Abstract
The CALGB conducted a series of clinical trials with azacitidine (Vidaza®) administered subcutaneously or intravenously in patients with MDS using the FAB classification (
Best Response using IWG Response Criteria for WHO AML Patients in Studies 8421, 8921, and 9221
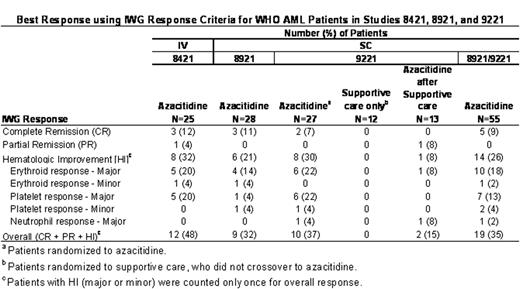
Median duration of any response (CR, PR or HI) in the 33 azacitidine-treated responders was 279 days (range: 61 to 724 days). The median duration of CR in the 8 azacitidine-treated responders was not achieved; however, the 25th percentile was 115 days (range: 92 to 274+ days). In Study 9221, the median duration of transfusion independence (defined as ≥56 days) in patients independent at baseline was significantly longer in the azacitidine group compared with supportive care for red blood cells (azacitidine [n=8]: 411 days vs. supportive care [n=9]: 133 days, p=0.02) and platelets (azacitidine [n=13]: 363 days vs. supportive care [n=18]: 125 days, p=0.004). In the azacitidine group, 22% (6/27) of patients had a hemoglobin improvement to >11 g/dL that was maintained for ≥56 days compared with 8% (2/25) in the supportive care group (p=0.2). The proportions of patients with ANC >1500/m3 and platelets >100,000/mm3 lasting for ≥56 days were similar between the treatment arms. Azacitidine patients with WHO AML had a longer median survival (19.3 months) compared with the supportive care group (12.9 months) (p=0.2). Further studies investigating azacitidine in patients with AML with dysplasia are warranted.
Author notes
Corresponding author

