Abstract
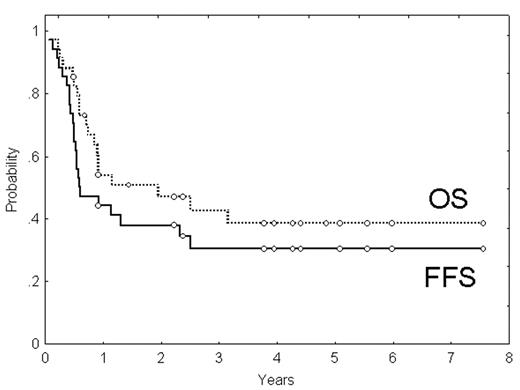
The outcome of patients (pts) with PTCL receiving conventional therapy is dismal. Because of this, there is an increasing interest to investigate intensive treatments in these pts. The aim of the study is to analyze the interim results, in terms of toxicity, response and outcome, of a phase II trial that includes high-dose chemotherapy (CT) plus ASCT as 1st-line treatment for pts with PTCL. Thirty-four pts (23M/11F; median age: 47 yrs.) diagnosed with PTCL (excluding cutaneous and anaplastic ALK+), in stages II–IV and ≤65 yrs, who have already finished planned therapy, are the subject of this analysis. Nine pts (26%) presented with primary extranodal disease, 24 (71%) were in stage IV, and 13 (38%) had bone marrow involvement. Fifty percent of the pts had high/intermediate or high-risk IPI, whereas 53% were in the groups 3 or 4 according to the Italian Index for PTCL. Pts received intensive CT (3 courses of high-dose CHOP [cyclophosphamide 2000 mg/m2 day 1, adriamycin 90 mg/m2 day 1, vincristine 2 mg day 1, prednisone 60 mg/m2/day, days 1 to 5, with mesnum and G-CSF], alternating with 3 courses of standard ESHAP). Responders (CR or PR) were submitted to ASCT. Median follow-up of surviving pts was 3.9 yrs (range, 0.5–7.6). Twenty four pts (71%) received the planned 6 courses of CT. Response rate after CT was: CR, 12 cases (35%); PR, 8 (23%); failure, 14 (42%), including one pt who died because of sepsis. Hematological toxicity of CT mainly consisted of neutropenia (grades 3–4 in 87 and 62% after high-dose CHOP and ESHAP, respectively) and thrombocytopenia (grades 3–4 in 63 and 68%, respectively). Severe infection requiring hospitalization was observed in 38 and 15% of courses of high-dose CHOP or ESHAP, respectively. Only 14 of the 20 candidates (70% of all candidates and 41% of all pts) received ASCT due to lack of stem-cell mobilization (3 cases), previous toxicity (2) and pt decision (1). No differences in the outcome were seen among these 20 pts according to whether or not they could eventually receive ASCT. No major toxicity was observed after ASCT. The overall response after the whole treatment was: CR, 14 cases (41%), PR, 5 (15%), failure, 15 (44%). Two of 14 pts in CR and the 5 pts in PR eventually progressed. Four-year failure-free survival (FFS) was 30%, whereas 4-year disease-free survival for pts achieving CR was 63%. Nineteen pts have died during follow-up, with a 4-yr overall survival (OS) of 38% (95%CI: 21–55%). Most patients died because of PTCL progression, but 2 pts died in CR due to a secondary acute lymphoblastic leukemia and to a lung cancer, respectively. IPI and Italian Index for PTCL were able to predict FFS and OS. In summary, in this series of patients with PTCL a relatively high CR rate was obtained with high-dose CHOP/ESHAP followed by ASCT. Toxicity was manageable. However, the prognosis of patients with PTCL, particularly of those not achieving CR, is still very unfavorable.
Author notes
Corresponding author