Abstract
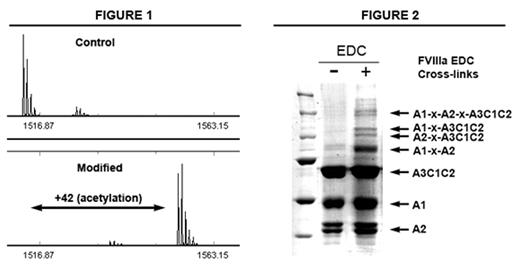
Activated factor VIII (FVIIIa) is a complex of three subunits, designated A1, A2, and A3C1C2, that serves as a cofactor for factor IXa in the proteolytic activation of factor X. The structure and surface interactions between factor VIIIa subunits have yet to be fully defined, but a homology-based model is available as a predictive tool (Stoylova-McPhie et al, Blood, 2002). To investigate FVIIIa inter-subunit interactions we used a chemical modification approach to determine surface-exposed and potentially interactive residues. The protein modification reagents acetic anhydride and diethylpyrocarbonate (DEPC), that modify lysine and histidine residues, respectively, were used to identify residues that are modified in the free subunits but protected in the bound complex. The modified samples were digested with various proteases and the resulting digests were analyzed by MALDI-TOF mass spectrometry to determine sites of peptide modification (Figure 1). The majority of data observed was in agreement with the A1/A3C1C2 interface in the predicted model. Protection was observed at Lys-89, Lys-142, and Lys-230 in the A1 subunit, all of which are near the A3C1C2 interface. While most His residues were completely modified by DEPC, partial protection was seen at His-1954, His-1957, and His-1961 in A3C1C2, which we have previously shown to be an A1-interactive region (Ansong and Fay, Biochemistry, 2005). In addition to this data, we attempted to define inter-subunit contacts by covalently cross-linking the FVIIIa complex. Using the zero-length cross-linker 1-ethyl-3-[3-dimethylaminopropyl] carbodiimide (EDC), which cross-links Glu/Asp residues to Lys, four distinct cross-linked bands were identified by SDS-PAGE (Figure 2). The composition of the cross-linked products was determined using proteolytic digestion and MALDI analysis. The four products correspond to each possible combination of the three subunits. Interestingly, there are no candidate cross-link sites between A1 and A2 in the model, yet this cross-link is the most predominant of the four. One alternative is that the A1–A2 cross-link may involve the A1 337–372 region that is not represented in the model. Taken together, the data are directed toward physically defining interactive regions between FVIIIa subunits and serve to test and supplement current structure models.
Author notes
Corresponding author