Abstract
Mycobacterium bovis bacillus Calmette-Guérin (BCG) has been used to treat bladder cancer for almost 30 years; however, the effector mechanism of the BCG-induced antitumor response remains enigmatic. Most BCG research has focused on the mononuclear-cell infiltrate, but growing evidence supports a role for neutrophils in the antitumor response. Previously, we demonstrated increased urinary tumor necrosis factor (TNF)–related apoptosis-inducing ligand (TRAIL/Apo-2L) levels from BCG-responsive patients compared to nonresponders. Interestingly, neutrophils isolated from the urine expressed TRAIL/Apo-2L, leading us to investigate the neutrophil response to BCG. BCG-stimulated neutrophils expressed surface-bound and released functional soluble TRAIL/Apo-2L. Whereas neither interferon α (IFN-α) nor IFN-γ directly induced TRAIL/Apo2L expression by neutrophils, IFN-α did stimulate TRAIL gene transcription, and IFN-primed neutrophils contained and released more TRAIL/Apo-2L after BCG stimulation than did unprimed neutrophils. In unstimulated neutrophils TRAIL/Apo-2L was present predominantly in the azurophilic granules and plasma-membrane–enriched/secretory-granule fraction. Finally, we observed that killed BCG, Toll-like receptor 2 (TLR2) and TLR4 agonists, and an M tuberculosis cell-wall fraction were each capable of inducing the release of soluble TRAIL/Apo-2L from neutrophils. These results further characterize the potential role neutrophils may play in initiating the antitumor response described with BCG treatment for superficial bladder cancer.
Introduction
Urothelial carcinoma of the bladder accounts for approximately 5% of all cancer deaths in humans.1 A majority of the tumors (70%-80%) are superficial at diagnosis and, after local surgical therapy, have a high rate of local recurrence (70%) and progression (30%). Thus, patients require lifelong medical follow-up examinations with inspections of their bladders, and additional surgical resection and prophylactic treatments are typically needed in the presence of recurrence. Current treatments extend time to recurrence but do not alter disease survival. The resulting economic burden on the US health care system is enormous, reaching over $4 billion annually. Indeed, as measured on the basis of cumulative per patient cost from diagnosis until death, the cost to treat bladder cancer exceeds all other forms of human cancer.
In 1921, bacillus Calmette-Guérin (BCG) was isolated from Mycobacterium bovis, a mycobacterium causing bovine tuberculosis.2 Since 1976, BCG has been used with increasing success for the treatment of superficial bladder cancer and has become the treatment of choice for this tumor entity.3 Despite its worldwide acceptance for the treatment of superficial bladder cancer, and many mechanistic studies performed, the antitumor effector mechanism remains incompletely defined. BCG therapy results in a massive local immune response characterized by the induced cytokine expression in the bladder tissue and urine,4 and an influx of granulocytes and mononuclear cells into the bladder wall.5,6 A proinflammatory, Th-1 cytokine (eg, interleukin 2 [IL-2], IL-12, interferon γ [IFN-γ]) response typically predominates following BCG stimulation, and the generation of a Th-1 cytokine response is often associated with a favorable response.7-9 Investigation of the antitumor effects of BCG has focused on the mononuclear infiltrate, and the contribution of the granulocyte infiltrate has been largely overlooked. Recently, our laboratory observed the expression of tumor necrosis factor (TNF)–related apoptosis-inducing ligand (TRAIL/Apo-2L) on neutrophils in voided urine following BCG therapy,10 suggesting a potential role for the granulocyte influx in the direct antitumor effects of BCG immunotherapy. TRAIL/Apo-2L, a member of the TNF superfamily, induces apoptotic cell death in a variety of tumor cells but has little to no cytotoxicity against normal cells or tissues.11,12 Expression of functional TRAIL/Apo-2L can be induced on T cells, B cells, natural killer (NK) cells, dendritic cells, Mϕ, and neutrophils after IFN-α and IFN-γ stimulation.13-19 Because BCG induces IFN production within the bladder during intravesical immunotherapy, studies were initiated to investigate whether BCG directly or indirectly induces the expression or release (or both) of TRAIL/Apo-2L from neutrophils.
Materials and methods
Mycobacterium bovis BCG and Mycobacterium fractions
MV261 BCG, a Pasteur strain previously transfected with the kanamycin resistance plasmid pMV261,20 was obtained from Dr Yi Luo (University of Iowa, Iowa City, IA) and maintained at 37°C in 7H9 Middlebrook broth (Difco, Detroit, MI) supplemented with 10% albumin dextrose concentrate (5% bovine serum albumin [BSA], 2% dextrose, and 0.85% NaCl), 0.05% Tween 80 (Sigma, St Louis, MO), and 30 μg/mL kanamycin. One absorbance unit at 600 nm for the BCG culture was calculated as 2.5 × 107 colony-forming units (CFUs) and represents 5 × 108 particles. Heat-killing was carried out by culturing the BCG at 70°C for 1 hour. Mycobacterium fractions were obtained from Dr John Belisle (Colorado State University, Ft Collins, CO) through the Tuberculosis Vaccine Testing and Research Materials Contract.
Isolation of neutrophils
Neutrophils were isolated from peripheral blood of healthy donors after informed consent was obtained in accordance with University of Iowa Institutional Review Board guidelines and in a manner consistent with the tenets of the Declaration of Helsinki. Neutrophils were separated from peripheral-blood mononuclear cells (PBMCs) by Ficoll-Paque Plus (Pharmacia, Uppsala, Sweden) centrifugation, followed by 3% dextran (Amersham, Piscataway, NJ) sedimentation as previously described.21 After hypotonic lysis of erythrocytes with ice-cold distilled H2O for 28 seconds, an equal volume of 1.8% NaCl was added to restore isotonicity. After centrifugation, neutrophils were cultured in RPMI supplemented with 10% fetal bovine serum (FBS), kanamycin, sodium pyruvate, nonessential amino acids, and HEPES (N-2-hydroxyetyhylpiperazine-N′-2-ethanesulfonic acid).
Neutrophil stimulation with BCG, TLR agonists, M tuberculosis fractions, or IFN
Neutrophils (3 × 106/mL) were incubated in the presence or absence of BCG or heat-killed BCG (1 CFU/cell); the Toll-like receptor (TLR) agonists Pam3CSK, ultra-pure lipopolysaccharide (LPS), or polyinosine-polycytidylic acid (poly I:C) (InvivoGen, San Diego, CA); purified M tuberculosis fractions; or IFN-α or IFN-γ (100 or 1000 U; PeproTech, Rocky Hill, NJ) for the indicated times. In some cases, neutrophils were pretreated with cycloheximide (10 μg/mL) or actinomycin-D (1 μg/mL) for 1 hour before adding BCG. After stimulation, the neutrophils were centrifuged, and supernatants/cell lysates (prepared by lysing the cells in phosphate-buffered saline [PBS] containing 1% Nonidet P-40 and Complete Mini protease inhibitors [Roche Diagnostics, Indianapolis, IN]) were analyzed by enzyme-linked immunosorbent assay (ELISA) for TRAIL/Apo-2L (Cell Sciences, Canton, MA) or IL-8 (R&D Systems, Minneapolis, MN).
Flow cytometry
To stain cells, 100 μL cells were combined in a 96-well round-bottom plate with 2 μL biotinylated RIK-2, IgG1 anti–human TRAIL (1 mg/mL; provided by Dr H. Yagita, Juntendo University, Tokyo, Japan); DREG-56, fluorescein isothiocyanate (FITC)–conjugated IgG1 anti–human CD62L (eBioscience, San Diego, CA); or IgG1-biotin or -FITC isotype controls (Caltag Laboratories, Burlingame, CA), and incubated at 4°C for 30 minutes. Following 3 washes with PBS containing 2 mg/mL BSA and 0.02% NaN3, 2 μL phycoerythrin (PE)–labeled streptavidin (1:50 dilution; Caltag Laboratories) was added for 30 minutes. To measure intracellular TRAIL/Apo-2L, neutrophils (106 cells/well) were stimulated with BCG for 4 hours and then incubated with 100 μL Cytofix/Cytoperm (BD Biosciences, San Diego, CA) solution for 20 minutes at 4°C in a round-bottom microplate. After washing with Perm/Wash buffer (BD Biosciences), 2 μL biotinylated RIK-2 or IgG1-biotin isotype controls diluted in Perm/Wash buffer was added for 30 minutes at 4°C. Following 2 washes with Perm/Wash buffer, 2 μL PE-labeled streptavidin (1:50 dilution; Caltag Laboratories) was added for 30 minutes. After washing 3 times in Perm/Wash buffer, cells were immediately analyzed. Cell analysis was performed on a FACScan (Becton Dickinson, Franklin Lakes, NJ) with more than 104 cells analyzed per sample.
Neutrophil fractionation by N2 cavitation
Subcellular fractionation of neutrophils was performed using nitrogen cavitation to disrupt cells and centrifugation on a Percoll gradient to isolate organelles as previously described.21 Isolated neutrophils were suspended at 30 × 106/mL in PBS and treated with diisopropylfluorophosphate (DFP; 1 mM) for 20 minutes at room temperature to inhibit endogenous serine proteases. Neutrophils were centrifuged, resuspended at 100 × 106/mL in 1 × RB (relaxation buffer [pH 7.3] 10 mM piperazine diethanesulfonic acid [PIPES], 100 mM KCl, 3 mM NaCl, and 3.5 mM MgCl2), and placed in ice-cold cavitation apparatus. The bomb (Parr Instrument, Moline, IL; no. 4639 bomb, 45 mL) was pressurized to 350 psi with N2 and allowed to equilibrate for 20 minutes at 4°C. The cells were then slowly expelled dropwise into a tube containing EGTA (ethylene glycol tetraacetic acid) for a final concentration of 1.25 mM. The sample was centrifuged at 200g for 10 minutes at 4°C to separate any unbroken cells and nuclei from the postnuclear supernatant. The postnuclear supernatant was layered atop a discontinuous Percoll gradient made of heavy Percoll (density, 1.12 g/mL) overlaid with light Percoll (density, 1.05 g/mL) and centrifuged at 48 400g for 15 minutes at 4°C. In addition to the organelle-free cytosol at the top of the gradient, neutrophil contents were separated into different fractions in the gradient, containing azurophilic granules (α-band), specific granules (β-band), and a fraction enriched for plasma membranes and secretory vesicles (γ-band). The fractions were aspirated from the gradient and washed free of Percoll with RBEGTA (relaxation buffer containing 1.25 mM EGTA).
Quantitative real-time PCR
For quantitative real-time polymerase chain reaction, total RNA was isolated from 107 untreated neutrophils or neutrophils stimulated for 20 hour with either BCG (1 CFU/cell) or IFN-α (1000 U/mL) with TRIzol reagent (Life Technologies, Gaithersburg, MD). Total RNA (1 μg) was reverse-transcribed using Superscript II reverse transcriptase (RT; Invitrogen, Carlsbad, CA). The real-time quantitative RT-PCR primer/probe sets for TRAIL/Apo-2L and rRNA were purchased from PE Applied Biosystems (Foster City, CA). cDNA (250 ng) was used as a template for TaqMan assay of TRAIL/Apo-2L transcripts and the internal control of rRNA.22
Immunoblotting
Freshly isolated neutrophils (5 × 106) were lysed in 300 μL lysis buffer (1% NP-40 and Complete Mini protease inhibitor tablet [Roche] in PBS). The lysates were boiled in 6 × nonreducing loading buffer for 5 minutes, separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose, and blocked overnight at 4°C in 5% nonfat dry milk in PBS-Tween 20 (0.05% vol/vol). The membrane was incubated with rabbit anti–human TRAIL antiserum (PeproTech), washed, and then incubated with a donkey anti–rabbit horseradish peroxidase (HRP) antibody (Amersham) and developed by chemiluminescence (SuperSignal West Pico Chemiluminescence Substrate; Pierce, Rockford, IL).
To demonstrate caspase-8 cleavage, neutrophils (5 × 106 cells/mL) were incubated in the absence or presence of BCG (1 CFU/cell) in polypropylene tubes for 15 hours, after which the supernatant was recovered by centrifugation. The neutrophil-conditioned medium was then added to the human bladder tumor cell line RT-4 (obtained from Dr Scott Crist, University of Iowa, Iowa City, IA) in 24-well plates (2 × 105 cells/well) for 8 hours. Cells were lysed, and the lysates were centrifuged at 14 000g to remove cellular debris. Samples were electrophoresed and transferred to nitrocellulose, and the membrane was incubated with an anti–caspase-8 monoclonal antibody (mAb; provided by Dr Marcus Peter, University of Chicago, IL) for 1 hour. After washing, the membrane was incubated with an anti–mouse HRP antibody (Amersham) for 1 hour. Following several washes, the blots were developed by chemiluminescence.
Killing of RT-4 bladder tumor cells using BCG-stimulated neutrophil-conditioned medium
Neutrophils (5 × 106 cells/mL) were incubated in the absence or presence of BCG (1 CFU/cell) in polypropylene tubes for 15 hours, after which the supernatant was recovered by centrifugation. The neutrophil-conditioned medium was then added to the human bladder tumor cell line RT-4 in microtiter plates (4 × 104 cells/well) for 18 hours, after which cell death was determined by crystal violet staining.23 To immunodeplete TRAIL/Apo-2L protein in the conditioned medium, the anti-TRAIL mAb M181 (Amgen, Seattle, WA; 20 μg) was added to equal volumes of supernatant for 2 hours at 4°C, followed by the addition of protein G Sepharose beads (Sigma) for 15 hours at 4°C. The beads were pelleted, and the TRAIL-depleted supernatant was then added to RT-4 cells. Caspase-8 activity was measured after 8 hours using a carboxyfluorescein-labeled leucine-glutamic acid-threonine-aspartic acid (LETD) fluoromethyl ketone (Immunochemistry Technologies, Bloomington, MN). Briefly, cells were incubated with the 1 × FLICA (fluorochrome inhibitor of caspases) solution for 1 hour at 37°C, and then immediately analyzed on a FACScan. In some experiments, zVAD-fmk (20 μM) was added to the RT-4 cells 15 minutes before adding neutrophil-conditioned medium.
Results
Stimulation of neutrophils with BCG results in TRAIL /Apo-2L expression
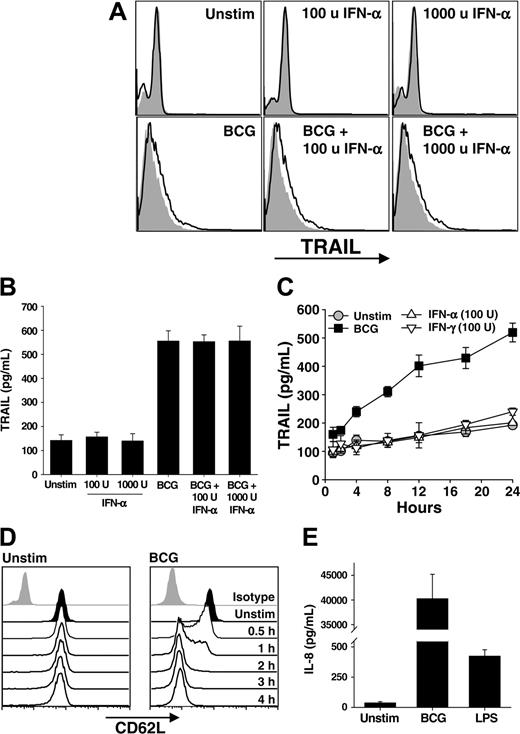
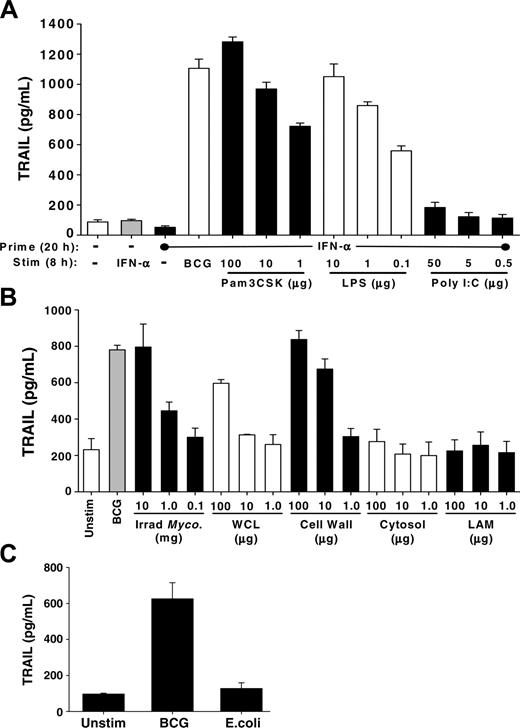
Following BCG instillation into the bladder, a massive influx of granulocytes occurs followed by the influx of mononuclear cells. The idea of an antitumor role for neutrophils in BCG immunotherapy is plausible with our recent demonstration that neutrophils isolated from the urine of a patient undergoing BCG immunotherapy expressed TRAIL/Apo-2L,10 leading us to hypothesize that the neutrophil response to BCG contributes to the antitumor activity seen in this clinical setting. Thus, we directly stimulated neutrophils with BCG, IFN-α, or the combination of BCG and IFN-α for 4 hours and then analyzed the cells for surface TRAIL/Apo-2L by flow cytometry and tested the supernatants for soluble TRAIL/Apo-2L using ELISA. There was a slight, but reproducible, increase in surface-bound TRAIL/Apo-2L on all groups of neutrophils stimulated with BCG (Figure 1A). Examination of the supernatants from these same groups revealed nearly a 4-fold increase in soluble TRAIL/Apo-2L detected compared with those from unstimulated neutrophils (Figure 1B). Interestingly, IFN-α alone did not increase TRAIL/Apo-2L expression on or release from neutrophils, which is contrary to previous reports,19,24 nor was there an additive or synergistic response when the combination of BCG and IFN-α was used. To further examine the response of neutrophils to BCG stimulation, we analyzed the response for 24 hours. Soluble TRAIL/Apo-2L increased with BCG stimulation throughout the 24 hours examined, whereas IFN-α or IFN-γ stimulation did not induce TRAIL/Apo-2L release (Figure 1C), indicating that the failure to see an IFN effect (Figure 1B) was not because of differences in the kinetics of TRAIL expression.
To verify that BCG was activating the neutrophils, we examined CD62L shedding and IL-8 secretion after stimulating with BCG or LPS. Neutrophils shed CD62L upon activation through proteolytic cleavage,25,26 and alterations in CD62L levels were seen as early as 30 minutes after addition of BCG, and CD62L was virtually absent by 2 hours (Figure 1D). Neutrophil activation with LPS produced similar kinetics of CD62L shedding (data not shown). Activated neutrophils secrete IL-8,27 and BCG-stimulated neutrophils produced approximately 40 ng/mL IL-8 after 24 hours (Figure 1E), which is consistent with previous reports.28 By comparison, just over 400 pg/mL IL-8 was measured after LPS stimulation. Collectively, these results demonstrate BCG to be a potent stimulator of TRAIL/Apo-2L expression and release from human neutrophils.
Neutrophils stimulated with BCG, but not IFN, release TRAIL /Apo-2L. (A-B) Human neutrophils were isolated from blood of healthy donors and placed into culture (3 × 106/mL) with BCG (1 CFU/cell), IFN-α (100 or 1000 U/mL), or both for 4 hours. Cells were then examined for surface TRAIL/Apo-2L expression by flow cytometry (A) or the culture supernatant was assayed for TRAIL/Apo-2L levels by ELISA (B). Filled histograms represent staining with isotype mAb; unfilled histograms, staining with anti-TRAIL mAb. (C) Neutrophils (3 × 106/mL) were stimulated in vitro with BCG (1 CFU/cell), IFN-α (100 U/mL), or IFN-γ (100 U/mL), and TRAIL/Apo-2L levels were measured in the culture supernatant over a 24-hour period. (D) Expression of CD62L on unstimulated and BCG-stimulated neutrophils as measured by flow cytometry. (E) IL-8 production by unstimulated, BCG-simulated (1 CFU/cell), or LPS-stimulated (100 ng/mL) neutrophils (3 × 106/mL) was measured by ELISA after 24 hours. Results in panels A, C, and D are representative of at least 3 independent experiments, whereas the results in B and E are averaged from 3 separate experiments.
Neutrophils stimulated with BCG, but not IFN, release TRAIL /Apo-2L. (A-B) Human neutrophils were isolated from blood of healthy donors and placed into culture (3 × 106/mL) with BCG (1 CFU/cell), IFN-α (100 or 1000 U/mL), or both for 4 hours. Cells were then examined for surface TRAIL/Apo-2L expression by flow cytometry (A) or the culture supernatant was assayed for TRAIL/Apo-2L levels by ELISA (B). Filled histograms represent staining with isotype mAb; unfilled histograms, staining with anti-TRAIL mAb. (C) Neutrophils (3 × 106/mL) were stimulated in vitro with BCG (1 CFU/cell), IFN-α (100 U/mL), or IFN-γ (100 U/mL), and TRAIL/Apo-2L levels were measured in the culture supernatant over a 24-hour period. (D) Expression of CD62L on unstimulated and BCG-stimulated neutrophils as measured by flow cytometry. (E) IL-8 production by unstimulated, BCG-simulated (1 CFU/cell), or LPS-stimulated (100 ng/mL) neutrophils (3 × 106/mL) was measured by ELISA after 24 hours. Results in panels A, C, and D are representative of at least 3 independent experiments, whereas the results in B and E are averaged from 3 separate experiments.
Subcellular distribution of TRAIL /Apo-2L in neutrophils
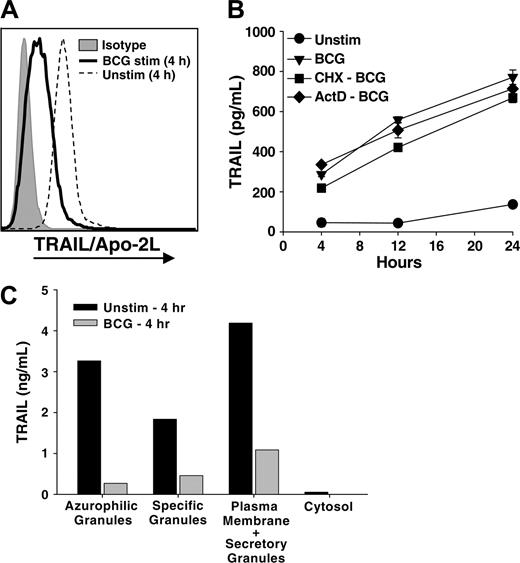
The rapid release of soluble TRAIL/Apo-2L could reflect the release of prefabricated TRAIL/Apo-2L from an intracellular store that is quickly depleted after stimulation or de novo synthesis. To investigate the former possibility, neutrophils were cultured in the absence or presence of BCG for 4 hours, and the intracellular levels of TRAIL/Apo-2L were measured by flow cytometry. Whereas naive neutrophils cultured for 4 hours contained intracellular TRAIL/Apo-2L, BCG-stimulated neutrophils had dramatically lower intracellular levels of TRAIL/Apo-2L (Figure 2A). Freshly isolated, unstimulated neutrophils displayed intracellular TRAIL/Apo-2L levels similar to neutrophils cultured for 4 hours (data not shown). These results suggested that neutrophils possess abundant intracellular stores of TRAILApo-2L; however, we also needed to determine if there was any contribution of de novo synthesis in the BCG-induced release of TRAIL/Apo-2L. To investigate this possibility, we treated neutrophils with the protein and RNA synthesis inhibitors cycloheximide (10 μg/mL)29 or actinomycin-D (1 μg/mL),30 respectively, for 1 hour before adding BCG. Neither agent suppressed the release of TRAIL/Apo-2L by the neutrophils (Figure 2B), indicating that de novo protein synthesis is not required. This result further supports the conclusion that neutrophils possess intracellular stores of prefabricated TRAIL/Apo-2L that can be quickly released with proper stimulation.
Subcellular distribution of TRAIL /Apo-2L in neutrophils. (A) Intracellular neutrophil TRAIL /Apo-2L levels decrease after BCG stimulation. Neutrophils (106/mL) were stimulated in vitro with BCG (1 CFU/cell) for 4 hours, then intracellular TRAIL/Apo-2L levels were determined as described in “Materials and methods.” (B) Inhibition of protein and RNA synthesis does not reduce TRAIL/Apo-2L release from BCG-stimulated neutrophils. Neutrophils (3 × 106/mL) were treated with cycloheximide (10 μg/mL; CHX-BCG) or actinomycin-D (1 μg/mL; ActD-BCG) for 1 hour before stimulation with BCG (1 CFU/cell). TRAIL/Apo-2L levels were measured in the culture supernatant at 4, 12, and 24 hours after adding BCG. (C) Neutrophils (200 × 106) were isolated from peripheral blood and equally aliquoted into 2 groups: one placed into culture for 4 hours without any stimulus, and the other stimulated with BCG for 4 hours. The cells were then fractionated by nitrogen cavitation and density gradient centrifugation as described in “Materials and methods.” The amount of TRAIL/Apo-2L protein in each fraction was quantitated by ELISA. Similar results were obtained in two independent experiments.
Subcellular distribution of TRAIL /Apo-2L in neutrophils. (A) Intracellular neutrophil TRAIL /Apo-2L levels decrease after BCG stimulation. Neutrophils (106/mL) were stimulated in vitro with BCG (1 CFU/cell) for 4 hours, then intracellular TRAIL/Apo-2L levels were determined as described in “Materials and methods.” (B) Inhibition of protein and RNA synthesis does not reduce TRAIL/Apo-2L release from BCG-stimulated neutrophils. Neutrophils (3 × 106/mL) were treated with cycloheximide (10 μg/mL; CHX-BCG) or actinomycin-D (1 μg/mL; ActD-BCG) for 1 hour before stimulation with BCG (1 CFU/cell). TRAIL/Apo-2L levels were measured in the culture supernatant at 4, 12, and 24 hours after adding BCG. (C) Neutrophils (200 × 106) were isolated from peripheral blood and equally aliquoted into 2 groups: one placed into culture for 4 hours without any stimulus, and the other stimulated with BCG for 4 hours. The cells were then fractionated by nitrogen cavitation and density gradient centrifugation as described in “Materials and methods.” The amount of TRAIL/Apo-2L protein in each fraction was quantitated by ELISA. Similar results were obtained in two independent experiments.
Resting neutrophils possess several membrane-bound intracellular compartments that contain potentially reactive molecules spatially segregated until cell activation. The predominant granule populations are categorized as azurophilic granules, specific granules, and secretory vesicles.31 Whereas the azurophilic granules house an array of hydrolytic enzymes and proteases, most notably myeloperoxidase and elastase, the matrix of specific granules lacks proteins with proteolytic activity.31 Furthermore, specific granule membranes possess several functionally important receptors and integral membrane proteins, including complement receptors, flavocytochrome b558, β2 integrins, and the formyl peptide receptor. Given this distribution of matrix and membrane proteins in the different granules, we next determined the intracellular location of TRAIL/Apo-2L within freshly isolated neutrophils. Unstimulated neutrophils were disrupted by N2 cavitation, the fractions separated on a discontinuous Percoll gradient,21 and the amount of TRAIL/Apo-2L in each fraction was quantitated. Unexpectedly, the azurophilic granules and the plasma-membrane–enriched/secretory-granule fraction contained the highest amount of TRAIL/Apo-2L (Figure 2C). We also examined the amount of TRAIL/Apo-2L in the same fractions isolated from neutrophils that had been stimulated with BCG for 4 hours and found much less TRAIL/Apo-2L in all fractions, further indicating that BCG stimulates the secretion/release of TRAIL/Apo-2L from neutrophils.
Heat-killed BCG activates neutrophils to release TRAIL/Apo-2L similar to viable BCG
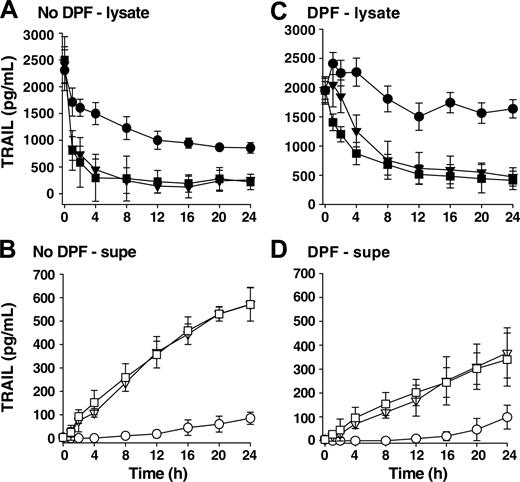
To determine whether the BCG needed to be viable to stimulate TRAIL/Apo-2L release, we stimulated neutrophils with either viable or heat-killed BCG and examined the amount of TRAIL/Apo-2L present within neutrophil cell lysates or culture supernatants. There was no difference in the amount of TRAIL/Apo-2L in either fraction when viable or heat-killed BCG was used (Figures 3A-B), suggesting that one or more subcomponent of BCG that is not dependent on bacteria viability is stimulatory in this setting. We attribute the decline in TRAIL/Apo-2L in unstimulated neutrophil lysates to proteolytic degradation within the cultured neutrophils because there is no concomitant increase in TRAIL/Apo-2L levels in the supernatants. Our conclusion for proteolytic degradation is supported by the observation that unstimulated neutrophils treated with the irreversible protease inhibitor DFP retained most of the TRAIL/Apo-2L within the cell (Figure 3C). Furthermore, there was less TRAIL/Apo-2L detected in the supernatant when neutrophils were stimulated with BCG (either viable or heat-killed) in the presence of DFP (Figure 3D), suggesting that protease activity is needed for maximal TRAIL/Apo-2L release after BCG stimulation.
Heat-killed BCG stimulates TRAIL/Apo-2L release from neutrophils to the same degree as viable BCG and protease activity is needed for maximum TRAIL/Apo-2L release. TRAIL/Apo-2L levels in neutrophil lysates (A,C) and the culture medium (B,D) were determined over a period of 24 hours from unstimulated (•, ○), BCG-stimulated (▪, □), or heat-killed (HK; ▾, ▿) BCG-stimulated neutrophils (3 × 106 neutrophils/mL; 1 CFU/cell). Heat-killed BCG was incubated at 70°C for 1 hour before adding to neutrophils. In addition, some neutrophils were treated with DFP (1 mM) for 20 minutes at room temperature (C-D) before placing into culture. Results are representative of 3 independent experiments.
Heat-killed BCG stimulates TRAIL/Apo-2L release from neutrophils to the same degree as viable BCG and protease activity is needed for maximum TRAIL/Apo-2L release. TRAIL/Apo-2L levels in neutrophil lysates (A,C) and the culture medium (B,D) were determined over a period of 24 hours from unstimulated (•, ○), BCG-stimulated (▪, □), or heat-killed (HK; ▾, ▿) BCG-stimulated neutrophils (3 × 106 neutrophils/mL; 1 CFU/cell). Heat-killed BCG was incubated at 70°C for 1 hour before adding to neutrophils. In addition, some neutrophils were treated with DFP (1 mM) for 20 minutes at room temperature (C-D) before placing into culture. Results are representative of 3 independent experiments.
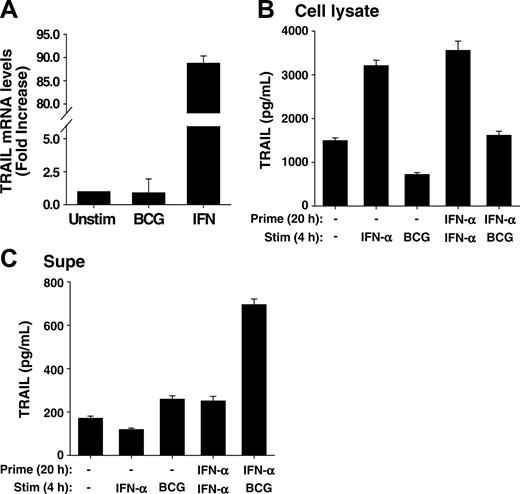
IFN-α increases transcription of the TRAIL gene in neutrophils, and IFN-α-primed neutrophils contain and release elevated amounts of TRAIL/Apo-2L after BCG stimulation. (A) Human neutrophils were isolated from blood of healthy donors and placed into culture (107/mL) with BCG (1 CFU/cell), IFN-α (1000 U/mL), or left unstimulated for 20 hours. Total RNA was then isolated and TRAIL/Apo-2L mRNA levels were quantitated by real-time PCR. (B-C) Neutrophils (3 × 106/mL) were incubated with or without IFN-α (1000 U/mL) for 20 hours, and then stimulated with either IFN-α (1000 U/mL) or BCG (1 CFU/cell) for an additional 4 hours. TRAIL/Apo-2L levels were measured in the neutrophil-cell lysate (B) and culture supernatant (C) by ELISA. All groups are averages of triplicates ± standard deviation.
IFN-α increases transcription of the TRAIL gene in neutrophils, and IFN-α-primed neutrophils contain and release elevated amounts of TRAIL/Apo-2L after BCG stimulation. (A) Human neutrophils were isolated from blood of healthy donors and placed into culture (107/mL) with BCG (1 CFU/cell), IFN-α (1000 U/mL), or left unstimulated for 20 hours. Total RNA was then isolated and TRAIL/Apo-2L mRNA levels were quantitated by real-time PCR. (B-C) Neutrophils (3 × 106/mL) were incubated with or without IFN-α (1000 U/mL) for 20 hours, and then stimulated with either IFN-α (1000 U/mL) or BCG (1 CFU/cell) for an additional 4 hours. TRAIL/Apo-2L levels were measured in the neutrophil-cell lysate (B) and culture supernatant (C) by ELISA. All groups are averages of triplicates ± standard deviation.
IFN stimulates TRAIL gene transcription in neutrophils, and IFN-α–primed neutrophils release elevated amounts of TRAIL/Apo-2L after BCG stimulation
In patients receiving BCG immunotherapy, the neutrophils producing TRAIL/Apo-2L were recruited from the circulation, and thus traversed several cellular barriers, including vascular endothelium and bladder epithelium at the very minimum. The process of transmigration itself primes neutrophils for a variety of agonist-dependent events, as previously demonstrated comparing the function of neutrophils recovered from skin windows or from the circulation of the same individual.32 Moreover, it is possible that IFN, which is also found at high levels in the urine of patients responsive to BCG therapy, provides additional priming cues to the neutrophils entering the bladder. We, therefore, first examined TRAIL/Apo-2L mRNA levels in neutrophils after stimulation with either BCG or IFN-α using quantitative real-time PCR. Whereas BCG did not induce any increase in TRAIL transcripts compared to unstimulated neutrophils, there was almost a 90-fold increase in TRAIL/Apo-2L mRNA levels after IFN-α stimulation (Figure 4A). Seeing this, we predicted IFN-primed neutrophils would have higher intracellular levels of TRAIL/Apo-2L and, consequently, release more TRAIL/Apo-2L after BCG stimulation versus unprimed neutrophils. This was indeed the case because there was roughly twice the amount of TRAIL/Apo-2L protein within IFN-primed neutrophils compared with unprimed cells (Figure 4B), and approximately 3 times as much TRAIL/Apo-2L present in the culture supernatant after IFN-α priming and BCG stimulation compared with neutrophils that were unprimed and stimulated with either IFN-α or BCG or primed and stimulated with IFN-α (Figure 4C). These results further indicate that neutrophils contain intracellular stores of TRAIL/Apo-2L that are readily released after stimulation with an appropriate secretogogue, like BCG, and demonstrate the priming function of IFN in the production of TRAIL/Apo-2L for release from human neutrophils.
Neutrophil stimulation with TLR2 or TLR4 agonists induces TRAIL /Apo-2L release
Neutrophils are the prototypic innate immune cell to respond to bacterial infections, and the effector functions initiated in response to bacteria are dependent on recognition of conserved molecular patterns found in and on the bacteria. The TLR family is an evolutionarily conserved group of proteins responsible for mediating innate immune reactions, especially against bacterial infections, through the recognition of pathogen-associated molecular patterns.33,34 Because TLR2 and TL4 have been implicated to play distinct roles in the host immune response to BCG,35,36 we tested whether specific agonists for these TLRs stimulated TRAIL/Apo-2L release from IFN-primed neutrophils. Indeed, high levels of TRAIL/Apo-2L were detected in the culture supernatant after IFN-primed neutrophils were stimulated with either Pam3CSK (TLR2) or LPS (TLR4; Figure 5A). In contrast, stimulation with poly I:C did not, which was expected because neutrophils do not express TLR3.37 These results suggest that neutrophil recognition of BCG by TLR2 or TLR4 may contribute to the induced release of TRAIL/Apo-2L.
Specific agonists to TLR2 or TLR4 and purified mycobacterial-cell wall stimulated TRAIL/Apo-2L release from neutrophils. (A) Human neutrophils were isolated from blood of healthy donors and placed into culture (3 × 106/mL) with or without IFN-α (1000 U/mL) for 20 hours, and then stimulated with either BCG (1 CFU/cell) or the TLR agonists Pam3CSK, poly I:C, or LPS, at the indicated concentrations, for an additional 8 hours. TRAIL/Apo-2L levels were then measured in the culture supernatant by ELISA. (B) Human neutrophils placed into culture (3 × 106/mL) with BCG (1 CFU/cell), irradiated M tuberculosis whole cells, M tuberculosis whole-cell lysate (WCL), M tuberculosis cell wall (27 000g) fraction, M tuberculosis cytosol fraction, and LAM of M tuberculosis, at the indicated concentrations, for 24 hours. The culture supernatant was assayed for TRAIL/Apo-2L levels by ELISA. ELISA results in panels A-B are averaged from 3 different donors. (C) Neutrophils (3 × 106/mL) were stimulated in vitro with BCG (1 CFU/cell) or E coli (1 CFU/cell), and TRAIL/Apo-2L levels were measured in the culture supernatant over a 24-hour period. Results are averaged from 3 different donors.
Specific agonists to TLR2 or TLR4 and purified mycobacterial-cell wall stimulated TRAIL/Apo-2L release from neutrophils. (A) Human neutrophils were isolated from blood of healthy donors and placed into culture (3 × 106/mL) with or without IFN-α (1000 U/mL) for 20 hours, and then stimulated with either BCG (1 CFU/cell) or the TLR agonists Pam3CSK, poly I:C, or LPS, at the indicated concentrations, for an additional 8 hours. TRAIL/Apo-2L levels were then measured in the culture supernatant by ELISA. (B) Human neutrophils placed into culture (3 × 106/mL) with BCG (1 CFU/cell), irradiated M tuberculosis whole cells, M tuberculosis whole-cell lysate (WCL), M tuberculosis cell wall (27 000g) fraction, M tuberculosis cytosol fraction, and LAM of M tuberculosis, at the indicated concentrations, for 24 hours. The culture supernatant was assayed for TRAIL/Apo-2L levels by ELISA. ELISA results in panels A-B are averaged from 3 different donors. (C) Neutrophils (3 × 106/mL) were stimulated in vitro with BCG (1 CFU/cell) or E coli (1 CFU/cell), and TRAIL/Apo-2L levels were measured in the culture supernatant over a 24-hour period. Results are averaged from 3 different donors.
Mycobacterium cell wall is a potent inducer of TRAIL/Apo-2L release from neutrophils
Our data in Figure 3 indicate that heat-killed and viable BCG each could induce neutrophils to release TRAIL/Apo-2L; thus, we reasoned that one or more BCG subcomponents may be sufficient to elicit a similar response. To investigate this, we tested the ability of several Mycobacterium fractions (irradiated M tuberculosis whole cells, M tuberculosis whole-cell lysate, M tuberculosis cell-wall [27 000g] fraction, M tuberculosis cytosol fraction, and lipoarabinomannan [LAM] of M tuberculosis) to stimulate TRAIL/Apo-2L release from purified neutrophils (Figure 5B). Under these conditions, irradiated M tuberculosis was equipotent as BCG at stimulating the release of TRAIL/Apo-2L at the highest concentration, as anticipated given that M bovis and M tuberculosis are closely related.38 The cell-wall fraction from M tuberculosis was the most potent cellular constituent in eliciting the release of TRAIL/Apo-2L, whereas the whole-cell lysate, cytosol, and LAM were less effective stimuli. Because several studies implicate LAM of Mycobacterium species in eliciting phagocyte responses and functioning as a virulence factor in mycobacteria, we predicted that LAM would induce TRAIL/Apo-2L release. We also looked for signs of neutrophil activation and found that the same fractions that induced TRAIL/Apo-2L release also induced CD62L shedding and IL-8 secretion (data not shown). Because neutrophils can respond to other inflammatory stimuli within the bladder, we also examined the ability of Escherichia coli, which is a common cause of bacterial urinary tract infections,39 to stimulate neutrophils to release TRAIL/Apo-2L. Examination of supernatant from neutrophils stimulated with E coli found TRAIL/Apo-2L levels to be similar to that present in the supernatant from unstimulated neutrophils (Figure 5C), suggesting that the unique molecular composition of BCG is important for stimulating the release of TRAIL/Apo-2L. Collectively, these results suggest that one or more components of the Mycobacterium species cell wall triggers the release of TRAIL/Apo-2L from neutrophils.
Neutrophils contain a cleaved form of TRAIL/Apo-2L that is functional when released after BCG stimulation
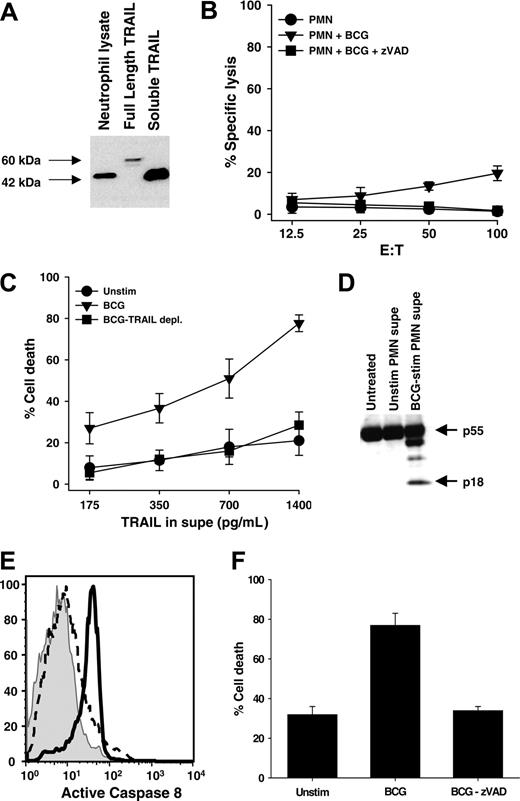
Full-length surface-bound TRAIL/Apo-2L can be cleaved by cysteine proteases to form a soluble form.40 Both the full-length and cleaved TRAIL/Apo-2L can induce apoptosis by binding with the cognate death receptors TRAIL-R1 and TRAIL-R2.41 To determine the predominant form of TRAIL/Apo-2L present in neutrophils, a cell lysate from freshly isolated, unstimulated neutrophils was probed. Amino acid sequence analysis of the full-length TRAIL cDNA predicts a weight of 32.5 kDa for TRAIL monomers,11,12 whereas soluble, recombinant TRAIL (rTRAIL) composed of the extracellular 169 amino acids yields a 19.6-kDa monomer. Dimers of these 2 TRAIL/Apo-2L forms migrate on SDS-PAGE at approximately 60 kDa and 42 to 45 kDa, respectively. With this in mind, we used a cell lysate derived from Ad5-TRAIL–infected tumor cells42 as a source of full-length TRAIL/Apo-2L protein and soluble, rTRAIL/Apo-2L corresponding to the extracellular domain as reference samples. The predominant form of TRAIL/Apo-2L present in unstimulated neutrophils was a cleaved dimer, as judged by SDS-PAGE under nonreducing conditions (Figure 6A).
Because TRAIL/Apo-2L causes apoptosis in numerous tumor cells, including bladder tumor cells, and high levels of soluble TRAIL have been found in the urine of patients following BCG instillation for the treatment of bladder cancer,10 we sought to determine if: (1) the small amount of TRAIL/Apo-2L present on the cell surface of BCG-stimulated neutrophils conferred any cell-mediated tumoricidal activity, and (2) the TRAIL/Apo-2L released following BCG stimulation also retained cytotoxic function. When BCG-stimulated neutrophils were used as effectors against 51Cr-labeled RT-4 bladder tumor cell targets, there was a small, but reproducible, amount of target-cell lysis that was inhibited with the inclusion of the pan-caspase inhibitor zVAD-fmk (Figure 6B). These results are consistent with those in Figure 1A, which showed a small amount of TRAIL/Apo-2L protein on the surface of BCG-stimulated neutrophils. Next, we tested the activity of the soluble TRAIL/Apo-2L released after BCG stimulation. When RT-4 cells were incubated with the culture supernatant of neutrophils that were stimulated with BCG, the tumor cells were killed, but not when cultured with medium from unstimulated neutrophils (Figure 6C). To demonstrate the killing was TRAIL/Apo-2L dependent, we immunodepleted TRAIL/Apo-2L from the BCG-stimulated neutrophil-conditioned medium with a TRAIL/Apo-2L–specific mAb. When this medium was added to the RT-4 cells, the amount to tumor-cell death was reduced to that seen when unstimulated neutrophil medium was added. The cell death induced by the BCG-stimulated neutrophil-conditioned medium was determined to be apoptotic as demonstrated by the activation of caspase-8 (Figure 6D-E), and by the addition of zVAD-fmk to the RT-4 target cells prior to incubation with the BCG-stimulated neutrophil-conditioned medium, which protected the RT-4 cells from apoptotic death and returned the level of tumor-cell death to background levels (Figure 6F).
Neutrophils contain a cleaved form of TRAIL/Apo-2L that retains tumoricidal activity when released into culture supernatant after BCG stimulation. (A) Freshly isolated neutrophils were lysed and TRAIL/Apo-2L was detected by immunoblotting. Full-length TRAIL/Apo-2L from Ad5-TRAIL–infected cells and recombinant soluble TRAIL/Apo-2L protein were used as reference. SDS-PAGE was performed under nonreducing conditions. (B) Cell-mediated tumoricidal activity of BCG-stimulated neutrophils (PMN). Neutrophils were cultured for 20 hours in the absence or presence of BCG (1 CFU/cell), and then added to 51Cr-labeled RT-4 target cells at the indicated effector-target cell ratios for 16 hours. Inclusion of the pan-caspase inhibitor zVAD-fmk (20 μM) inhibited killing of RT-4 target cells. (C-F) Conditioned medium from unstimulated or BCG-stimulated neutrophils was added to RT-4 cells, after which cell death and caspase-8 activation were determined. (C) Crystal violet staining was performed to determine cell death after 24 hours, and a portion of the BCG-stimulated neutrophil supernatant was immunodepleted of TRAIL/Apo-2L, as described in “Materials and methods,” and the resultant killing returning to baseline levels. (D) RT-4 cells were harvested after 8 hours and caspase-8 cleavage was determined by immunoblotting. (E) RT-4 cells were harvested after 6 hours and assayed for caspase-8 activity using carboxyfluorescein-labeled LETD fluoromethyl ketone. (F) The addition of the pan-caspase inhibitor zVAD-fmk (20 μM) blocked cell death back to control levels. Data are representative of 3 independent experiments showing similar results.
Neutrophils contain a cleaved form of TRAIL/Apo-2L that retains tumoricidal activity when released into culture supernatant after BCG stimulation. (A) Freshly isolated neutrophils were lysed and TRAIL/Apo-2L was detected by immunoblotting. Full-length TRAIL/Apo-2L from Ad5-TRAIL–infected cells and recombinant soluble TRAIL/Apo-2L protein were used as reference. SDS-PAGE was performed under nonreducing conditions. (B) Cell-mediated tumoricidal activity of BCG-stimulated neutrophils (PMN). Neutrophils were cultured for 20 hours in the absence or presence of BCG (1 CFU/cell), and then added to 51Cr-labeled RT-4 target cells at the indicated effector-target cell ratios for 16 hours. Inclusion of the pan-caspase inhibitor zVAD-fmk (20 μM) inhibited killing of RT-4 target cells. (C-F) Conditioned medium from unstimulated or BCG-stimulated neutrophils was added to RT-4 cells, after which cell death and caspase-8 activation were determined. (C) Crystal violet staining was performed to determine cell death after 24 hours, and a portion of the BCG-stimulated neutrophil supernatant was immunodepleted of TRAIL/Apo-2L, as described in “Materials and methods,” and the resultant killing returning to baseline levels. (D) RT-4 cells were harvested after 8 hours and caspase-8 cleavage was determined by immunoblotting. (E) RT-4 cells were harvested after 6 hours and assayed for caspase-8 activity using carboxyfluorescein-labeled LETD fluoromethyl ketone. (F) The addition of the pan-caspase inhibitor zVAD-fmk (20 μM) blocked cell death back to control levels. Data are representative of 3 independent experiments showing similar results.
Discussion
When BCG is used clinically to treat superficial bladder cancer, the first and most abundant cells infiltrating the bladder are neutrophils. Recent in vivo studies demonstrated the importance of neutrophils in the immune response to mycobacterial infections,43-45 yet neutrophils have not been recognized as playing any role in the BCG-induced antitumor response within the bladder. Within the context of the BCG response in the treatment of bladder cancer, neutrophils express fatty acid-synthetase ligand and exert nonspecific but effective bystander effects, and produce an array of cytokines, such as IL-1, IL-6, IL-8, IL-12, granulocyte-macrophage colony-stimulating factor (GM-CSF), and TNF, essential for recruiting and activating other immune cells within the bladder.46 Previous studies from our laboratory demonstrated that neutrophils recruited to the bladder by BCG immunotherapy also express TRAIL/Apo-2L,10 and the current study was designed to further investigate the ability of neutrophils to produce TRAIL/Apo-2L when stimulated with BCG. Our results show that neutrophils naturally possess large intracellular stores of a cleaved, but functional, form of TRAIL/Apo-2L that is rapidly released when directly stimulated with BCG. In contrast, only a very low, but reproducible, increase in surface TRAIL/Apo-2L expression occurred after BCG stimulation. Because a proinflammatory, Th1 cytokine (especially IFN-γ) response predominates during BCG immunotherapy, and the Th1 response is often associated with a favorable clinical response,7-9 it was also necessary to see how neutrophils responded to IFN in our hands. Contrary to previous reports,18,19,24 we were unable to detect any increase in surface or soluble TRAIL/Apo-2L after stimulation with IFN-α or IFN-γ. The amount of surface TRAIL/Apo-2L reported in those studies was not overwhelming, and the differences from those studies may be related to donor variability, mAb used, or even neutrophil isolation techniques. Koga et al18 and Tecchio et al19 used the same brand of ELISA kit as we did, but they used different cell numbers (Koga et al, 107/mL; Tecchio et al, 5 × 106/mL) and only measured just over 500 and 400 pg/mL soluble TRAIL/Apo-2L after IFN stimulation, making it possible that cell number and donor variability led to the inconsistency between our results and theirs. Additional studies with IFN-α found it was a potent inducer of TRAIL transcription within neutrophils, and IFN-α–primed neutrophils released greater amounts of TRAIL/Apo-2L after BCG stimulation. The intriguing priming/stimulating combination of IFN and BCG is attractive clinically because it provides additional support for the correlation between the high urinary levels of proinflammatory cytokines, including IFN, high urinary TRAIL/Apo-2L levels, and responsiveness to BCG therapy.
Most antimicrobial responses by neutrophils occur rapidly, within minutes, and rely on redistribution of proteins within the neutrophil rather than de novo synthesis of bioactive molecules.47 For example, oxygen-dependent killing of ingested microbes reflects the generation of toxic molecules by the interaction of myeloperoxidase, a protein synthesized during the promyelocytic stage of neutrophil maturation in the bone marrow, stored in azurophilic granules, and released into the phagosome by agonist-dependent degranulation, and reactive oxygen species generated by nicotinamide adenine dinucleotide phosphate (NADPH) oxidase. The storage of TRAIL/Apo-2L within intracellular granules follows the same paradigm, although its detection in azurophilic granules of resting neutrophils was unexpected. The presence of TRAIL/Apo-2L within this intracellular compartment may provide an explanation why the TRAIL/Apo-2L is present in a cleaved form within neutrophils, because azurophilic granules of neutrophils contain numerous proteolytic enzymes, including serine proteases, cathepsin G, proteinase 3, elastase, and cysteine proteases.31 Alternatively, the cleaved form might be generated during the disruption and subcellular fractionation of neutrophils. Mariani and Krammer48 suggested that membrane-bound TRAIL/Apo-2L is cleaved by cysteine proteases, not by metalloproteases. Incubation of neutrophils with the protease inhibitor DFP prior to BCG stimulation also retarded the release of TRAIL/Apo-2L into the culture supernatant, suggesting a protease function in this part of the neutrophil response also. At this time, it is unknown which protease is responsible for cleaving TRAIL/Apo-2L within the cell and facilitating its release after BCG stimulation.
It was intriguing to see that the BCG-stimulated neutrophil-conditioned medium was able to be such a potent inducer of bladder tumor cell death, despite containing a relatively low concentration of TRAIL/Apo-2L. Our results indicate that TRAIL/Apo-2L was the sole inducer of tumor-cell death because the immunodepletion of TRAIL/Apo-2L from BCG-stimulated neutrophil-conditioned medium abolished all tumoricidal activity (Figure 6C). We have observed significant RT-4–cell death with as little as 1 ng/mL rTRAIL/Apo-2L (T.J.K. and T.S.G., unpublished observation, January 2005); however, this does not preclude the possibility that BCG-stimulated neutrophils cannot produce or secrete other factors that could aid in the induction of tumor-cell death. For example, neutrophils stimulated with Mycobacterium can secrete TNF,49,50 which not only has tumoricidal activity but can also help to sensitize cells to death-receptor–mediated apoptosis.51 In addition, Mycobacterium-stimulated neutrophils can secrete reactive oxygen species that could also increase tumor-cell sensitivity to apoptosis-inducing molecules. Further study is needed to investigate such possibilities.
From the clinical perspective one complication of using BCG for bladder cancer is infection, including both local and disseminated disease. As a result, thought has been put into identifying an equally effective nonviable preparation of BCG free of any potential side effects. Data in Figure 3 demonstrate that heat-killed and viable BCG are equipotent in eliciting TRAIL/Apo-2L release from neutrophils, suggesting that one or more BCG subcomponents may be sufficient to elicit the beneficial effects of inducing TRAIL/Apo-2L release from neutrophils, but free of the risks of unintended complicating infection. Furthermore, data derived from studies of BCG-infected murine macrophages indicate that mycobacterial surface proteins are released from the microbe and incorporated into the phagosomal membrane and other subcellular compartments of the phagocyte.52 Such components may mediate biologic effects seen during BCG-phagocyte interactions. Mycobacterial antigens can be divided into several groups based on subcellular localization, whether they are cytosolic, and whether they are cell-wall associated or extracellular (ie, secreted in the culture medium). Zlotta et al53 isolated several mycobacterial antigens from BCG and analyzed their effectiveness in stimulating cytokine production from PBMCs in vitro. Our investigation determined that the cell-wall fraction of Mycobacterium strongly induced the release of TRAIL/Apo-2L. The inability of LAM to induce TRAIL/Apo-2L release was surprising because it was a potent inducer of IFN-γ from PBMCs,53 and the immunostimulatory capacity of LAM has been implicated in several studies by eliciting phagocyte responses and functioning as a virulence factor in mycobacteria. It is possible that the purified LAM was ineffective because it was not internalized by the neutrophils and compartmentalized in the same context, as it would have been with the whole cells or cell wall.
In patients receiving BCG immunotherapy, the intravesical neutrophils that produce TRAIL/Apo-2L have been recruited from the circulation, and thus already traversed several cellular barriers, including vascular endothelium and bladder epithelium at the very minimum. The process of transmigration itself primes neutrophils for a variety of agonist-dependent events, as previously demonstrated comparing the function of neutrophils recovered from skin windows or from the circulation of the same individual.32 Understanding the changes that occur in neutrophils as they migrate into the bladder after BCG instillation is important for understanding their role in the BCG response and TRAIL/Apo-2L production/release. Furthermore, after repeated BCG installations the early accumulation of granulocytes is followed by the influx of Mϕ and lymphocytes (both T and B cells).54,55 Given that multiple immune-cell types, and even bladder-tumor cells, express TRAIL/Apo-2L after IFN-stimulation,13-19,56 and PBMCs stimulated in vitro with BCG produce IFN-α and IFN-γ (T.S.G., unpublished observation, August 2004), we predict that later in the clinical response to BCG, these populations will also express TRAIL/Apo-2L as they enter the bladder with its increasing IFN levels. Additionally, whereas other inflammatory stimuli recruit neutrophils to the bladder (eg, bacterial urinary tract infection with E coli),39 they are presumably not effective as anticancer agents because, unlike BCG, they do not contain the same immunostimulatory proteins or polysaccharides that induce TRAIL/Apo-2L release. Most studies of mycobacteria-phagocyte interactions have focused on M tuberculosis and the monocyte or macrophage, reflecting the clinically relevant biology. Much less is known about the human neutrophil-BCG interface and no studies have focused on the precise kinetics and consequences of phagosome maturation during neutrophil engagement of BCG. Furthermore, understanding the subcellular fate of BCG after phagocytosis may be important in understanding why viable BCG is needed for effective bladder cancer therapy. It is possible that the active process of phagocytosis or passage of BCG into the intracellular compartment of neutrophils induces TRAIL/Apo-2L release.
In conclusion, we present a mechanism by which neutrophils partake in the antitumor response within the bladder after BCG instillation. These studies will be a guide to better understanding neutrophil biology in this clinical situation, and could serve as a basis for investigating the potential use and role of BCG subcomponents in the antitumor response noted in the treatment of bladder cancer.
Prepublished online as Blood First Edition Paper, July 21, 2005; DOI 10.1182/blood-2005-03-1327.
Supported by a Carver Medical Research Initiative Grant administered through the University of Iowa Carver College of Medicine (T.S.G.) and National Institutes of Health grants CA109446-01 (T.S.G.) and AI 034879-17 (W.M.N.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors wish to thank Drs. Bennett Elzey, Michael O'Donnell, and Timothy Ratliff for careful reading of the manuscript.