Abstract
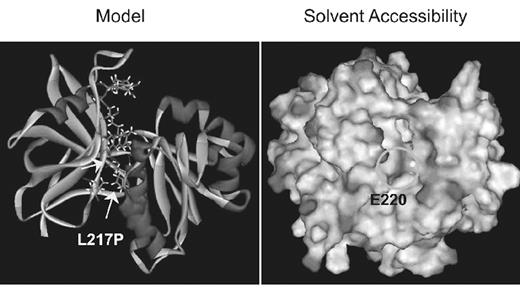
Congenital methemoglobinemia caused by cytochrome b5 reductase(b5R) deficiency is sporadic worldwide, although clusters of this disorder have been identified in certain ethnic groups. Several case reports of congenital methemoglobinemia have been reported from India, most with cytochrome b5 reductase deficiency. However, as of yet, no cytochrome b5 reductase Indian mutation has been identified. Here we describe the first molecular basis of b5R deficiency to cause methemoglobinemia in India. The propositus is a 22 year old Indian male who was born from a consanguineous union in Southern India. He was cyanotic since birth. He had no neurologic deficits and no known exposures to drugs or toxins. Congenital methemoglobinemia was suspected after an extensive cardiopulmonary workup was unrevealing. He had an elevated methemoglobin level of 16%. Hemoglobin M was not present. Spectrophotometric assay of b5R activity in erythrocytes was 4% of normal mean, while his father had normal enzyme activity and his mother and sister had 35 and 45% of normal, respectively. b5R activity in the propositus’ platelets and granulocytes was normal, consistent with a diagnosis of type 1 methemoglobinemia. Genomic DNA was PCR amplified and sequenced. A mutation was found in exon 8 in the second position of codon 217 which is a T to C transversion at position 25985 in the gene which changes the amino acid at that position from leucine to proline(L217P). The propositus was homozygous for the mutation, while both parents and sister were heterozygous for this mutation, confirming the autosomal recessive pattern of inheritance of type 1 methemoglobinemia. b5R mRNA levels were elevated in all family members, a finding not previously reported with any b5R mutation. We also show that measurement of enzyme activity is not reliable for detection of heterozygosity of b5R deficiency as his father had normal enzyme activity. The L217P mutation, located in the NADH binding domain, lies in a region of the sequence that is highly conserved throughout evolution from yeast to man, suggesting its essential importance in enzyme function. We examined the effect of this mutation by replacing the wild-type residue with the mutant one in the human b5R structure(PDB:1UMK) using the program Insight II. As residue 217 lies at the base of alpha helix Nα3, the L217P mutation causes steric hindrance with contacts between the proline side-chain and the main chain before energy minimization. Following energy minimization, no major changes in main-chain path were observed. However, contact alterations with residues within 3.0A radius of residue 217 were recorded. Since residue 217 is located within a hydrophobic pocket we also examined the effect of the mutation on solvent accessibility. Our model predicts that glutamate 220, a polar amino acid, is buried deeper in the hydrophobic pocket in the mutant protein. Assuming our model is accurate, the mutation is predicted to result in enzyme instability, thus explaining the type 1 phenotype of the propositus. Biochemical studies are underway to test the validity of our model. It remains to be seen if this disorder is endemic in India as it is in other ethnic groups.
Author notes
Corresponding author