Abstract
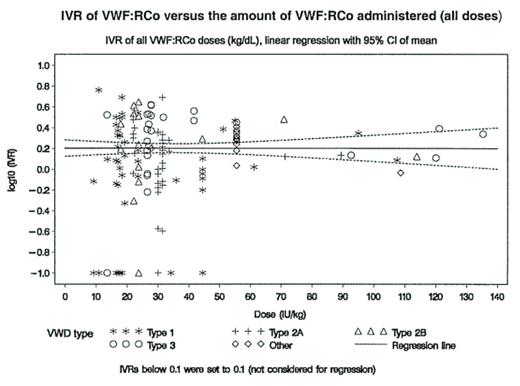
The VWF/FVIII concentrate Humate-P® (ZLB Behring) was studied in a prospective, uncontrolled, open-label clinical trial in VWD patients undergoing elective surgery. An interim analysis was performed after 24 patients (18 female, 6 male) with the following types of VWD had been included into the study: type 1 (n=11), type 2A (n=2), type 2B (n=4), type 2M (n=2) and type 3 (n=5). All patients underwent the pre-operative PK assessment to individualize the loading and maintenance doses of Humate-P® and 18 patients had completed surgery. The primary objective of the study - to demonstrate safety and hemostatic efficacy - was achieved in 100% of the patients. All patients received at least two infusions: a single infusion of 60 IU VWF:RCo per kg b.w. for a pharmacokinetic (PK) assessment prior to surgery and a PK guided loading dose before start of surgery. In addition, all patients received more infusions for further treatment after surgery. PK results: VWF:RCo: The overall median IVR was 2.1 [% per IU/kg] (range 1.1–4.2). The overall median response was 75.3% (range 37.0–169.4). Type 3 VWD patients had a slightly lower median response of 73.4% (range 63.7–96.1). Median value of Cmax was 142 IU/dL (range 84–330). Median effective half-life was 6.7 h (range 0.2–74.9), median clearance 3.6 [mL/kg/h] (range 1.0–16.6). FVIII:C: The median IVR was 2.8 [% per IU/kg] (range 1.4–4.9). Type 3 VWD patients had a median IVR of 3.4 [% per IU/kg] (range 1.7–4.9). The median response was 96.4% (range 63.0–164.8). Type 3 VWD patients had a median response of 104.5% (range 63.0–155.3). Dose-linearity: Graphical presentation of the relation between IVR and dose administered demonstrated the dose linearity of the VWF concentrate within a wide dose range.
IVR of VWF:RCo versus the amount of VWF:RCo administered (all doses)
IVR of VWF:RCo versus the amount of VWF:RCo administered (all doses)
Statistical modeling using all doses (loading dose and maintenance infusions) supported this finding. Using the log-transformed IVR plotted against the dose administered the slope was 0.0004 (95% confidence interval: −0.0045 to 0.0053. Conclusion: The PK results are comparable to PK data from other clinical studies with Humate-P®/Haemate P® and given the interindividual variability underline the importance of pharmacokinetic studies prior to surgical interventions. Dose linearity of VWF:RCo with Humate-P® has been shown over a wide range of dosages (10–135 IU/kg b.w.). These results increase our confidence in the treatment of VWD patients with Humate-P®.
Author notes
Corresponding author