Comment on Gale et al, page 3658
An elegant analysis of FLT3 mutational status in a large cohort of acute myeloid leukemia patients assesses the role of transplantation for these poor-prognosis patients.
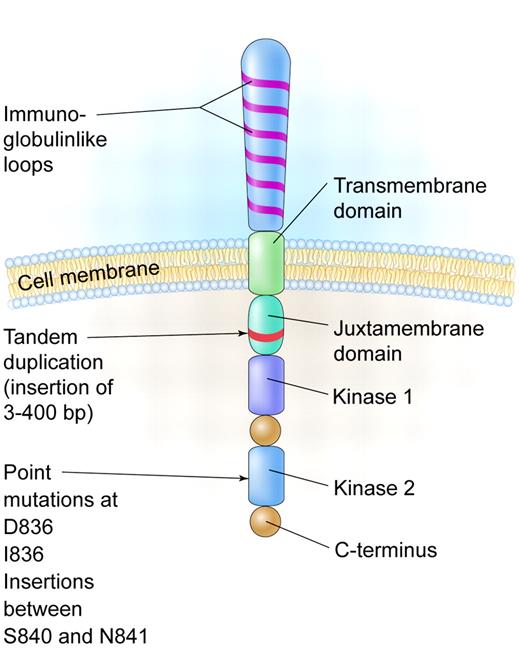
The FMS-like tyrosine kinase 3 (FLT3) is expressed on the cell surface of hematopoietic progenitors and its ligand can be found in either a soluble form or a membrane-bound form on bone marrow stromal cells. In 1996, it was reported that an internal tandem duplication (ITD) of base pairs within the juxtamembrane coding portion or point mutations in the second kinase domain (KDM) could result in constitutive activation of the gene in acute myeloid leukemia (AML) patients (see figure). These abnormalities are seen in up to 30% of patients with AML and especially in those with normal cytogenetics and with acute promyelocytic leukemia, making it one of the most frequently identified molecular abnormalities in AML.1
To assess the significance of these mutations, large observational studies of cohorts of AML patients were evaluated to determine the frequency of FLT3 mutations and its impact on prognosis. A meta-analysis of 4 of the largest series has confirmed that FLT3 mutations have an adverse impact on disease-free and overall survival in patients with AML.2
In clinical practice, a frequent approach to patients with poor prognostic AML is to offer allogeneic stem cell transplantation (SCT) in the hopes that the graft-versus-leukemia effect can overcome the high rate of relapse in these patients. However, a subset analysis of large trials examining different modes of consolidation therapy for patients with AML including chemotherapy or SCT has not born out that allogeneic SCT can overcome the adverse prognosis.3
Gale and colleagues from the Medical Research Council (MRC) of the United Kingdom have now addressed the issue of whether patients with AML with a FLT3/ITD have an improved outcome if they undergo SCT compared with patients receiving chemotherapy. Patient material from the MRC AML-10 and -12 trials was retrospectively analyzed for an FLT3/ITD mutation, and patient outcomes were compared depending on the type of therapy received. Patients with FLT3/KDM were not included in this study, although it appears that these patients have a similar prognosis to patients with an ITD.2 As one might predict, the relapse rate in FLT/ITD+ patients receiving either an autograft or an allograft compared with chemotherapy was lower, but these lower relapse rates did not translate into an overall survival advantage. Though in the allografted patients there was a suggestion that allografting reduced the risk of relapse, the authors rightly point out that these data may be subject to bias based on the retrospective nature of the study and relatively small numbers. This trend of a reduced relapse risk was not born out in the donor–versus–no donor analysis. Thus, while it is not clear from this study that SCT is uniquely effective in patients who are FLT3/ITD+, SCT clinicians may still want to take FLT/ITD or KDM status into account when deciding whether a patient should undergo transplantation given the overall poorer prognosis of these patients and lack of good alternative therapies at the present time.FIG1
A schematic diagram of the FLT3 receptor tyrosine kinase showing the location of the internal tandem duplication of genes within the juxtamembrane domain and point mutations and gene insertions in the second kinase domain. Illustration by Kenneth Probst.
A schematic diagram of the FLT3 receptor tyrosine kinase showing the location of the internal tandem duplication of genes within the juxtamembrane domain and point mutations and gene insertions in the second kinase domain. Illustration by Kenneth Probst.
Several inhibitors of FLT3 have entered clinical trials and demonstrated leukemic cytoreduction, although most responses have been modest.4 These modest responses have raised the question as to how central a role FLT3 mutations play in the leukemogenesis of AML. The presence of an FLT3 mutation in AML patients is sometimes not stable between diagnosis and relapse and suggests the possibility that these mutations may be secondary rather than primary events.5
The FLT3 small molecule inhibitors described in the preceding paragraph are currently being studied in combination with chemotherapy in patients with AML, and it is hoped that these studies will improve the response rate in these poor-prognosis patients. ▪