
Discussion
Our studies show that ectopic expression of hTERT in human CD4+ T cells can be used to increase the replicative lifespan of such cells and obtain large numbers in culture. Nevertheless, none of the hTERT-transduced CD4+ T-cell populations could be expanded for more than 200 PDs in contrast to human fibroblasts transduced with the same vector (results not shown). Furthermore, despite high levels of telomerase activity throughout the culture period, a progressive loss of telomeric DNA was observed and the telomere length in late passages of such cells was extremely short. However, compared to GFP controls, the telomere loss in hTERT-transduced CD4+ T cells was less per PD. These observations suggest that hTERT overexpression in human CD4+ T lymphocytes increases the replicative lifespan of such cells but does not prevent overall loss of telomere length. Of note, the consequences of telomerase overexpression appear to differ between CD4+ T cells (this study) and CD8+ T cells.23,27,28 Whereas hTERT overexpression in CD8+ T cells frequently resulted in net telomere elongation and immortalization, we observed only an increased replicative potential and no net telomere elongation in CD4+ T cells. These observations suggest that hTERT overexpression has different consequences in CD4+ and CD8+ T cells, which warrant further studies in direct comparisons.
The finding that ectopic expression of telomerase can extend the replicative lifespan of certain cell types without causing a net elongation of telomere length is not without precedent.19,20,26,35 Furthermore, the telomere length in some human fibroblasts transduced with hTERT were also found to continue to shorten to an average length below those of untransduced cells that entered replicative senescence and crisis.36,37 Our observations support the notion that hTERT and telomerase have other roles in the viability and growth of cells beyond preventing a decline in the average telomere length.38-42 One possibility is that telomerase is required not only for elongation of the 3′ end of chromosomes but has a role in the repair of damage to telomeric DNA as well.43
Overexpression of hTERT in CD4+ T cells did not result in marked global changes in gene expression and the expression of genes involved in cell-cycle regulation or repair of DNA damage was not notably changed (Table 3). Among the genes that did show an overall change in expression pattern, the HERC5 gene (up-regulated in hTERT-transduced T cells; Table 1) appears of interest. HERC5 plays a role in regulation of cyclin-dependent protein kinase activity and the ubiquitin cycle (like HERC6) and is associated with cell-cycle regulation (G1- to S-phase transition). HERC genes contain a domain that is involved in chromosome condensation (RCC1).44 We further found down-regulation of several chemokine receptors. This might indicate that hTERT-transduced lymphocytes are less dependent on chemokine stimulation. Especially CCR5 was down-regulated. Activation of CCR5 can lead to both the de novo expression of FAS ligand (FASL) and induction of susceptibility to Fas-mediated apoptosis in CD4+ T cells.45
The average telomere length in a cell will reflect the balance between factors that elongate and those that shorten telomeric DNA at individual chromosome ends in the cells of interest and its immediate progenitors. The level of telomerase activity, subcellular localization of telomerase, recruitment of telomerase to the telomere, the structure of terminal telomeric DNA, levels of telomere-binding proteins, and levels of telomerase RNA all have been proposed to influence the elongation of the telomeres by telomerase.2,14 Likewise, the loss of telomeric DNA is expected to have many causes.8 In antigen-specific CD4+ T cells the loss of telomeres and telomerase activity could be subject to further control by the action of cytokines.46 The data described in this report as well as these previous studies support the notion that telomerase plays a key role in the biology of T cells. Given the many levels at which the telomere length in T cells appears to be controlled, a clear picture of the precise role of exogenous telomerase in the biology of CD4+ T cells is not expected in the near future.
Telomerase activity is believed to promote chromosomal stability primarily by acting at the very ends of chromosomes. Our results suggest that overexpression of hTERT in cells with critically short telomeres does not result in cell-cycle arrest or apoptosis43,47 and allows further proliferation despite chromosomal instability eventually leading to telomere dysfunction and anaphase arrest.48-50 The observed telomeric dysfunction may trigger chromosomal fragmentation through bridge-breakage events and could lead to a transformed phenotype.51 The risk of such transformation events is additive to the risk of insertional mutagenesis inherent in gene transfer strategies.52 These risks of ectopic hTERT expression in T cells have to be balanced against the advantage of increased expansion of T-cell clones that specifically react with tumor or viral antigens. Such cells could be used for adoptive transfer therapy in cancer patients to increase the number of effector cells that may mediate regression of tumors or influence severe viral infections.53-58
Prepublished online as Blood First Edition Paper, March 1, 2005; DOI 10.1182/blood-2004-10-4144.
Supported by grants from the National Institutes of Health (AI29524) and the National Cancer Institute of Canada with funds from the Terry Fox Run. A.R. was funded by a grant from the Deutsche Forschungsgemeinschaft. G.M.B. was funded by a grant from the Swiss National Science Foundation (31-53774-98) and the Bernese Cancer League (AI29524).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Keith Humphries (Terry Fox Laboratory, Vancouver, BC, Canda) for help with the construction of retroviral vectors, Dr Robert Weinberg (Massachusetts Institute of Technology, Boston, MA) for hTERT cDNA, and Dr Ludger Klein-Hitpass (Institute for Cell Biology, Essen, Germany) for help with gene expression analysis.

![Figure 3. Overexpression of hTERT increases the lifespan of CD4+ T lymphocytes. Polyclonal populations as well as cells derived from a single sorted naive and memory CD4+ lymphocyte from 2 different donors (aged 34 years [A] and 24 years [B]) were transduced with hTERT (right panels) and a GFP control vector (left panels) on the indicated days and cultured until no further growth was obtained. Population doublings (PDs) were determined by counting viable cells once a week. The proliferative lifespan of the hTERT-transduced populations was increased to over 120 PDs in polyclonal as well as clonally derived naive CD4+ cells (A and B) as well as CD4+ memory T cells (C, results obtained with cells from donor B) transduced with hTERT compared with GFP controls that stopped proliferation after 25 to 60 PDs. Accumulated total PDs are shown in panel C, whereas the number of PDs relative to the days in culture are shown in panels A and B.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/1/10.1182_blood-2004-10-4144/4/m_zh80130580350003.jpeg?Expires=1765887834&Signature=n5yXkI8fFzgITMTR6E-PQmEuNNVJ-8~l70~-Ct6rj08PfUxK6cBVYnKjSB7oI6sKMh5RF~RRxCaZNSGJGfpUp4QmYKLczhQk113H511bYRbbPz0OmknKlsfsoynenJbGrrJYKkHABzQ~ChgnRuL4q79Mrcc1-iSbZ-xVx-iQBZfPHMtfhTuMqLxFL12g2PC3TdyW7DWK4kEiWo2KcFCWvPC1caanuTNG7uMkbDhvUhuSb2-4ycP0zKB9fRzkQmqBiYgokPjYObFf8eA3f~IJBP01j6-MjpcMjCZPXVoMpiEt2Ufh8Yl3wJobhKcmAIwfzkY~sXAPytBaZ4KOTP9CmQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Telomere length analysis of transduced CD4+ T lymphocytes. Representative results of polyclonal CD4+ T cells transduced with GFP control (A) and hTERT (B) as well as a clone transduced with hTERT (C) measured by automated flow-FISH. The GFP control-transduced polyclonal population exhibited a decline of telomere length (111 bp/PD) and stopped at 54.0 PDs as indicated at a telomere length of approximately 3.4 ± 0 kb. There was also a decline of telomere length for the hTERT-transduced polyclonal population. The telomere loss was less over time compared to the GFP control (59 bp/PD [B], 58 bp/PD [C]). At later passages, hTERT-transduced polyclonal populations and clones exhibited shorter telomeres than were ever measured in the GFP controls (2.9 ± 0.25 kb [B], 2.3 ± 0.3 kb [C] versus 3.4 ± 0 kb [(A]). Error bars indicate mean ± standard deviation of duplicate measurements.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/1/10.1182_blood-2004-10-4144/4/m_zh80130580350005.jpeg?Expires=1765887834&Signature=nkt1sBhf962eNOVkjxkA1NWlzDxTztd0S5BehT5ktBSDxuImmVGMVib2m8dOI~P4UtZmWY1c4mn075Ta52M-DI2P82zjzW0f1mTGBpjYalK5gHXEaD2PaPdT126U6ZguZ1Vu7a5GI0Jxn5zcoBdOSKwVCU6ikn3jrklsxYKHmCiQZ21Ec1YNNzaq-xotCttPRDT1x-orBfPXd6oWS1qMoHjNLWsCs08zM9G7Ols8kzSxg2cDYmjBzBjArwIPD4eeK1CO5-yL6idUhIHAwd-9QUnfcY2eFBLmzd-~cI7d4aAGDoMCQ0~6dffQ3ijJzt28S-RMM4zGw5SImyek5lhgVw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)